Sex-Specific Behavioral and Molecular Responses to Maternal Lipopolysaccharide-Induced Immune Activation in a Murine Model: Implications for Neurodevelopmental Disorders
Abstract
1. Introduction
2. Results
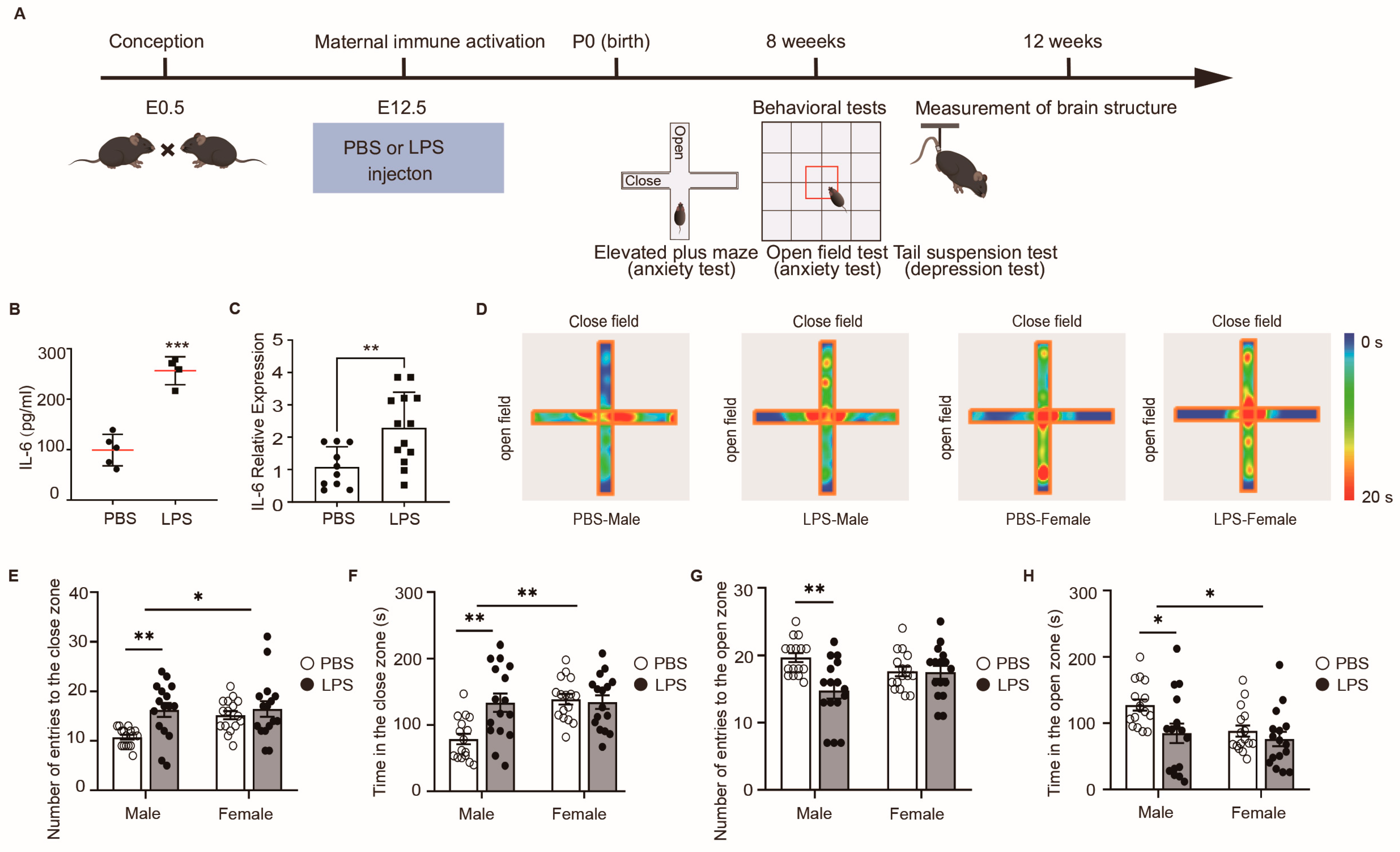
2.1. LPS-Induced MIA Male Offspring Showed Elevated Anxiety in Elevated Plus Maze Test
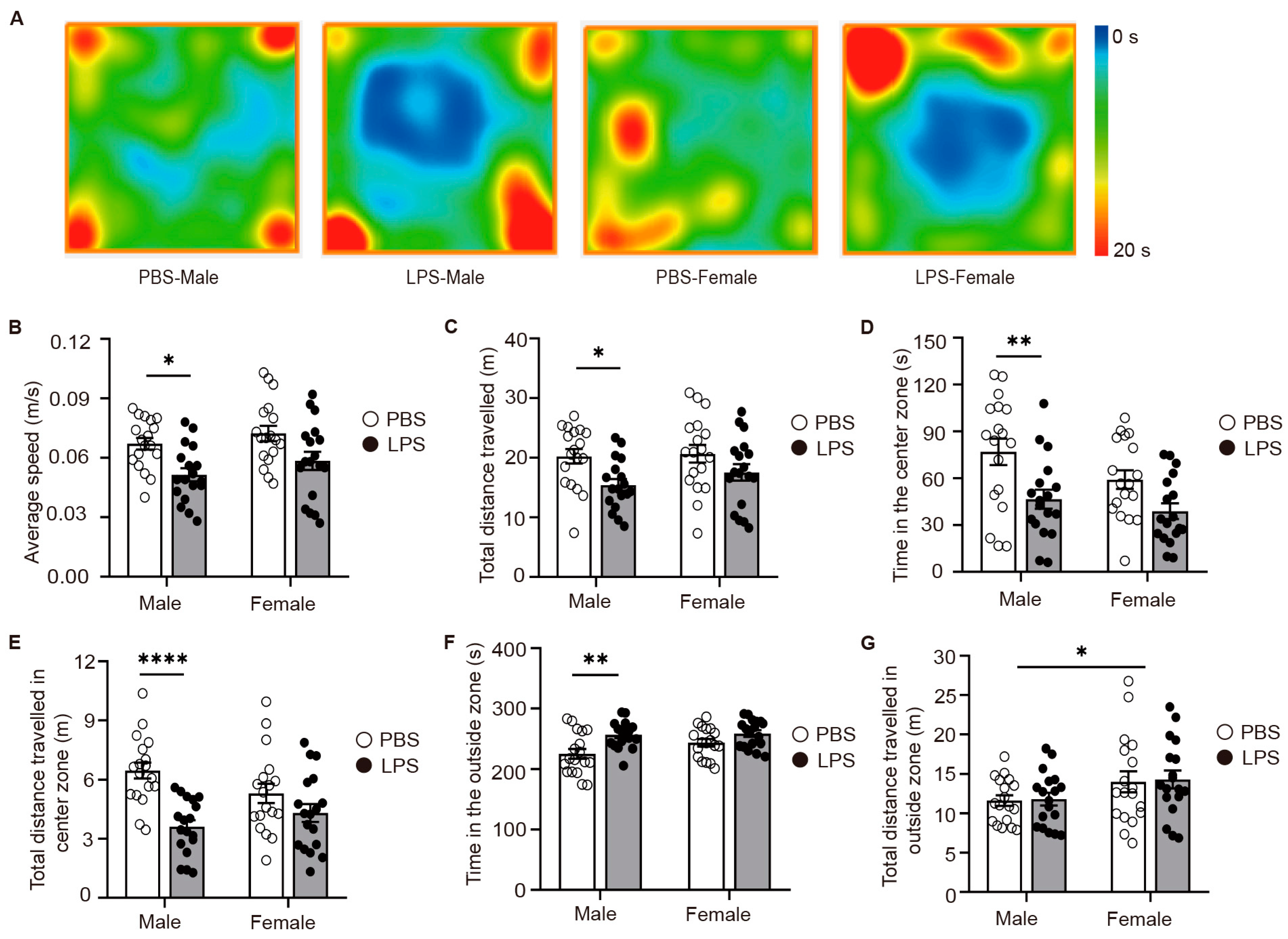
2.2. LPS-Induced MIA Male Offspring Showed Elevated Anxiety in Open-Field Test
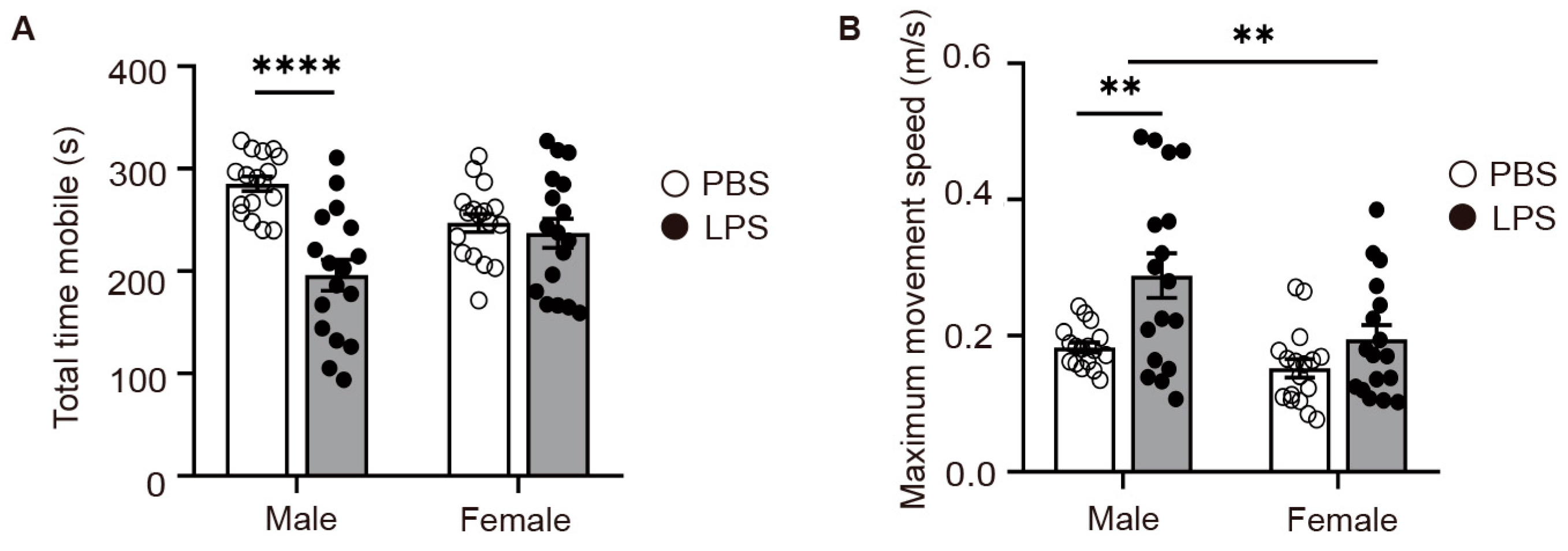
2.3. LPS-Induced MIA Male Offspring Showed Elevated Levels of Depression in Tail Suspension Test
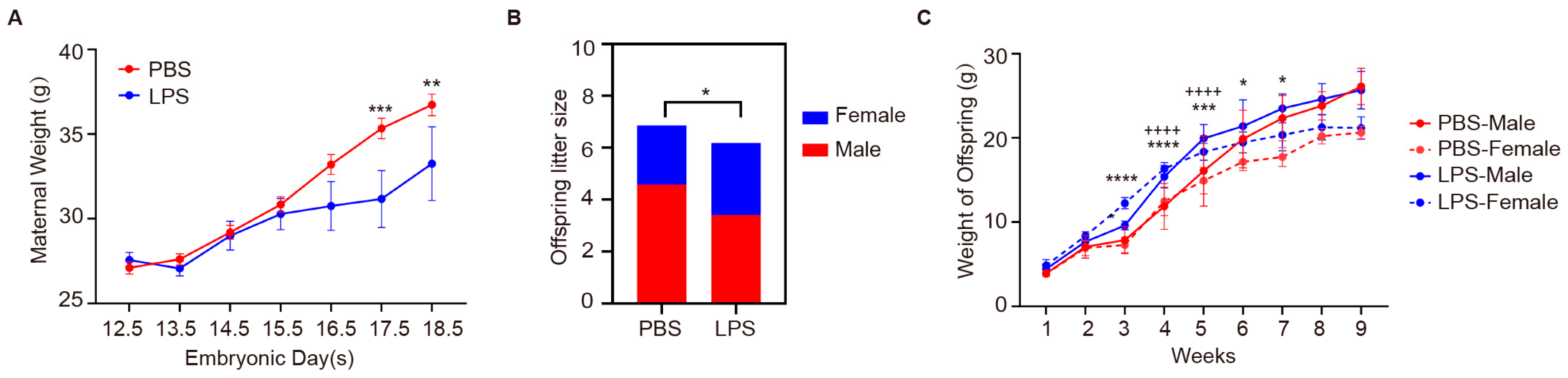
2.4. Effects of Maternal LPS Exposure on Maternal Weight and Offspring Development
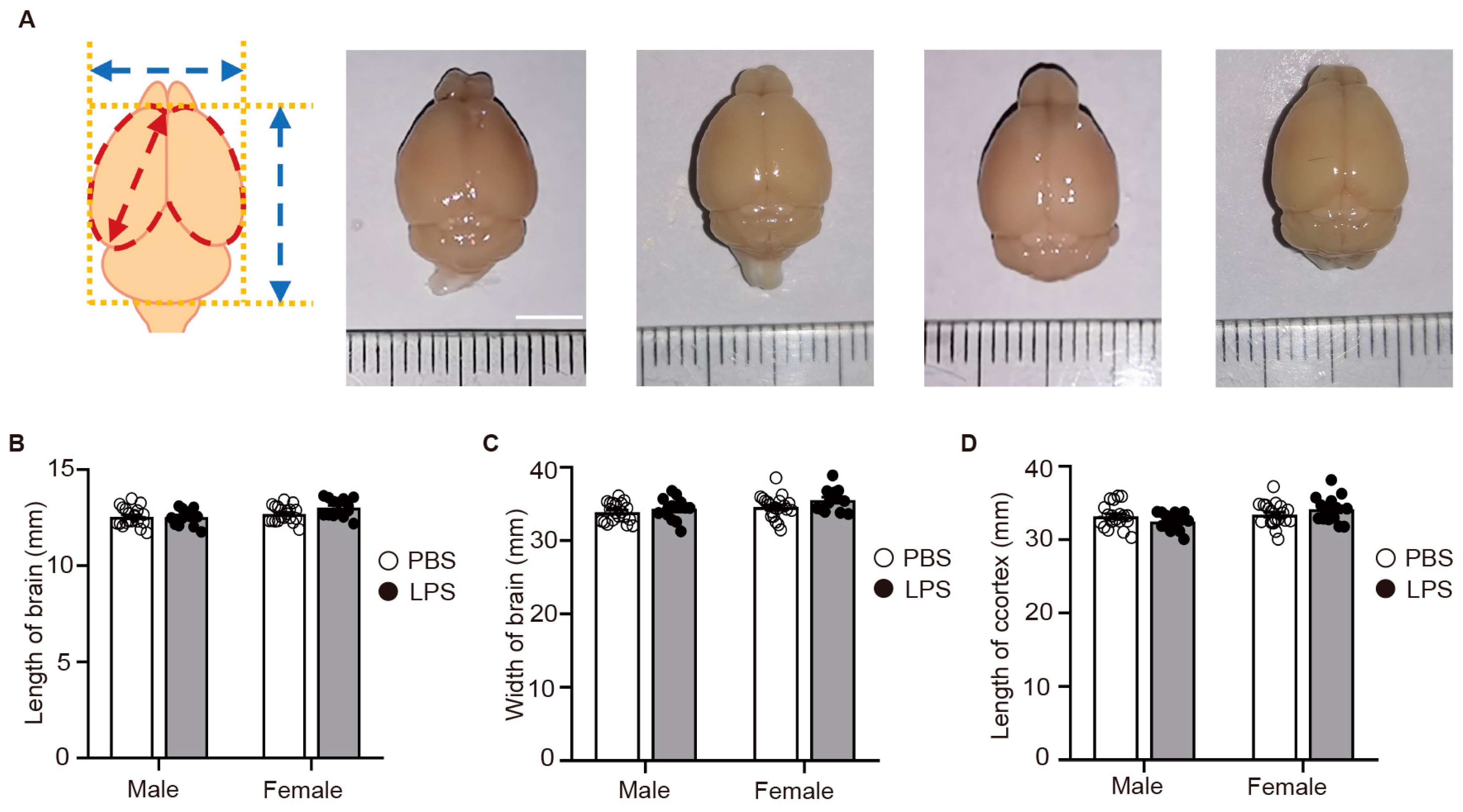
2.5. LPS-Induced MIA Did Not Alter the Size of the Offspring’s Brain
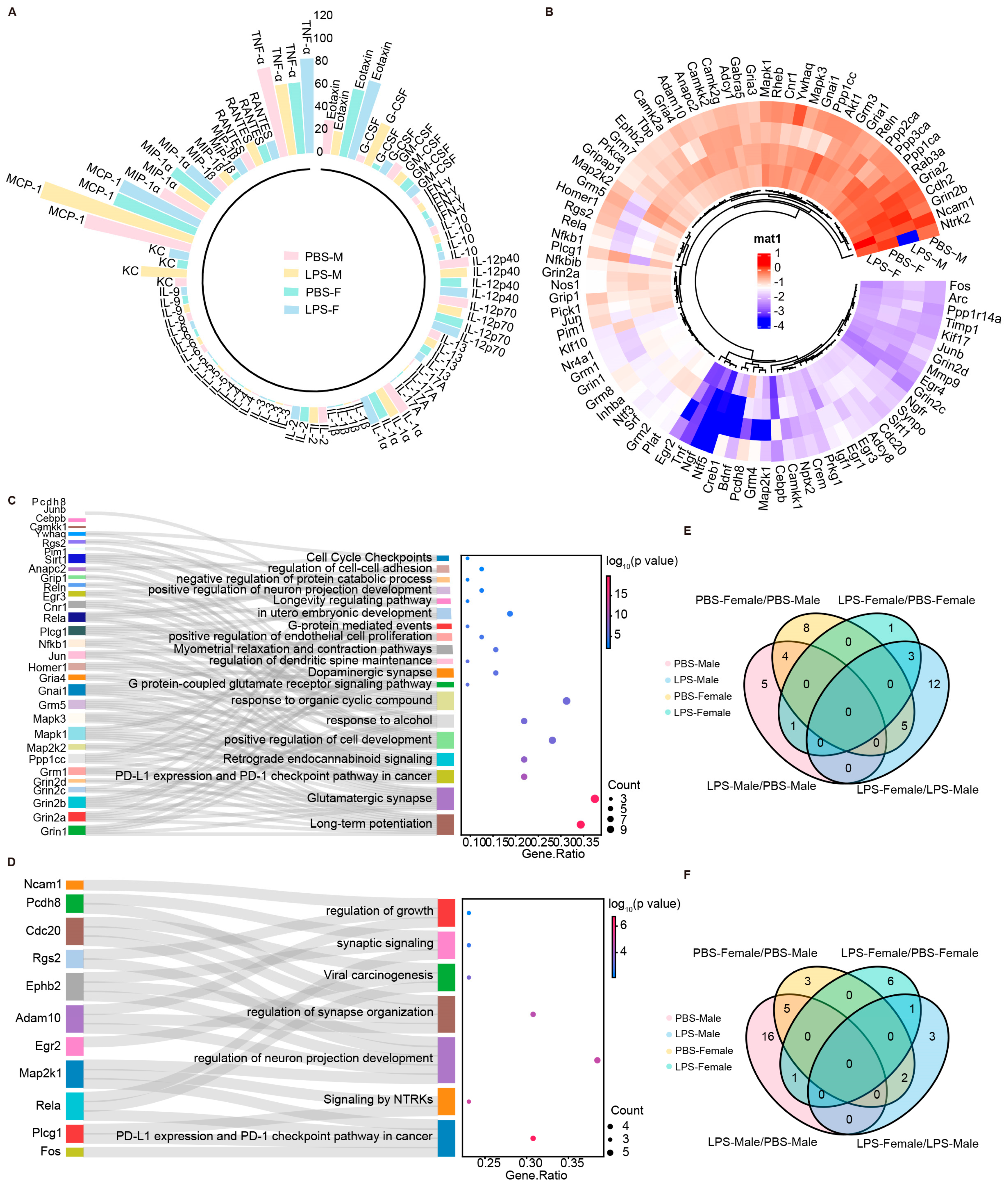
2.6. LPS-Induced MIA Promoted Significant Alterations in Synaptic-Related Genes of Male Offspring
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. LPS Injection and Treatment
4.3. Elevated Plus-Maze Test
4.4. Open-Field Test
4.5. Tail Suspension Test
4.6. Isolating the Cerebral Cortex of P0 Mice
4.7. Measurement of Different Indicators of Brain Structure in Adult Offspring
4.8. Luminex Multiplex Analysis
4.9. RNA Extraction and Real-Time PCR
4.10. PCR Array
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Z.; Liu, J.; Li, Y.; Liu, L.; Huang, P.; Wang, W.; Shan, Z.; Sun, R.; Shen, J.; et al. Dysregulation of immune and metabolism pathways in maternal immune activation induces an increased risk of autism spectrum disorders. Life Sci. 2023, 324, 121734. [Google Scholar] [CrossRef]
