Preparation of NH2-MIL-101(Fe) Metal Organic Framework and Its Performance in Adsorbing and Removing Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of NH2-MIL-101(Fe)
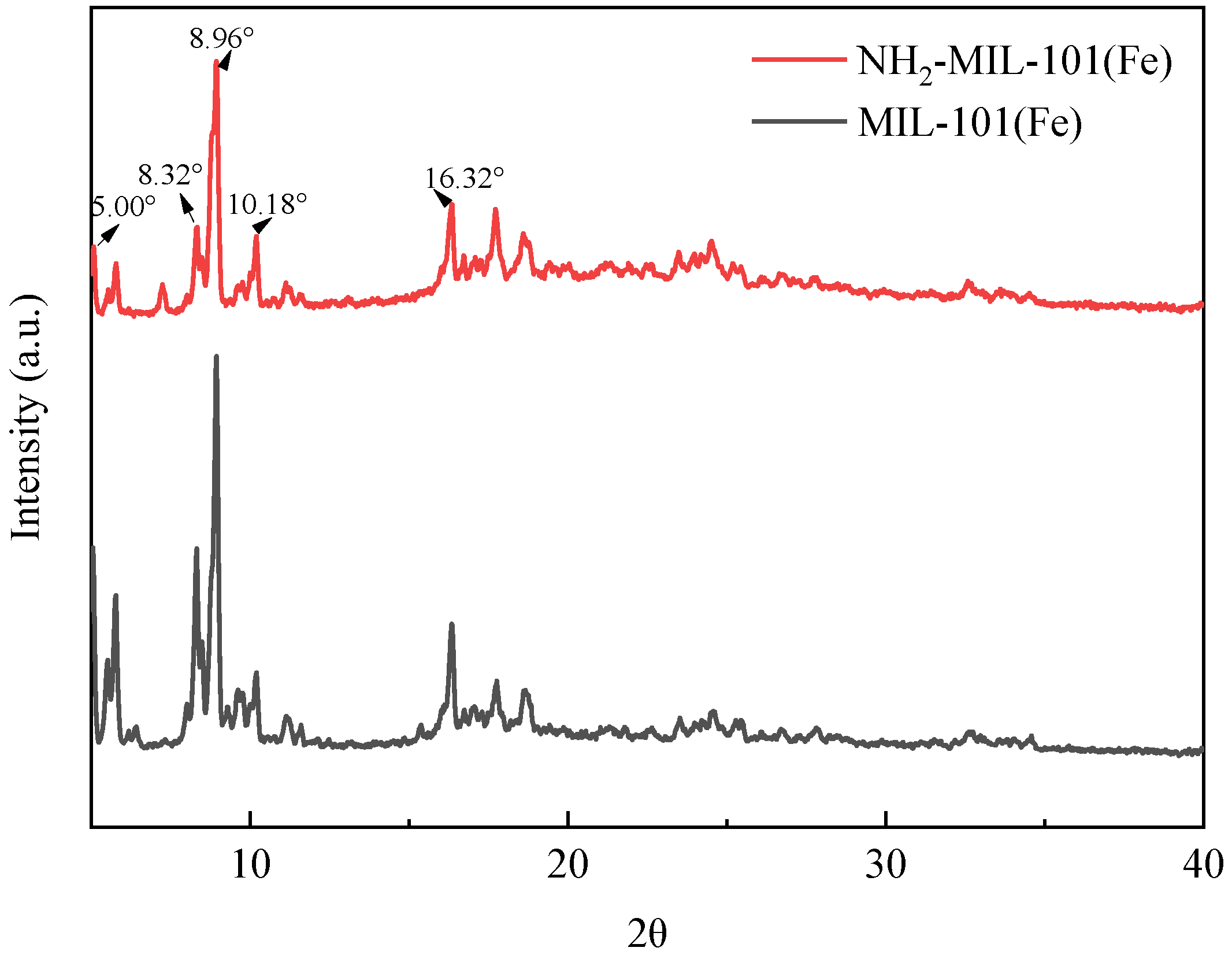
2.1.1. X-ray Diffraction Analysis (XRD)
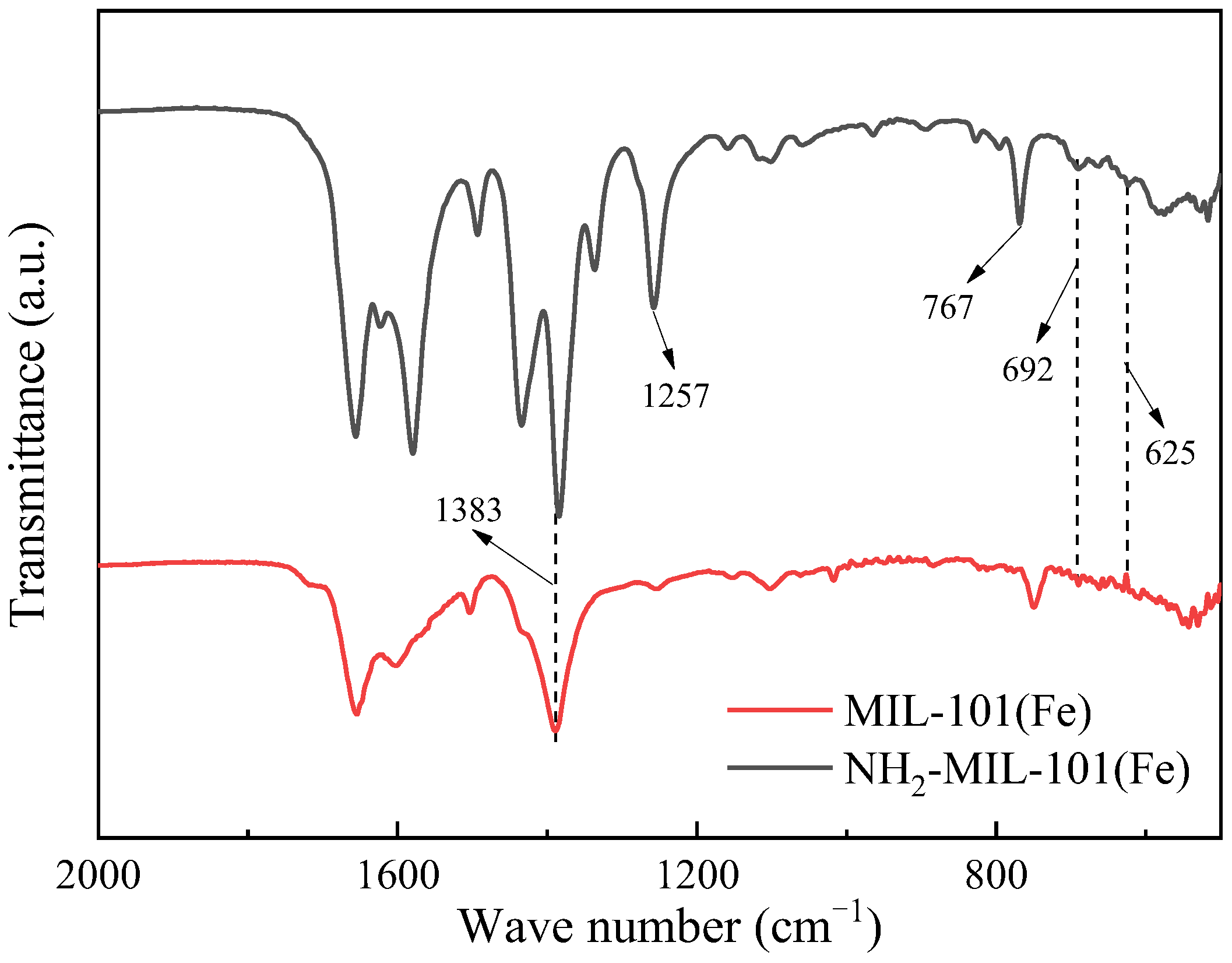
2.1.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
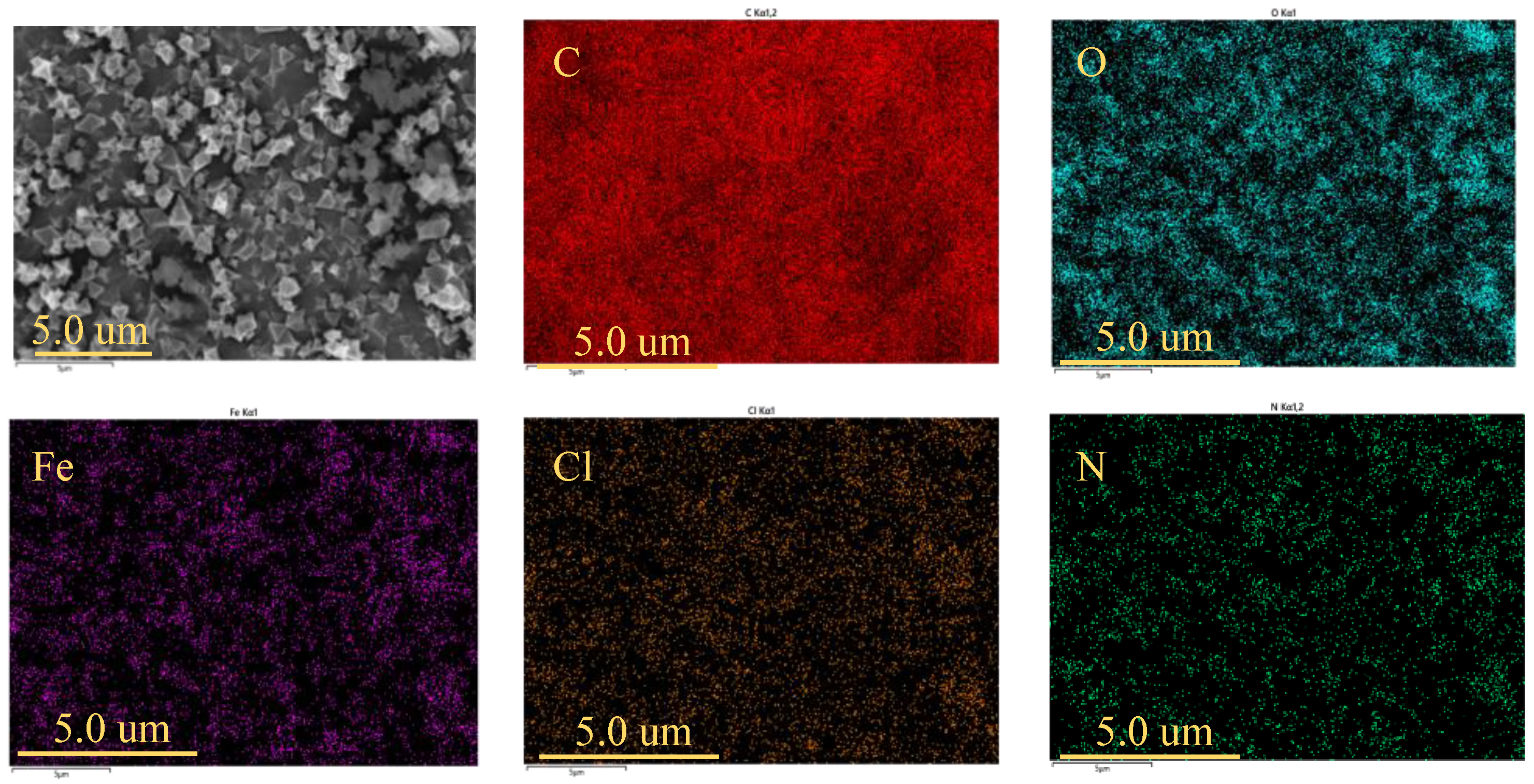
2.1.3. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy (EDS)
2.1.4. X-ray Photoelectron Spectroscopy (XPS)
2.1.5. Specific Surface Area and Pore Size Analysis (BET)
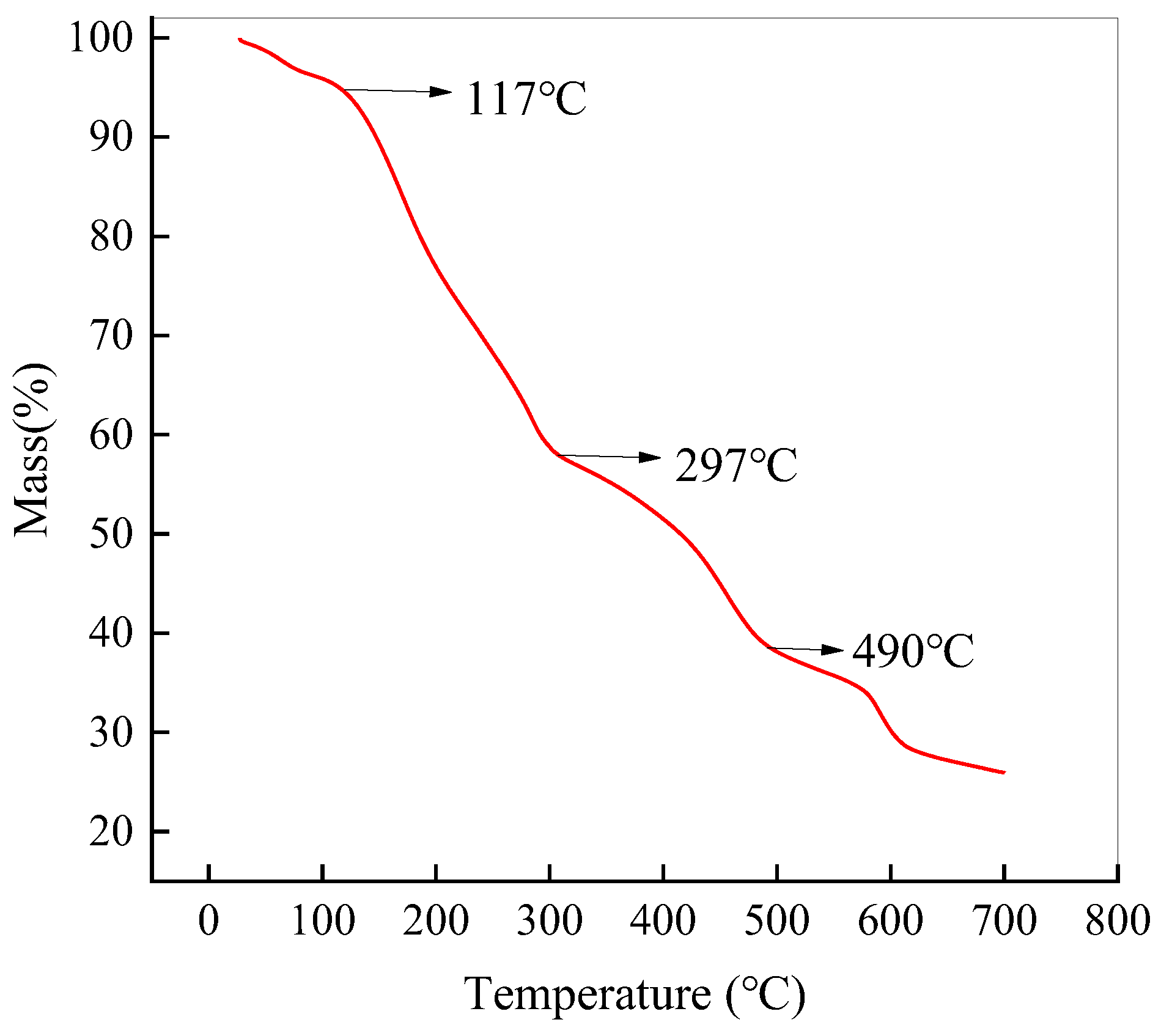
2.1.6. Thermogravimetric Analysis (TGA)
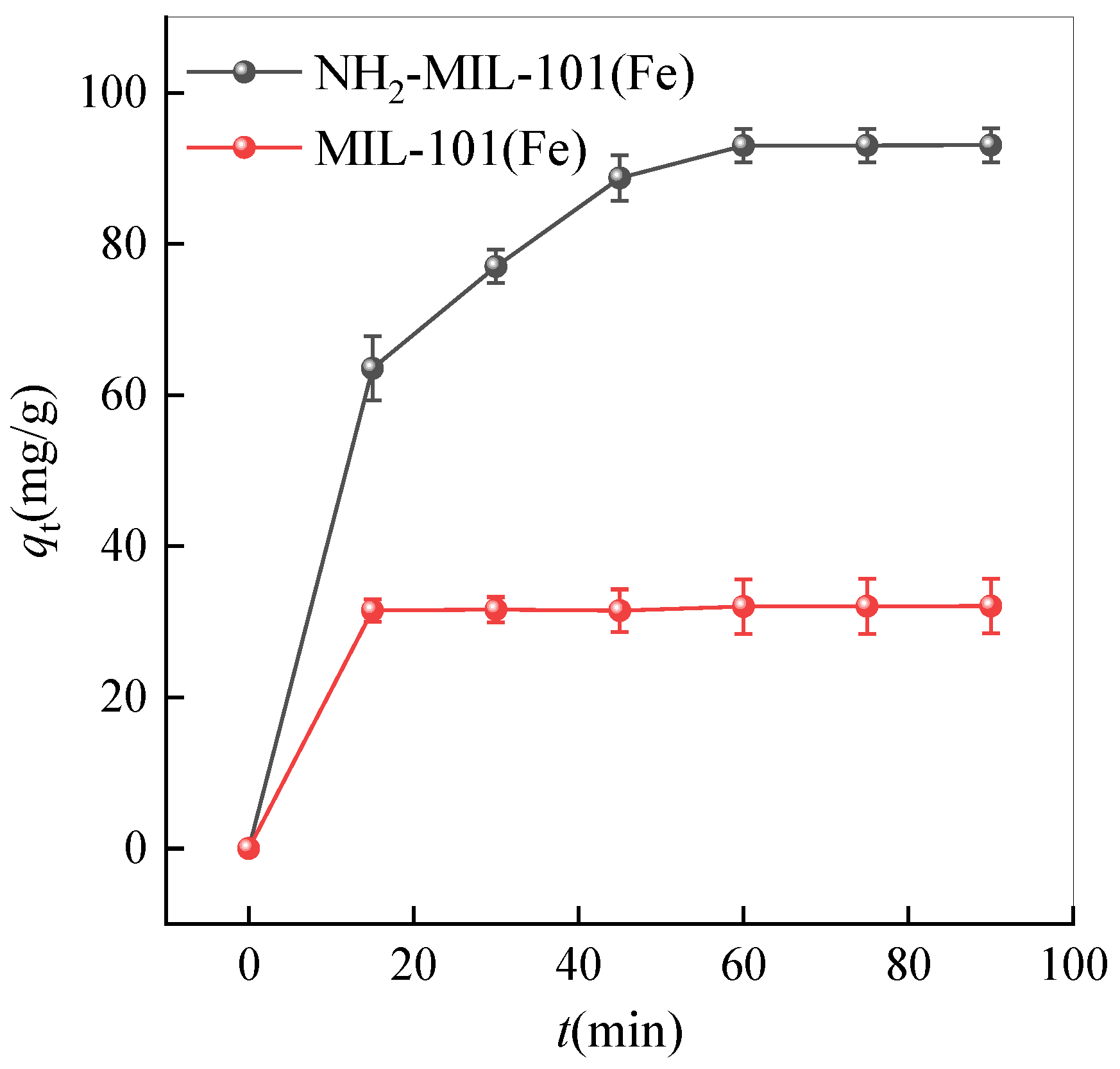
2.2. Adsorption Kinetics Analysis
2.2.1. Pseudo-First-Order Kinetic Model
2.2.2. Pseudo-Second-Order Kinetic Model
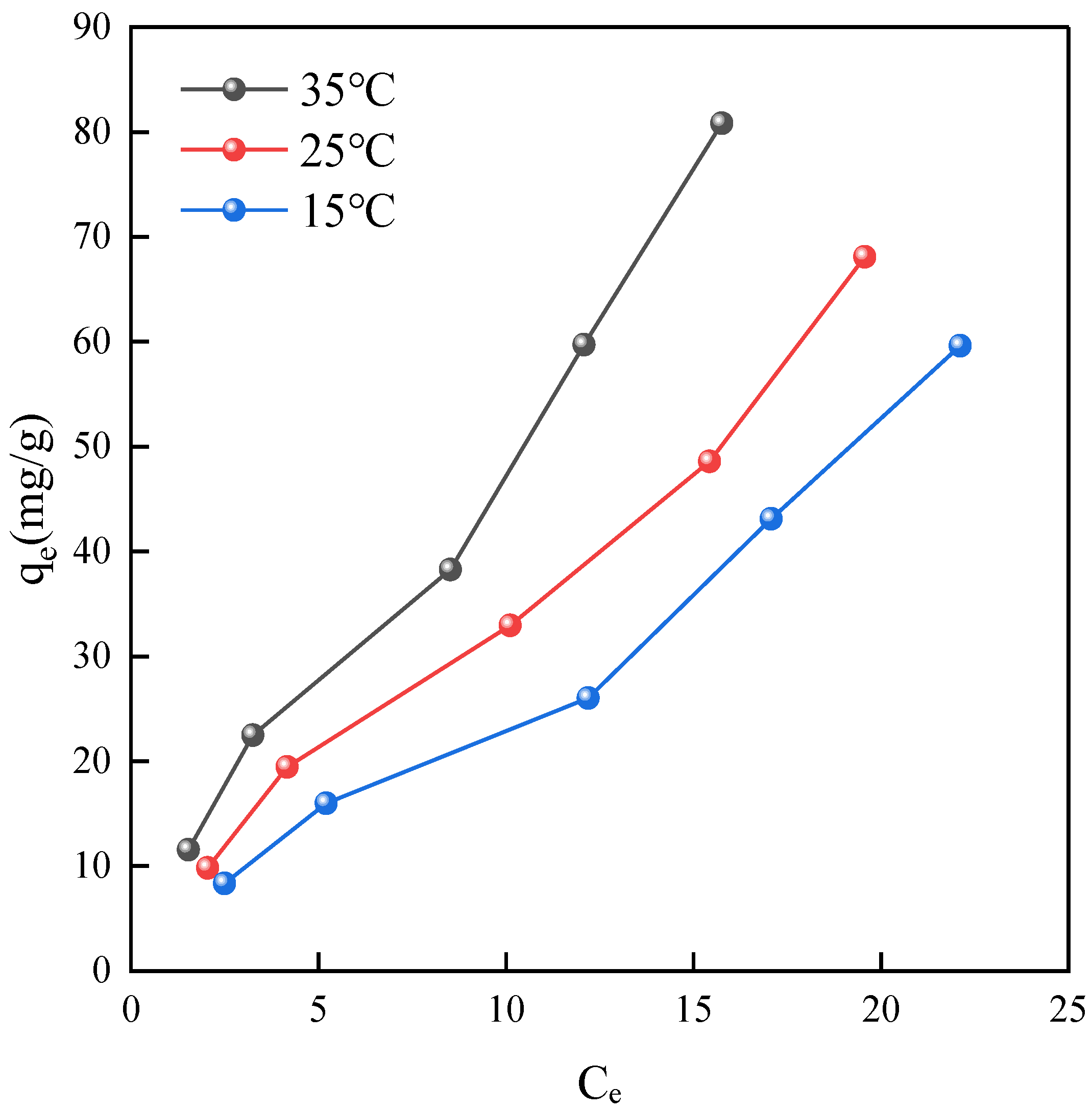
2.3. Adsorption Isotherm Analysis
2.3.1. Adsorption Isotherms
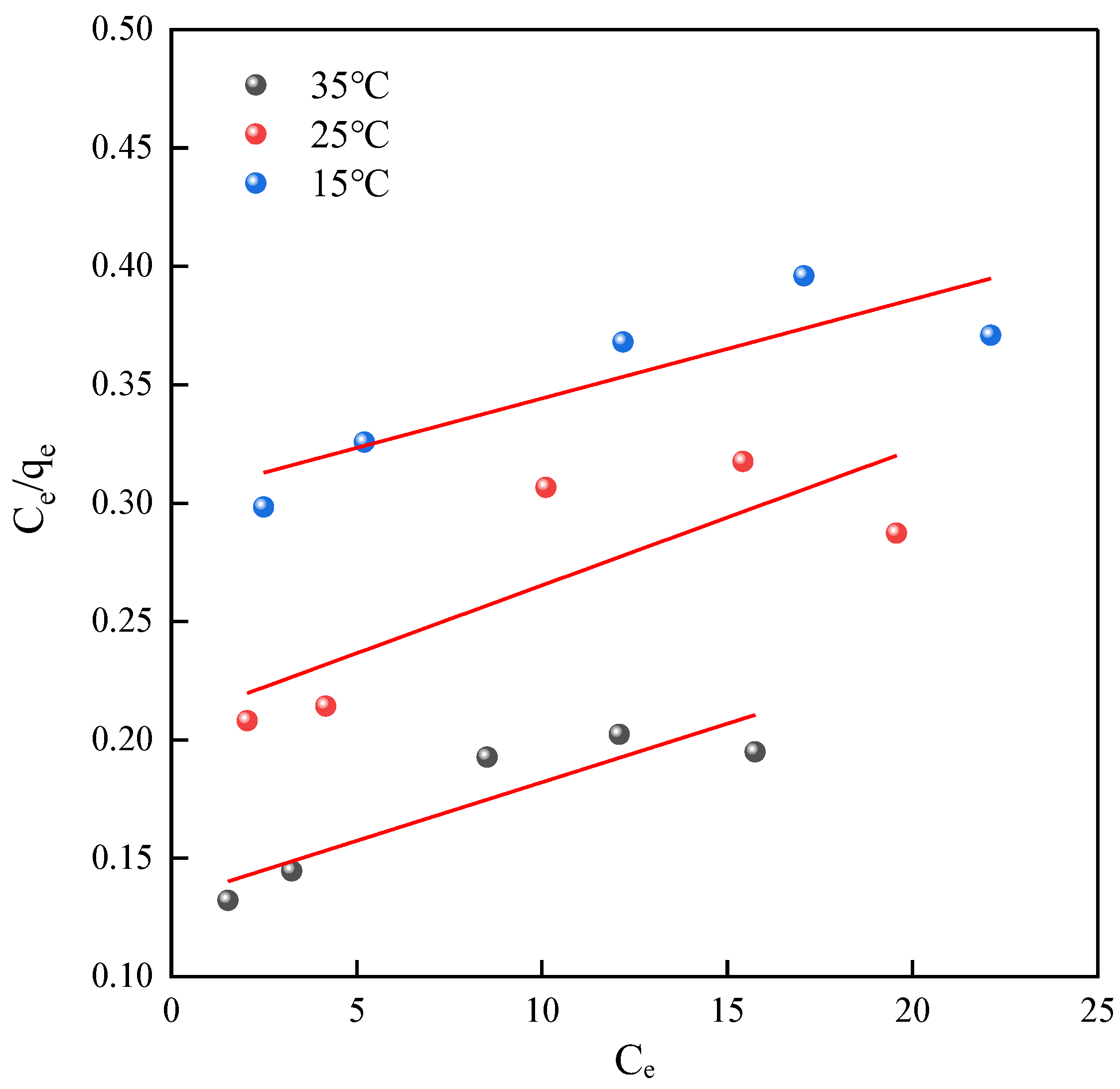
2.3.2. Langmuir Model Adsorption Isotherm Fitting
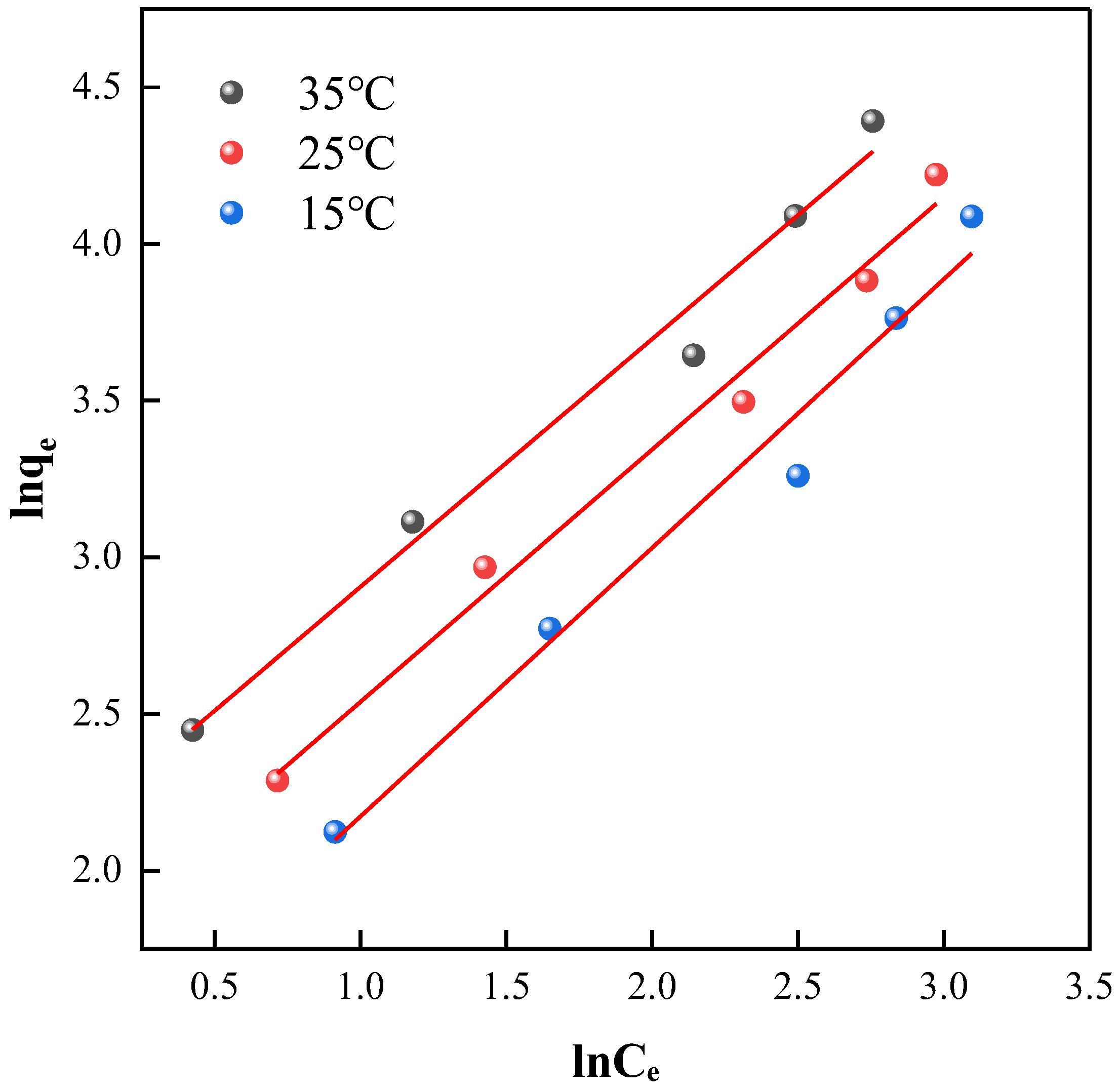
2.3.3. Freundlich Model Adsorption Isotherm Fitting
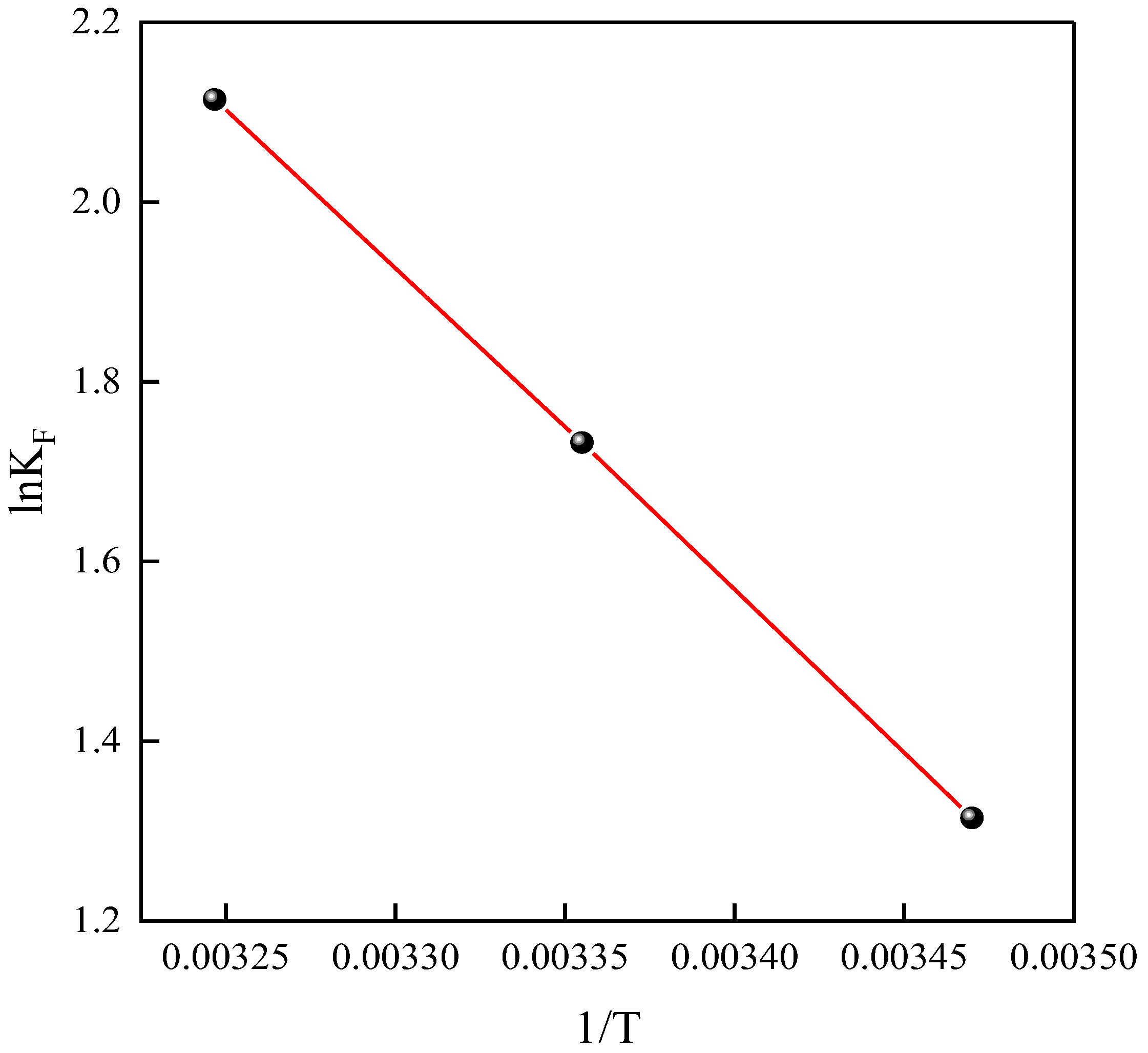
2.4. Thermodynamics
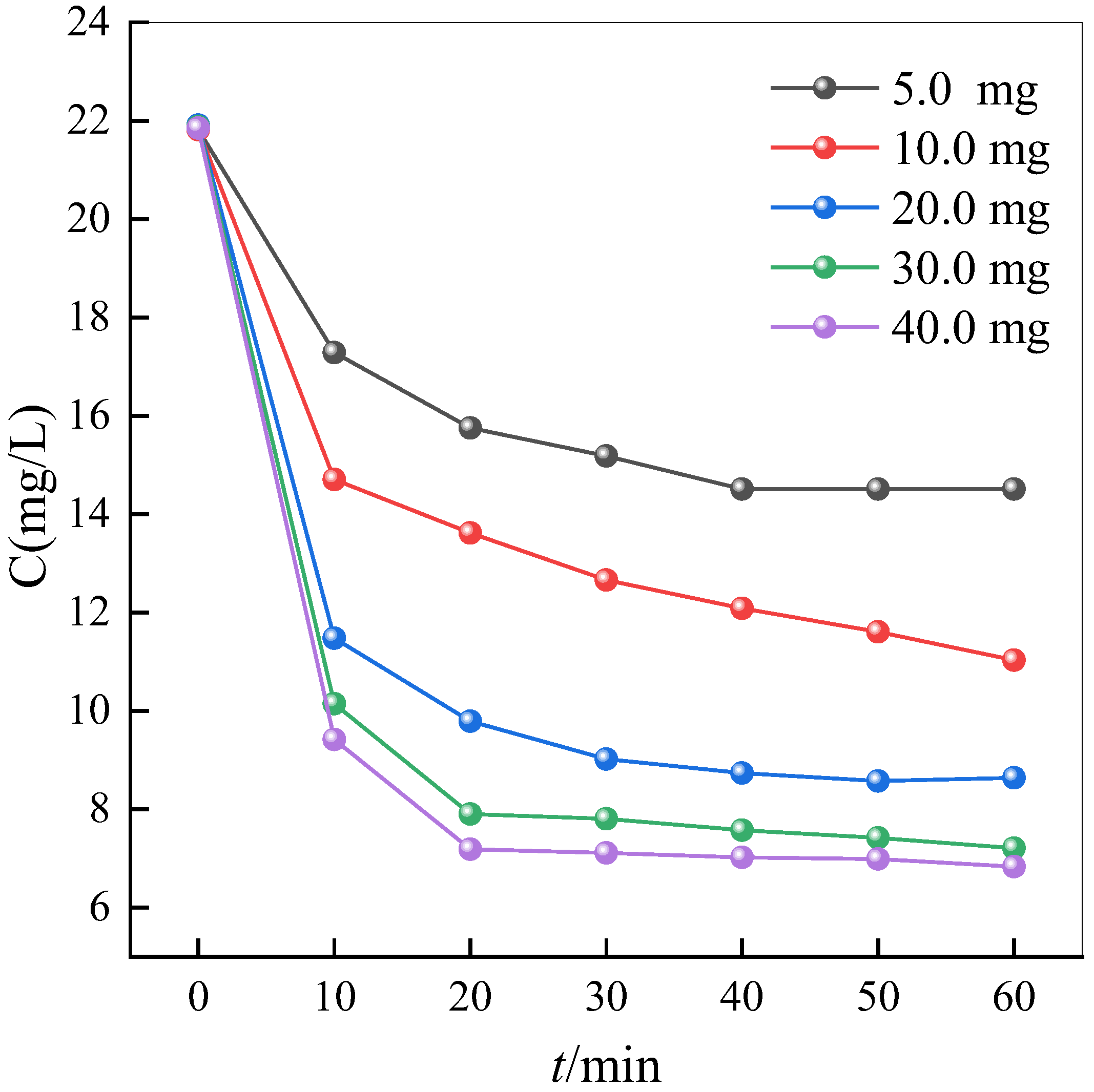
2.5. Effect of Material Dosage on Adsorption Efficiency
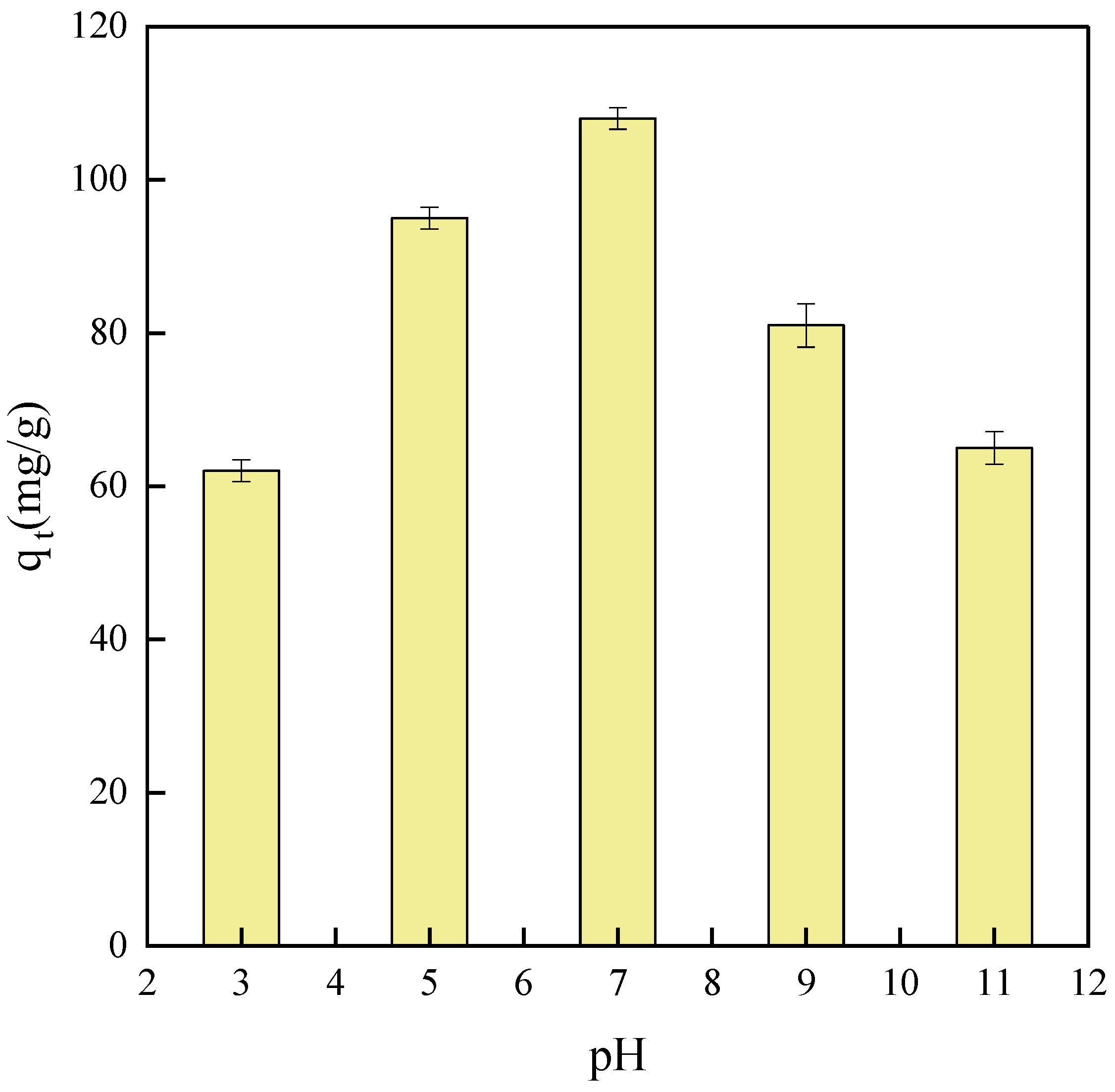
2.6. Effect of pH on Adsorption Capacity
3. Materials and Methods
3.1. Instruments and Experimental Reagents
3.1.1. Instruments
3.1.2. Experimental Reagents
3.2. Experimental Methods
3.2.1. Material Preparation
3.2.2. Calculation of Adsorption Capacity
3.3. Material Characterization Methods
3.3.1. X-ray Diffraction Analysis (XRD)
3.3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.3.3. Scanning Electron Microscopy
3.3.4. Energy-Dispersive Spectroscopy (EDS)
3.3.5. X-ray Photoelectron Spectroscopy (XPS)
3.3.6. Brunauer–Emmett–Teller Analysis (BET)
3.3.7. Thermogravimetric Analysis (TGA)
3.4. Analysis of Adsorption Mechanisms
3.4.1. Adsorption Kinetics Models
Pseudo-First-Order Kinetic Model
Proposed Second-Order Kinetic Model
