Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles Improve Antimicrobial Activity
Abstract
:1. Introduction
2. Results
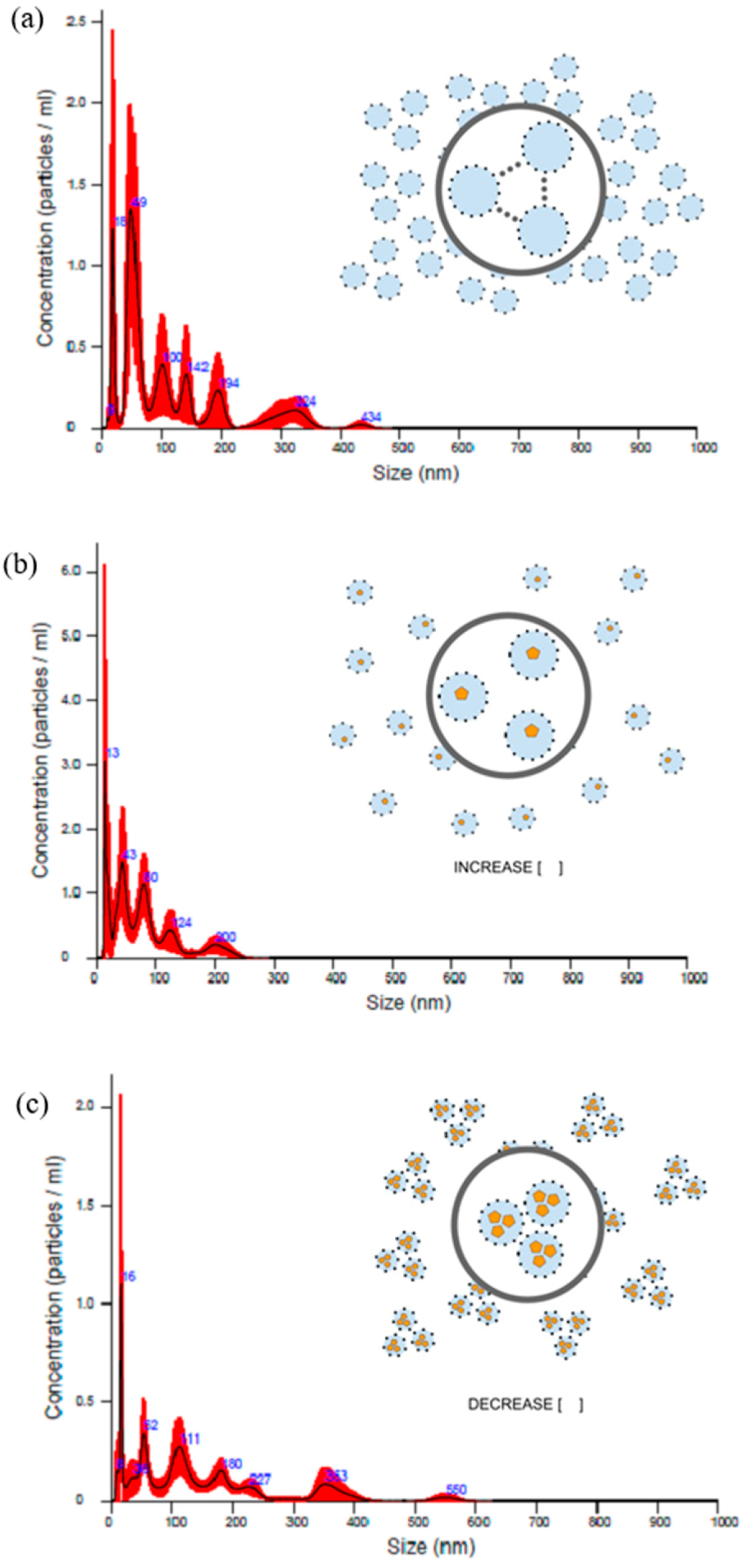
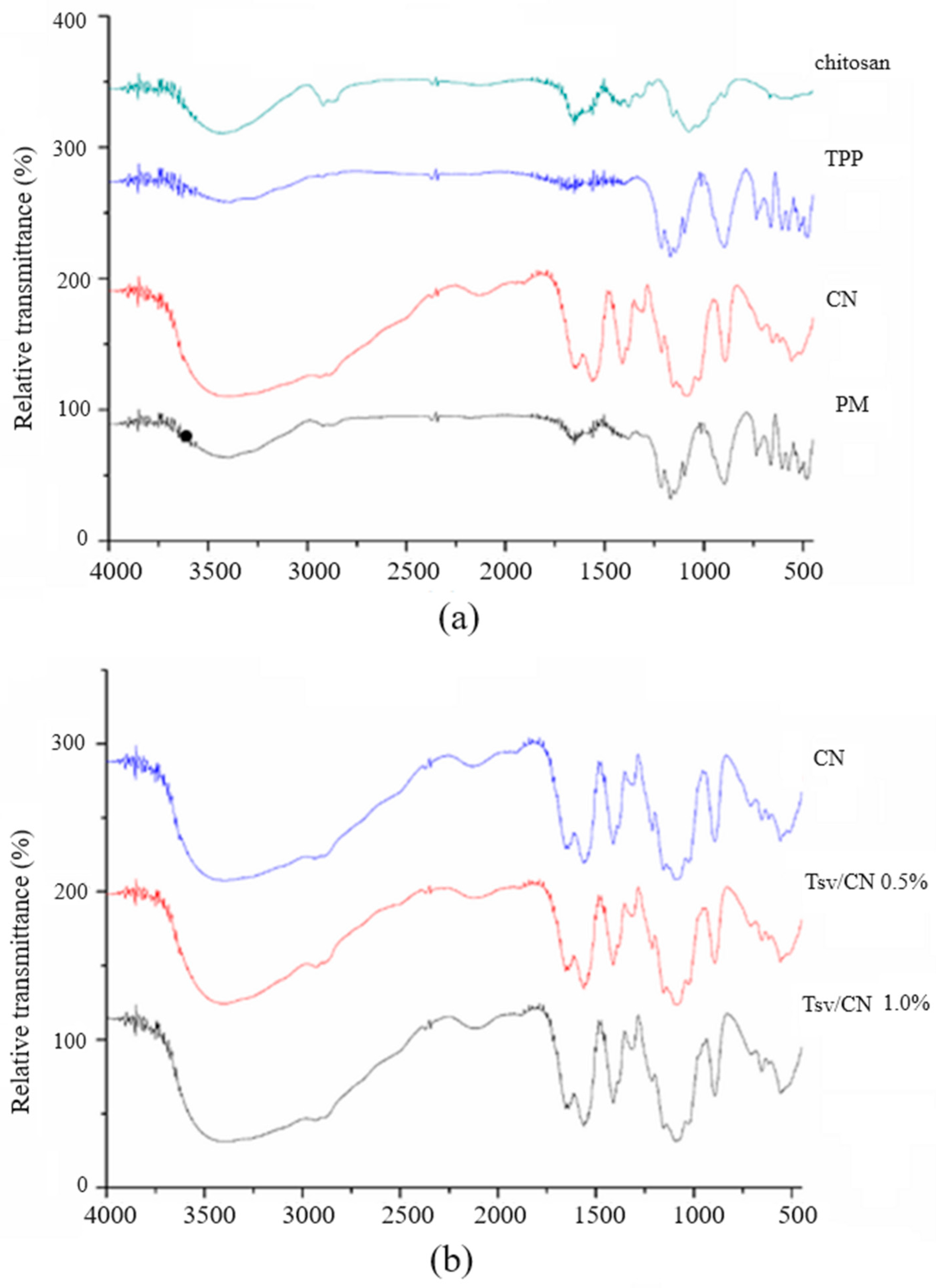
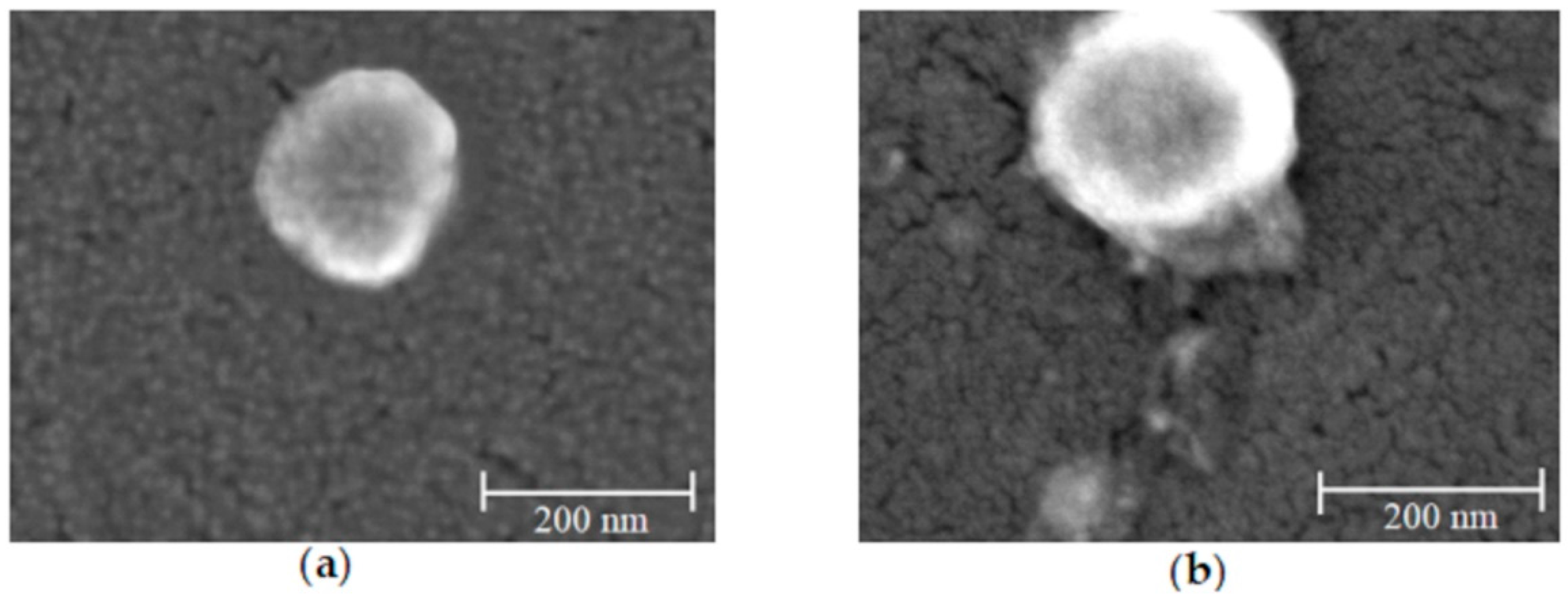
2.1. Preparation and Physicochemical Characterization of Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles
2.2. Antibacterial and Antifungal Effect of Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Obtaining of Tityus stigmurus Scorpion Venom
4.2. Preparation of T. stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles
4.3. Physical–Chemical Aspects of Nanoparticles
4.3.1. Determination of Zeta Potential, Particle Size and Polydispersity Index
4.3.2. Venom-Loading Efficiency
4.3.3. Nanoparticle Tracking Analyzer
4.3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