- Jash, S.; Sharma, S. Pathogenic Infections during Pregnancy and the Consequences for Fetal Brain Development. Pathogens 2022, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Cherkerzian, S.; Seidman, L.J.; Papandonatos, G.D.; Savitz, D.A.; Tsuang, M.T.; Goldstein, J.M.; Buka, S.L. Maternal Bacterial Infection During Pregnancy and Offspring Risk of Psychotic Disorders: Variation by Severity of Infection and Offspring Sex. Am. J. Psychiatry 2020, 177, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kotajima-Murakami, H.; Tanaka, M.; Katoh, Y.; Ikeda, K. Influence of Prenatal Drug Exposure, Maternal Inflammation, and Parental Aging on the Development of Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 821455. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Masi, A.; Dale, R.C.; Whitehouse, A.J.O.; Pokorski, I.; Alvares, G.A.; Hickie, I.B.; Breen, E.; Guastella, A.J. Social impairments in autism spectrum disorder are related to maternal immune history profile. Mol. Psychiatry 2017, 23, 1794–1797. [Google Scholar] [CrossRef]
- Bodnar, T.S.; Raineki, C.; Wertelecki, W.; Yevtushok, L.; Plotka, L.; Zymak-Zakutnya, N.; Honerkamp-Smith, G.; Wells, A.; Rolland, M.; Woodward, T.S.; et al. Altered maternal immune networks are associated with adverse child neurodevelopment: Impact of alcohol consumption during pregnancy. Brain Behav. Immun. 2018, 73, 205–215. [Google Scholar] [CrossRef]