3.4.2. Adsorption Isotherm Model
Langmuir Adsorption Isotherm Model
Freundlich Adsorption Isotherm Model
3.4.3. Adsorption Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.J.; Kiwi, J.; Zivkovic, I.; Ronnow, H.M.; Wang, T.H.; Rtimi, S. Quantification of the local magnetized nanotube domains accelerating the photocatalytic removal of the emerging pollutant tetracycline. Appl. Catal. B-Environ. 2019, 248, 450–458. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjorklund, G. The impact of tetracycline pollution on the aquatic environment and removal strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Dong, Y.W.; Luan, Y.X.; Tian, S.N.; Li, C.; Li, Y.; Zhou, J.H. Integrated assessment of the spatial distribution, sources, degradation, and human risk of tetracyclines in honey in China. J. Hazard. Mater. 2024, 473, 134681. [Google Scholar] [CrossRef]
- Loffler, P.; Escher, B.I.; Baduel, C.; Virta, M.P.; Lai, F.Y. Antimicrobial transformation products in the aquatic environment: Global occurrence, ecotoxicological risks, and potential of antibiotic resistance. Environ. Sci. Technol. 2023, 57, 9474–9494. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R. Environmental impact of waste treatment and synchronous hydrogen production: Based on life cycle assessment method. Toxics 2024, 12, 652. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen reduction reaction in the field of water environment for application of nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef]
- Su, R.K.; Li, Z.S.; Cheng, F.H.; Dai, X.R.; Wang, H.Q.; Luo, Y.T.; Huang, L. Advances in the degradation of emerging contaminants by persulfate oxidation technology. Water Air Soil. Poll. 2023, 234, 754. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R. Cobalt-based mof material activates persulfate to degrade residual ciprofloxacin. Water 2024, 16, 2299. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.D.; Elliott, A.G.