4.3.5. Study of Shape and Surface of Nanoparticles
4.4. Evaluation of the In Vitro Antibacterial Activity
4.5. Evaluation of the In Vitro Antifungal Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar] [CrossRef]
- Sonmez, E.; Kekecoglu, M.; Bozdeveci, A.; Alpay, S. Chemical Profiling and Antimicrobial Effect of Anatolian Honey Bee Venom. Toxicon 2022, 213, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic Pollution and Associated Antimicrobial Resistance in the Environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Abbasmanthiri, R.; Abdo Osman, N.M.; Siddiqui, Y.; Al-Bannah, F.A.; Al-Rawi, A.M.; Al-Asmari, S.A. Assessment of the Antimicrobial Activity of Few Saudi Arabian Snake Venoms. Open Microbiol. J. 2015, 9, 18–25. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sharma, B.K.; Subedi, N.; Ranabhat, S.; Baral, M.P. Burden of Serious Bacterial Infections and Multidrug-Resistant Organisms in an Adult Population of Nepal: A Comparative Analysis of Minimally Invasive Tissue Sampling Informed Mortality Surveillance of Community and Hospital Deaths. Clin. Infect. Dis. 2021, 73, S415–S421. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Raza, A.; un Nisa, Z.; Abdel-Massih, R.M.; Al Bakain, R.; Cabrerizo, F.M.; Dela Cruz, T.E.; Aziz, R.K.; Musharraf, S.G. Detection of Antimicrobial Resistance (AMR) and Antimicrobial Susceptibility Testing (AST) Using Advanced Spectroscopic Techniques: A Review. TrAC Trends Anal. Chem. 2024, 172, 117562. [Google Scholar] [CrossRef]
- World Health Organization No Time to Wait: Securing the Future from Drug-Resistant Infections. Available online: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections (accessed on 11 September 2024).