- Murakami, Y.; Imamura, Y.; Kasahara, Y.; Yoshida, C.; Momono, Y.; Fang, K.; Sakai, D.; Konishi, Y.; Nishiyama, T. Maternal Inflammation with Elevated Kynurenine Metabolites Is Related to the Risk of Abnormal Brain Development and Behavioral Changes in Autism Spectrum Disorder. Cells 2023, 12, 1087. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Xu, L.-L.; Shao, L.; Xia, R.-M.; Yu, Z.-H.; Ling, Z.-X.; Yang, F.; Deng, M.; Ruan, B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef]
- Talukdar, P.M.; Abdul, F.; Maes, M.; Binu, V.; Venkatasubramanian, G.; Kutty, B.M.; Debnath, M. Maternal Immune Activation Causes Schizophrenia-like Behaviors in the Offspring through Activation of Immune-Inflammatory, Oxidative and Apoptotic Pathways, and Lowered Antioxidant Defenses and Neuroprotection. Mol. Neurobiol. 2020, 57, 4345–4361. [Google Scholar] [CrossRef]
- de Cossío, L.F.; Lacabanne, C.; Bordeleau, M.; Castino, G.; Kyriakakis, P.; Tremblay, M. Lipopolysaccharide-induced maternal immune activation modulates microglial CX3CR1 protein expression and morphological phenotype in the hippocampus and dentate gyrus, resulting in cognitive inflexibility during late adolescence. Brain Behav. Immun. 2021, 97, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Labrousse, V.; Leyrolle, Q.; Amadieu, C.; Aubert, A.; Sere, A.; Coutureau, E.; Grégoire, S.; Bretillon, L.; Pallet, V.; Gressens, P.; et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav. Immun. 2018, 73, 427–440. [Google Scholar] [CrossRef]
- Nakamura, J.P.; Gillespie, B.; Gibbons, A.; Jaehne, E.J.; Du, X.; Chan, A.; Schroeder, A.; Buuse, M.v.D.; Sundram, S.; Hill, R.A. Maternal immune activation targeted to a window of parvalbumin interneuron development improves spatial working memory: Implications for autism. Brain Behav. Immun. 