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Shao, J.Y.; Qian, G.S.; Adeleye, S.A.; Hao, T.W. Removal of tetracycline by aerobic granular sludge from marine aquaculture wastewater: A molecular dynamics investigation. Bioresour. Technol. 2022, 355, 127286. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Peng, L.; Gu, Y.; Liang, C.Z.; Pott, R.W.M.; Xu, Y.F. Insights into biodegradation of antibiotics during the biofilm-based wastewater treatment processes. J. Clean. Prod. 2023, 393, 136321. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Liu, Y.W.; Shan, X.; Nghiem, L.D.; Nguyen, L.N. Removal process of antibiotics during anaerobic treatment of swine wastewater. Bioresour. Technol. 2020, 300, 122707. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Peng, Z.; Liu, Z.F.; Shao, B.B.; Liang, Q.H.; He, Q.Y.; Wu, T.; Zhang, X.S.; Zhao, C.H.; Liu, Y.; et al. Activation of peroxydisulfate by bimetal modified peanut hull-derived porous biochar for the degradation of tetracycline in aqueous solution. J. Environ. Chem. Eng. 2022, 10, 107366. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef]
- Ren, S.; Wang, S.; Liu, Y.; Wang, Y.; Gao, F.; Dai, Y. A review on current pollution and removal methods of tetracycline in soil. Sep. Sci. Technol. 2023, 58, 2578–2602. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole degradation by UV and UV/H2O2 advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Du, L.; Ahmad, S.; Liu, L.; Wang, L.; Tang, J. A review of antibiotics and antibiotic resistance genes (ARGs) adsorption by biochar and modified biochar in water. Sci. Total Environ. 2023, 858, 159815. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938, Erratum in Environ. Health Perspect. 2000, 108, 598. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Xue, R.; Ma, X.; Zeng, Z.; Li, L.; Wang, S. Targeted improvement of narrow micropores in porous carbon for enhancing trace benzene vapor removal: Revealing the adsorption mechanism via experimental and molecular simulation. J. Colloid. Interf. Sci. 2024, 671, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Bai, X.; Li, Y.; Yang, B.; Yang, P.; Yu, F.; Ma, J. HKUST-1 derived carbon adsorbents for tetracycline removal with excellent adsorption performance. Environ. Res. 2022, 205, 112425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Z.; Liang, Y.; Wu, T.; Chen, Y.; Yan, J.; Zhu, Y.; Ding, D. Adsorptive decontamination of antibiotics from livestock wastewater by using alkaline-modified biochar. Environ. Res. 2023, 226, 115676. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Liu, S.; Cao, X.; Wu, K.; Cheong, W.-C.; Chen, Z.; Wang, Y.; Li, Y.; Liu, Y.; et al. Core-Shell ZIF-8@ZIF-67-Derived CoP Nanoparticle-Embedded N-Doped Carbon Nanotube Hollow Polyhedron for Efficient Overall Water Splitting. J. Am. Chem. Soc. 2018, 140, 2610–2618. [Google Scholar] [CrossRef]
- Almáši, M. A review on state of art and perspectives of Metal-Organic frameworks (MOFs) in the fight against coronavirus SARS-CoV-2. J. Coord. Chem. 2021, 74, 2111–2127. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Gyepes, R.; Zukal, A.; Čejka, J. Synthesis, characterization and sorption properties of zinc(II) metal–organic framework containing methanetetrabenzoate ligand. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 101–107. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Li, L.; Jiang, J. Iron terephthalate metal-organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl. Catal. B-Environ. 2014, 148, 191–200. [Google Scholar] [CrossRef]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.; Weireld, G.D. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) metal−organic frameworks at room temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef]
- Xie, D.; Ma, Y.; Gu, Y.; Zhou, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Bifunctional NH2-MIL-88(Fe) metal–organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J. Mater. Chem. A 2017, 5, 23794–23804. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.-Z.; Huang, Y.-Q.; Yang, J.