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Taghizadeh, S.; Hadi, N.; Ghanbariasad, A.; Berenjian, A.; Shiong, K.; Varjani, S.; Loke, P. Synthesis of Mesoporous Antimicrobial Herbal Nanomaterial-Carrier for Silver Nanoparticles and Antimicrobial Sensing. Food Chem. Toxicol. 2022, 165, 113077. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Campeiro, J.D.; Yonamine, C.M. Revisiting the Potential of South American Rattlesnake Crotalus Durissus Terrificus Toxins as Therapeutic, Theranostic and/or Biotechnological Agents. Toxicon 2022, 206, 1–13. [Google Scholar] [CrossRef]
- Schwartz, E.F.; Camargos, T.S.; Zamudio, F.Z.; Silva, L.P.; Schwartz, C.A.; Possani, L.D.; Bloch, C. Mass Spectrometry Analysis, Amino Acid Sequence and Biological Activity of Venom Components from the Brazilian Scorpion. Toxicon 2008, 51, 1499–1508. [Google Scholar] [CrossRef]
- Tamura, M.; Tatsushiro, C.; Hayato, E.; Ohki, S. Structural and Functional Studies of LaIT2, an Antimicrobial and Insecticidal Peptide from Liocheles Australasiae. Toxicon 2022, 214, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Almaaytah, A.; Albalas, Q. Scorpion Venom Peptides with No Disulfide Bridges: A Review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility in Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Martin-Serrano, Á.; Gómez, R.; Ortega, P.; de la Mata, F.J. Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (Amps). Pharmaceutics 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Kyoung, H.; Park, E.; Kyung, M.; Park, Y. Bactericidal Activities and Action Mechanism of the Novel Antimicrobial Peptide Hylin A1 and Its Analog Peptides against Acinetobacter Baumannii Infection. Eur. J. Pharm. Sci. 2022, 175, 106205. [Google Scholar] [CrossRef]
- Bertani, R.; Bonini, R.K.; Toda, M.M.; Isa, L.S.; Figueiredo, J.V.A.; dos Santos, M.R.; Ferraz, S.C. Alien Scorpions in the Municipality of São Paulo, Brazil—Evidence of Successful Establishment of Tityus Stigmurus (Thorell, 1876) and First Records of Broteochactas Parvulus Pocock, 1897, and Jaguajir Rochae (Borelli, 1910). BioInvasions Rec. 2018, 7, 89–94. [Google Scholar] [CrossRef]
- Nencioni, A.L.A.; Neto, E.B.; de Freitas, L.A.; Dorce, V.A.C. Effects of Brazilian Scorpion Venoms on the Central Nervous System. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 3. [Google Scholar] [CrossRef]
- Oliveira da Mata, D.; Tibery, D.V.; Fernandes-Pedrosa, M.F.; Schwartz, E.F. Modulation of HNav by Tst1, a β-Toxin Purified from the Scorpion Tityus Stigmurus. Biochimie 2023, 204, 118–126. [Google Scholar] [CrossRef]
- Almeida, D.D.; Scortecci, K.C.; Kobashi, L.S.; Agnez-Lima, L.F.; Medeiros, S.R.B.; Silva-Junior, A.A.; Junqueira-de-Azevedo, I.; Fernandes-Pedrosa, M. Profiling the Resting Venom Gland of the Scorpion Tityus Stigmurus through a Transcriptomic Survey. BMC Genom. 2012, 13, 362. [Google Scholar] [CrossRef]
- Dombu, C.Y.; Betbeder, D. Airway Delivery of Peptides and Proteins Using Nanoparticles. Biomaterials 2013, 34, 516–525. [Google Scholar] [CrossRef] [PubMed]
- AlGabbani, Q. Nanotechnology: A Promising Strategy for the Control of Parasitic Infections. Exp. Parasitol. 2023, 250, 108548. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-Based Nanoparticles: An Overview of Biomedical Applications and Its Preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Varma, R.S. A Review of Chitosan Nanoparticles: Nature’s Gift for Transforming Agriculture through Smart and Effective Delivery Mechanisms. Int. J. Biol. Macromol. 2024, 260, 129522. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; El-Saadony, M.T.; Sha, M.E.; Zabermawi, N.M.; Arif, M.; Elsaber, G.; Khafaga, A.F.; El-hakim, Y.M.A.; Al-sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approv. Stand. Ed. Clin. Lab. Stand. Inst. Doc. M07-A10 2015, 35, 3–110. [Google Scholar]