2020, 91, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Aria, F.; Bonini, S.A.; Cattaneo, V.; Premoli, M.; Mastinu, A.; Maccarinelli, G.; Memo, M. Brain Structural and Functional Alterations in Mice Prenatally Exposed to LPS Are Only Partially Rescued by Anti-Inflammatory Treatment. Brain Sci. 2020, 10, 620. [Google Scholar] [CrossRef]
- de Cossío, L.F.; Guzmán, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Nonoguchi, H.A.; Kouo, T.W.S.; Kortagere, S.; Hillman, J.; Boyle, D.L.; Mandyam, C.D. Lipopolysaccharide Exposure Differentially Alters Plasma and Brain Inflammatory Markers in Adult Male and Female Rats. Brain Sci. 2022, 12, 972. [Google Scholar] [CrossRef]
- Vojtechova, I.; Maleninska, K.; Kutna, V.; Klovrza, O.; Tuckova, K.; Petrasek, T.; Stuchlik, A. Behavioral Alterations and Decreased Number of Parvalbumin-Positive Interneurons in Wistar Rats after Maternal Immune Activation by Lipopolysaccharide: Sex Matters. Int. J. Mol. Sci. 2021, 22, 3274. [Google Scholar] [CrossRef] [PubMed]
- Xuan, I.C.Y.; Hampson, D.R. Gender-Dependent Effects of Maternal Immune Activation on the Behavior of Mouse Offspring. PLoS ONE 2014, 9, e104433. [Google Scholar] [CrossRef]
- Csatlosova, K.; Bogi, E.; Durisova, B.; Grinchii, D.; Paliokha, R.; Moravcikova, L.; Lacinova, L.; Jezova, D.; Dremencov, E. Maternal immune activation in rats attenuates the excitability of monoamine-secreting neurons in adult offspring in a sex-specific way. Eur. Neuropsychopharmacol. 2020, 43, 82–91. [Google Scholar] [CrossRef]
- Dehay, C.; Kennedy, H. Cell-cycle control and cortical development. Nat. Rev. Neurosci. 2007, 8, 438–450. [Google Scholar] [CrossRef]