-M. Effect of the synergetic interplay between the electrostatic interactions, size of the dye molecules, and adsorption sites of MIL-101(Cr) on the adsorption of organic dyes from aqueous solutions. Cryst. Growth Des. 2018, 18, 7533–7540. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.Z.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.Y.; Wang, Y.; Wang, X.; Han, S.Y.; Liu, X.G.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nature Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Udourioh, G.A.; Solomon, M.M.; Matthews-Amune, C.O.; Epelle, E.I.; Okolie, J.A.; Agbazue, V.E.; Onyenze, U. Current trends in the synthesis, characterization and application of metal-organic frameworks. React. Chem. Eng. 2023, 8, 278–310. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Lim, S.S.; Foo, C.Y.; Haw, C.Y.; Chiu, W.S.; Chia, C.H.; Khiew, P.S. Solvothermal synthesis of nanostructured nickel-based metal–organic frameworks (Ni-MOFs) with enhanced electrochemical performance for symmetric supercapacitors. J. Mater. Sci. 2023, 58, 11894–11913. [Google Scholar] [CrossRef]
- Dehghankar, M.; HMTShirazi, R.; Mohammadi, T.; Tofighy, M.A. Synthesis and modification methods of metal-organic frameworks and their application in modification of polymeric ultrafiltration membranes: A review. J. Environ. Chem. Eng. 2023, 11, 109954. [Google Scholar] [CrossRef]
- Bernt, S.; Guillerm, V.; Serre, C.; Stock, N. Direct covalent post-synthetic chemical modification of Cr-MIL-101 using nitrating acid. Chem. Commun. 2011, 47, 2838–2840. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv. Mater. 2011, 23, 3294–3297. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Li, L.; Li, G.; Li, G.; Shi, Z.; Feng, S. A strategy toward constructing a bifunctionalized MOF catalyst: Post-synthetic modification of MOFs on organic ligands and coordinatively unsaturated metal sites. Chem. Commun. 2012, 48, 6151–6153. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, D. Post-synthetic modification of metal-organic framework-based membranes for enhanced molecular separations. Coord. Chem. Rev. 2023, 491, 215259. [Google Scholar] [CrossRef]