- Batista, C.V.F.; Roman-Gonzalez, S.A.; Salas-Castillo, S.P.; Zamudio, F.Z.; Gomez-Lagunas, F.; Possani, L.D. Proteomic Analysis of the Venom from the Scorpion Tityus Stigmurus: Biochemical and Physiological Comparison with Other Tityus Species. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 147–157. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-C.; Wang, S.; Nie, Y.; Zhang, L.; Luo, X. Characterization of BmKbpp, a Multifunctional Peptide from the Chinese Scorpion Mesobuthus Martensii Karsch: Gaining Insight into a New Mechanism for the Functional Diversification of Scorpion Venom Peptides. Peptides 2012, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesaro, A. Chitosan Nanoparticles: Preparation, Size Evolution and Stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Rocha Soares, K.S.; Oliveira, A.R.; Daniele-Silva, A.; Gláucia-Silva, F.; Caroni, A.L.P.; Fernandes-Pedrosa, M.F.; Silva-Júnior, A.A. Self-Assembled Scorpion Venom Proteins Cross-Linked Chitosan Nanoparticles for Use in the Immunotherapy. J. Mol. Liq. 2017, 241, 540–548. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Gláucia-Silva, F.; Rocha Soares, K.S.; Souza, L.B.F.C.; Damasceno, I.Z.; Santos-Silva, E.; Lacerda, A.F.; Chaves, G.M.; Silva-Júnior, A.A.; Fernandes-Pedrosa, M.F. Biodegradable Cross-Linked Chitosan Nanoparticles Improve Anti-Candida and Anti-Biofilm Activity of TistH, a Peptide Identified in the Venom Gland of the Tityus Stigmurus Scorpion. Mater. Sci. Eng. C 2019, 103, 109830. [Google Scholar] [CrossRef] [PubMed]
- Rocha Soares, K.S.; Fonseca, J.L.C.; Bitencourt, M.A.O.; Santos, K.S.C.R.; Silva-Júnior, A.A.; Fernandes-Pedrosa, M.F. Serum Production against Tityus Serrulatus Scorpion Venom Using Cross-Linked Chitosan Nanoparticles as Immunoadjuvant. Toxicon 2012, 60, 1349–1354. [Google Scholar] [CrossRef]
- Rocha Soares, K.S.; Gláucia-Silva, F.; Daniele-Silva, A.; Torres-Rêgo, M.; Araújo, N.K.; Menezes, Y.A.S.; Damasceno, I.Z.; Tambourgi, D.V.; Silva-Júnior, A.A.; Fernandes-Pedrosa, M.F. Antivenom Production against Bothrops Jararaca and Bothrops Erythromelas Snake Venoms Using Cross-Linked Chitosan Nanoparticles as an Immunoadjuvant. Toxins 2018, 10, 158. [Google Scholar] [CrossRef]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide Colloidal Particles as Delivery Systems for Macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Koppolu, B.; Zaharoff, D.A. The Effect of Antigen Encapsulation in Chitosan Particles on Uptake, Activation and Presentation by Antigen Presenting Cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T. Chitosan Nanoparticle as Protein Delivery Carrier—Systematic Examination of Fabrication Conditions for Efficient Loading and Release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Dangor, Z.; Benson, N.; Berkley, J.A.; Bielicki, J.; Bijsma, M.W.; Broad, J.; Buurman, E.T.; Cross, A.; Duffy, E.M.; Holt, K.E.; et al. Vaccine Value Profile for Klebsiella Pneumoniae. Vaccine 2024, 42, S125–S141. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, M.; Pashmforoosh, N. Peptides with Diverse Functions from Scorpion Venom: A Great Opportunity for the Treatment of a Wide Variety of Diseases. Iran. Biomed. J. 2023, 27, 84–99. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics 2024, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan Nanoparticles Loaded with the Antimicrobial Peptide Temporin B Exert a Long-Term Antibacterial Activity in Vitro against Clinical Isolates of Staphylococcus Epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef]