- Casanova, M.F.; El-Baz, A.S.; Kamat, S.S.; Dombroski, B.A.; Khalifa, F.; Elnakib, A.; Soliman, A.; Allison-McNutt, A.; Switala, A.E. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol. Commun. 2013, 1, 67. [Google Scholar] [CrossRef] [PubMed]
- Stoner, R.; Chow, M.L.; Boyle, M.P.; Sunkin, S.M.; Mouton, P.R.; Roy, S.; Wynshaw-Boris, A.; Colamarino, S.A.; Lein, E.S.; Courchesne, E. Patches of Disorganization in the Neocortex of Children with Autism. N. Engl. J. Med. 2014, 370, 1209–1219. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Elliott, R.M.; Anderson, M.P. Maternal Immune Activation Increases Neonatal Mouse Cortex Thickness and Cell Density. J. Neuroimmune Pharmacol. 2012, 7, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Gąssowska-Dobrowolska, M.; Jęśko, H.; Czapski, G.A.; Wilkaniec, A.; Zawadzka, A.; Dominiak, A.; Polowy, R.; Filipkowski, R.K.; Boguszewski, P.M.; et al. Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring. Int. J. Mol. Sci. 2020, 21, 4097. [Google Scholar] [CrossRef]
- Antonson, A.M.; Radlowski, E.C.; Lawson, M.A.; Rytych, J.L.; Johnson, R.W. Maternal viral infection during pregnancy elicits anti-social behavior in neonatal piglet offspring independent of postnatal microglial cell activation. Brain Behav. Immun. 2016, 59, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Martín-González, N.S.; Castro-Quintas, Á.; Marques-Feixa, L.; Ayesa-Arriola, R.; López, M.; Fañanás, L. Maternal respiratory viral infections during pregnancy and offspring’s neurodevelopmental outcomes: A systematic review. Neurosci. Biobehav. Rev. 2023, 149, 105178. [Google Scholar] [CrossRef]