- Liu, T.; Feng, J.; Wan, Y.; Zheng, S.; Yang, L. ZrO2 nanoparticles confined in metal organic frameworks for highly effective adsorption of phosphate. Chemosphere 2018, 210, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.-J.; Guan, Z.-Y.; Ma, Y.-W.; Wan, J.-Q.; Wang, Y.; Qu, Y.-F. A novel catalyst of MIL-101(Fe) doped with Co and Cu as persulfate activator: Synthesis, characterization, and catalytic performance. Chem. Pap. 2018, 72, 235–250. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. A novel and universal metal-organic frameworks sensing platform for selective detection and efficient removal of heavy metal ions. Chem. Eng. J. 2019, 375, 122111. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H.; Moghadam, M.; Masoudinia, M. Nano-rod catalysts: Building MOF bottles (MIL-101 family as heterogeneous single-site catalysts) around vanadium oxide ships. J. Mol. Catal. A Chem. 2013, 374, 46–52. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Mehri Lighvan, Z.; Hosseini, S.R.; Norouzbahari, S.; Sadatnia, B.; Ghadimi, A. Synthesis, characterization, and selective gas adsorption performance of hybrid NH2-MIL-101(Fe)/ZIF-8 metal organic framework (MOF). Fuel 2023, 351, 128991. [Google Scholar] [CrossRef]
- Taha, A.A.; Huang, L.; Ramakrishna, S.; Liu, Y. MOF [NH2-MIL-101(Fe)] as a powerful and reusable Fenton-like catalyst. J. Water Process Eng. 2019, 33, 101004. [Google Scholar] [CrossRef]
- Mo, L.J.; Chen, G.Z.; Wang, H. Degradation of orange g using pms triggered by NH2-MIL-101(Fe): An amino-functionalized metal-organic framework. Molecules 2024, 29, 1488. [Google Scholar] [CrossRef]
- Zhang, H. Fabrication of NH2-MIL-101(Fe) Metal-Organic Framework and Its Photocatalytic Performance for Degrading Antibiotics. Master’s Thesis, Donghua University, Shanghai, China, 2022. [Google Scholar]