- Sahariah, P.; Sorensen, K.K.; Hjalmarsdottir, M.A.; Sigurjonsson, O.E.; Jensen, K.J.; Masson, M.; Thygesen, M.B. Antimicrobial Peptide Shows Enhanced Activity and Reduced Toxicity upon Grafting to Chitosan Polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; de Lima Gualque, M.W.; da Fonseca, F.H.; Pavan, F.R.; Santos-Filho, N.A. Nanobiotechnology with Therapeutically Relevant Macromolecules from Animal Venoms: Venoms, Toxins, and Antimicrobial Peptides. Pharmaceutics 2022, 14, 891. [Google Scholar] [CrossRef]
- Auwal, S.; Zarei, M.; Tan, C.; Basri, M.; Saari, N. Improved in Vivo Efficacy of Anti-Hypertensive Biopeptides Encapsulated in Chitosan Nanoparticles Fabricated by Ionotropic Gelation on Spontaneously Hypertensive Rats. Nanomaterials 2017, 7, 421. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and Vascular Relaxant Effects of Phospholipase A2 Toxins from Papuan Taipan (Oxyuranus Scutellatus) Venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
| Samples | Size (nm) | PdI | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| CN | 134.40 ± 0.75 | 0.391 ± 0.068 | +23.20 ± 1.47 | --- |
| Tsv/CN 0.5% | 176.16 ± 1.45 | 0.465 ± 0.027 | +28.63 ± 0.58 | 81.36 |
| Tsv/CN 1.0% | 106.03 ± 1.94 | 0.430 ± 0.014 | +26.96 ± 0.58 | 78.67 |
| Strains | Tsv (µg/mL) | CN (µg/mL) | Tsv/CN 0.5% (µg/mL) | Tsv/CN 1.0% (µg/mL) |
|---|---|---|---|---|
| MIC 100% | MIC 100% | MIC 100% | MIC 100% | |
| Staphylococcus aureus (ATCC 29213) | >500 | 178.5 | 89.2 | 44.6 |
| Escherichia coli (ATCC 25922) | >500 | 178.5 | >178.5 | >178.5 |
| Strains | Tsv (µg/mL) | CN (µg/mL) | Tsv/CN 0.5% (µg/mL) | Tsv/CN 1.0% (µg/mL) |
|---|---|---|---|---|
| MIC 100% | MIC 100% | MIC 100% | MIC 100% | |
| C. albicans (ATCC 90028) | >178.5 | 178.5 | 178.5 | 178.5 |
| C. tropicalis (ATCC 13803) | >178.5 | 11.1 | 11.1 | 5.5 |
| C. dubliniensis (CBS 7987) | >178.5 | 178.5 | 178.5 | 178.5 |
| C. glabrata (ATCC 2001) | >178.5 | >178.5 | >178.5 | >178.5 |
| C. parapsilosis (ATCC 22019) | >178.5 | 22.2 | 22.2 | 11.1 |
| C. krusei (ATCC 6258) | >178.5 | >178.5 | >178.5 | >178.5 |
| C. rugosa (ATCC 10571) | >178.5 | 178.5 | 178.5 | 178.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gláucia-Silva, F.; Torres, J.V.P.; Torres-Rêgo, M.; Daniele-Silva, A.; Furtado, A.A.; Ferreira, S.d.S.; Chaves, G.M.; Xavier-Júnior, F.H.; Rocha Soares, K.S.; Silva-Júnior, A.A.d.; et al. Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles Improve Antimicrobial Activity. Int. J. Mol. Sci. 2024, 25, 9893. https://doi.org/10.3390/ijms25189893
Gláucia-Silva F, Torres JVP, Torres-Rêgo M, Daniele-Silva A, Furtado AA, Ferreira SdS, Chaves GM, Xavier-Júnior FH, Rocha Soares KS, Silva-Júnior AAd, et al. Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles Improve Antimicrobial Activity. International Journal of Molecular Sciences. 2024; 25(18):9893. https://doi.org/10.3390/ijms25189893
Chicago/Turabian StyleGláucia-Silva, Fiamma, João Vicente Pereira Torres, Manoela Torres-Rêgo, Alessandra Daniele-Silva, Allanny Alves Furtado, Sarah de Sousa Ferreira, Guilherme Maranhão Chaves, Francisco Humberto Xavier-Júnior, Karla Samara Rocha Soares, Arnóbio Antônio da Silva-Júnior, and et al. 2024. "Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles Improve Antimicrobial Activity" International Journal of Molecular Sciences 25, no. 18: 9893. https://doi.org/10.3390/ijms25189893