- Silveira, V.; Medeiros, D.C.; Ropke, J.; Guidine, P.A.; Rezende, G.H.; Moraes, M.; Mendes, E.; Macedo, D.; Moreira, F.A.; and Oliveira, A.D. Effects of early or late prenatal immune activation in mice on behavioral and neuroanatomical abnor-malities relevant to schizophrenia in the adulthood. Int. J. Dev. Neurosci. 2017, 58, 1–8. [Google Scholar] [CrossRef]
- Short, S.J.; Lubach, G.R.; Karasin, A.I.; Olsen, C.W.; Styner, M.; Knickmeyer, R.C.; Gilmore, J.H.; Coe, C.L. Maternal Influenza Infection During Pregnancy Impacts Postnatal Brain Development in the Rhesus Monkey. Biol. Psychiatry 2010, 67, 965–973. [Google Scholar] [CrossRef]
- Lawrenson, I.D.; Krebs, D.L.; Linossi, E.M.; Zhang, J.-G.; McLennan, T.J.; Collin, C.; McRae, H.M.; Kolesnik, T.B.; Koh, K.; Britto, J.M.; et al. Cortical Layer Inversion and Deregulation of Reelin Signaling in the Absence of SOCS6 and SOCS7. Cereb. Cortex 2017, 27, 576–588. [Google Scholar] [CrossRef]
- Kadam, S.D.; French, B.M.; Kim, S.-T.; Morris-Berry, C.M.; Zimmerman, A.W.; Blue, M.E.; Singer, H.S. Altered Postnatal Cell Proliferation in Brains of Mouse Pups Prenatally Exposed to IgG from Mothers of Children with Autistic Disorder. J. Exp. Neurosci. 2013, 7, JEN.S12979. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Menard, C. Inflamed Astrocytes: A Path to Depression Led by Menin. Neuron 2018, 100, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.L.; Saijo, K. Estrogen Receptor β as a Candidate Regulator of Sex Differences in the Maternal Immune Activation Model of ASD. Front. Mol. Neurosci. 2021, 14, 717411. [Google Scholar] [CrossRef] [PubMed]