- Gecgel, C.; Simsek, U.B.; Gozmen, B.; Turabik, M. Comparison of MIL-101 (Fe) and amine-functionalized MIL-101 (Fe) as photocatalysts for the removal of imidacloprid in aqueous solution. J. Iran. Chem. Soc. 2019, 16, 1735–1748. [Google Scholar] [CrossRef]
- Pandey, A.; Sahoo, S. Synthesis and dielectric characterization of Iron-Doped forsterite coated titanium-based medical implants. IEEE Sens. J. 2023, 23, 31171–31177. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, T.; Pudukudy, M.; Su, H.; Jiang, L.; Shan, S.; Jia, Q.J.M.C. Influence of microwave-assisted synthesis on the structural and textural properties of mesoporous MIL-101 (Fe) and NH2-MIL-101 (Fe) for enhanced tetracycline adsorption. Mater. Chem. Phys. 2020, 251, 123060. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Chen, C.-W.; Hung, C.-M.; Chang, J.-H.; Dong, C.-D. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour. Technol. 2019, 284, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, X.; Yin, C.; Zhang, B.; Zhang, Q. Facile fabrication of hierarchical porous ZIF-8 for enhanced adsorption of antibiotics. J. Hazard. Mater. 2019, 367, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Lu, S.; Cheng, W.; Ren, J.; Wang, M.; Hu, B.; Jia, Z.; Li, Y.; Sun, Y. A spectroscopic and theoretical investigation of interaction mechanisms of tetracycline and polystyrene nanospheres under different conditions. Environ. Pollut. 2019, 249, 398–405. [Google Scholar] [CrossRef]
- Vinothkumar, K.; Balakrishna, R.G. One-pot synthesis of NH2-MIL-101(Fe) and α-Fe2O3 composite as efficient heterojunction for multifunctional photocatalytic membranes: Towards zero waste generation. Appl. Catal. B-Environ. 2024, 340, 123199. [Google Scholar] [CrossRef]
- Bauer, S.; Serre, C.; Devic, T.; Horcajada, P.; Marrot, J.; Férey, G.; Stock, N. High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system. Inorg. Chem. 2008, 47, 7568–7576. [Google Scholar] [CrossRef]

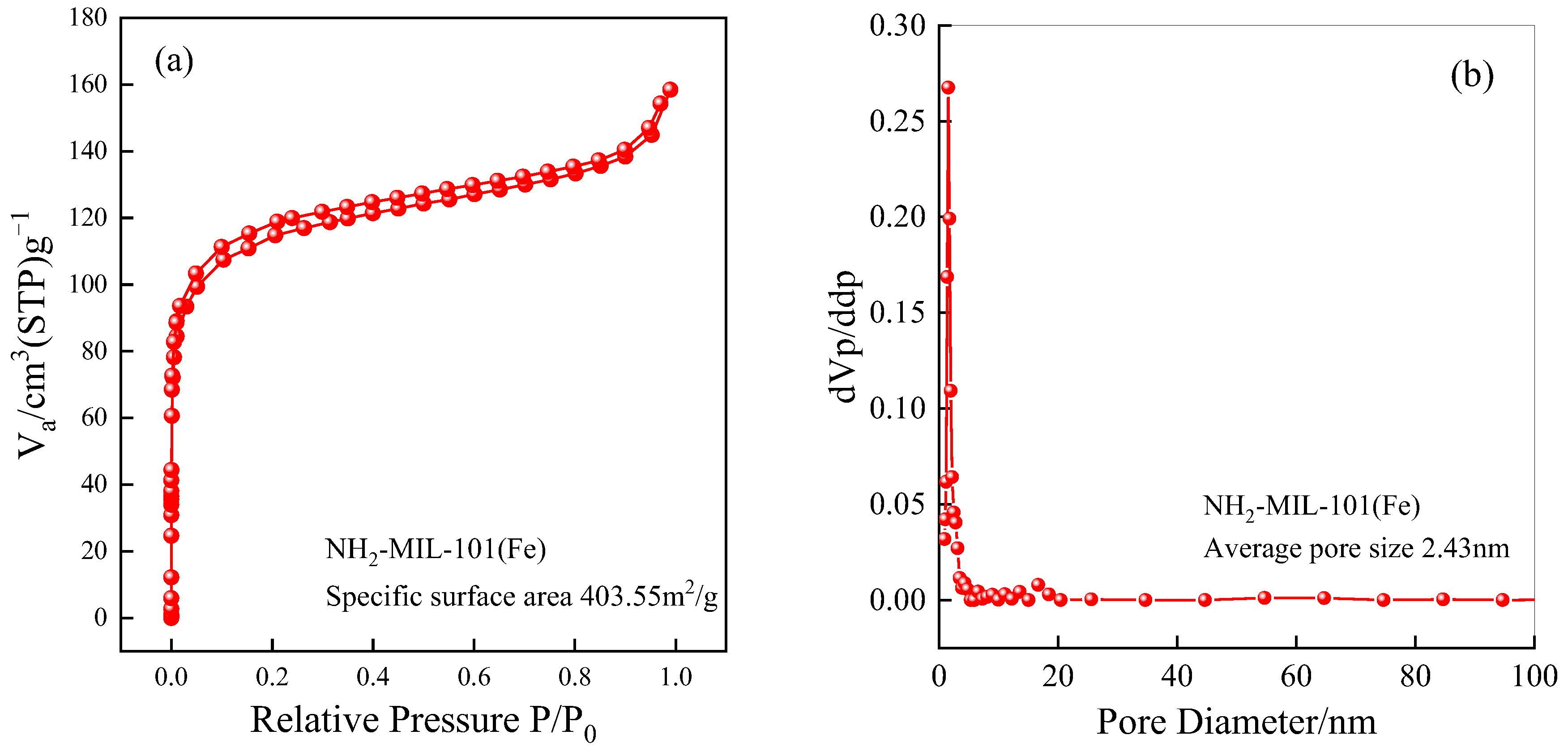
| Material | Specific Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|
| NH2-MIL-101(Fe) | 403.55 | 2.43 |
| MIL-101(Fe) | 1236.71 | 2.75 |