- Smolders, S.; Notter, T.; Smolders, S.M.; Rigo, J.-M.; Brône, B. Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain Behav. Immun. 2018, 73, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Zari, A.; Redwan, E.M.; Raszek, M.; Cowley, D.; Hromić-Jahjefendić, A.; Uversky, V.N.; Fabrowski, M.; Brogna, C.; Piscopo, M.; Rubio-Casillas, A. Interplay between Multisystem Inflammatory Syndrome in Children, Interleukin 6, Microbiome, and Gut Barrier Integrity. Immuno 2024, 4, 226–246. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Feng, X.; Song, M.; Shao, M.; Yang, Y.; Zhang, L.; Liu, Q.; Lv, L.; Su, X. Maternal immune activation alters adult behavior, intestinal integrity, gut microbiota and the gut inflammation. Brain Behav. 2021, 11, e02133. [Google Scholar] [CrossRef]
| Mouse | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Adam10 | Adcy1 | Adcy8 | Akt1 | Anapc2 | Arc | Bdnf | Camk2a | Camk2g | Camkk1 | Camkk2 | Cdc20 |
| B | Cdh2 | Cebpb | Cnr1 | Creb1 | Crem | Egr1 | Egr2 | Egr3 | Egr4 | Ephb2 | Fos | Gabra5 |
| C | Gnai1 | Gria1 | Gria2 | Gria3 | Gria4 | Grin1 | Grin2a | Grin2b | Grin2c | Grin2d | Grip1 | Gripap1 |
| D | Grm1 | Grm2 | Grm3 | Grm4 | Grm5 | Grm7 | Grm8 | Homer1 | Igf1 | Inhba | Jun | Junb |
| E | Kif17 | Klf10 | Map2k1 | Map2k2 | Mapk1 | Mapk3 | Mmp9 | Ncam1 | Nfkb1 | Nfkbib | Ngf | Ngfr |
| F | Nos1 | Nptx2 | Nr4a1 | Ntf3 | Ntf5 | Ntrk2 | Pcdh8 | Pick1 | Pim1 | Plat | Plcg1 | Ppp1ca |
| G | Ppp1cc | Ppp1r14a | Ppp2ca | Ppp3ca | Prkca | Prkg1 | Rab3a | Rela | Reln | Rgs2 | Rheb | Sirt1 |
| H | Srf | Synpo | Tbp | Timp1 | Tnf | Ywhaq | Actb | Gapdh | Hprt1 | B2m | NTC | NTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhao, R.; Yan, M.; Zhou, M.; Liu, H.; Wang, X.; Lu, C.; Li, Q.; Mo, Y.; Zhang, P.; et al. Sex-Specific Behavioral and Molecular Responses to Maternal Lipopolysaccharide-Induced Immune Activation in a Murine Model: Implications for Neurodevelopmental Disorders. Int. J. Mol. Sci. 2024, 25, 9885. https://doi.org/10.3390/ijms25189885
Xu J, Zhao R, Yan M, Zhou M, Liu H, Wang X, Lu C, Li Q, Mo Y, Zhang P, et al. Sex-Specific Behavioral and Molecular Responses to Maternal Lipopolysaccharide-Induced Immune Activation in a Murine Model: Implications for Neurodevelopmental Disorders. International Journal of Molecular Sciences. 2024; 25(18):9885. https://doi.org/10.3390/ijms25189885
Chicago/Turabian StyleXu, Jing, Rujuan Zhao, Mingyang Yan, Meng Zhou, Huanhuan Liu, Xueying Wang, Chang Lu, Qiang Li, Yan Mo, Paihao Zhang, and et al. 2024. "Sex-Specific Behavioral and Molecular Responses to Maternal Lipopolysaccharide-Induced Immune Activation in a Murine Model: Implications for Neurodevelopmental Disorders" International Journal of Molecular Sciences 25, no. 18: 9885. https://doi.org/10.3390/ijms25189885
APA StyleXu, J., Zhao, R., Yan, M., Zhou, M., Liu, H., Wang, X., Lu, C., Li, Q., Mo, Y., Zhang, P., Ju, X., & Zeng, X. (2024). Sex-Specific Behavioral and Molecular Responses to Maternal Lipopolysaccharide-Induced Immune Activation in a Murine Model: Implications for Neurodevelopmental Disorders. International Journal of Molecular Sciences, 25(18), 9885. https://doi.org/10.3390/ijms25189885