| Material | Pseudo-First-Order Kinetic Model | |||
|---|---|---|---|---|
| qe | q1 | K1 | R2 | |
| NH2-MIL-101(Fe) | 107.815 | 102.3604 | 0.1533 | 0.918 |
| Material | Pseudo-Second-Order Kinetic Model | |||
|---|---|---|---|---|
| qe | q2 | K2 | R2 | |
| NH2-MIL-101(Fe) | 107.8150 | 110.3752 | 0.0021 | 0.9834 |
| Material | T/°C | Langmuir Equation | qm | KL | R2 |
|---|---|---|---|---|---|
| NH2-MIL-101(Fe) | 15 | y = 0.0041x + 0.3024 | 244 | 0.0136 | 0.6736 |
| 25 | y = 0.0057x + 0.2079 | 175 | 0.0275 | 0.5515 | |
| 35 | y = 0.0050x + 0.1326 | 200 | 0.03771 | 0.7637 |
| Material | T/°C | Freundlich Equation | n | KF | R2 |
|---|---|---|---|---|---|
| NH2-MIL-101(Fe) | 15 | y = 0.8578x + 1.3145 | 1.1658 | 3.7229 | 0.9694 |
| 25 | y = 0.8057x + 1.7323 | 1.2412 | 5.6536 | 0.9832 | |
| 35 | y = 0.7914x + 2.1139 | 1.2636 | 8.2805 | 0.9774 |
| Material | T/K | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J/mol/K) |
|---|---|---|---|---|
| NH2-MIL-101(Fe) | 288 | −3.15 | 29.38 | 112.97 |
| 298 | −4.29 | |||
| 308 | −5.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Su, R. Preparation of NH2-MIL-101(Fe) Metal Organic Framework and Its Performance in Adsorbing and Removing Tetracycline. Int. J. Mol. Sci. 2024, 25, 9855. https://doi.org/10.3390/ijms25189855
Luo Y, Su R. Preparation of NH2-MIL-101(Fe) Metal Organic Framework and Its Performance in Adsorbing and Removing Tetracycline. International Journal of Molecular Sciences. 2024; 25(18):9855. https://doi.org/10.3390/ijms25189855
Chicago/Turabian StyleLuo, Yiting, and Rongkui Su. 2024. "Preparation of NH2-MIL-101(Fe) Metal Organic Framework and Its Performance in Adsorbing and Removing Tetracycline" International Journal of Molecular Sciences 25, no. 18: 9855. https://doi.org/10.3390/ijms25189855
APA StyleLuo, Y., & Su, R. (2024). Preparation of NH2-MIL-101(Fe) Metal Organic Framework and Its Performance in Adsorbing and Removing Tetracycline. International Journal of Molecular Sciences, 25(18), 9855. https://doi.org/10.3390/ijms25189855






