The Chronic Toxicity of Endocrine-Disrupting Chemical to Daphnia magna: A Transcriptome and Network Analysis of TNT Exposure
Abstract
:1. Introduction
2. Results
2.1. Fucntional Enrichment Analysis
2.2. Comprehensively Functional Enrichment Analysis for DEGs
2.3. Toxicity Mechanisms of Chronic TNT Exposure in D. magna
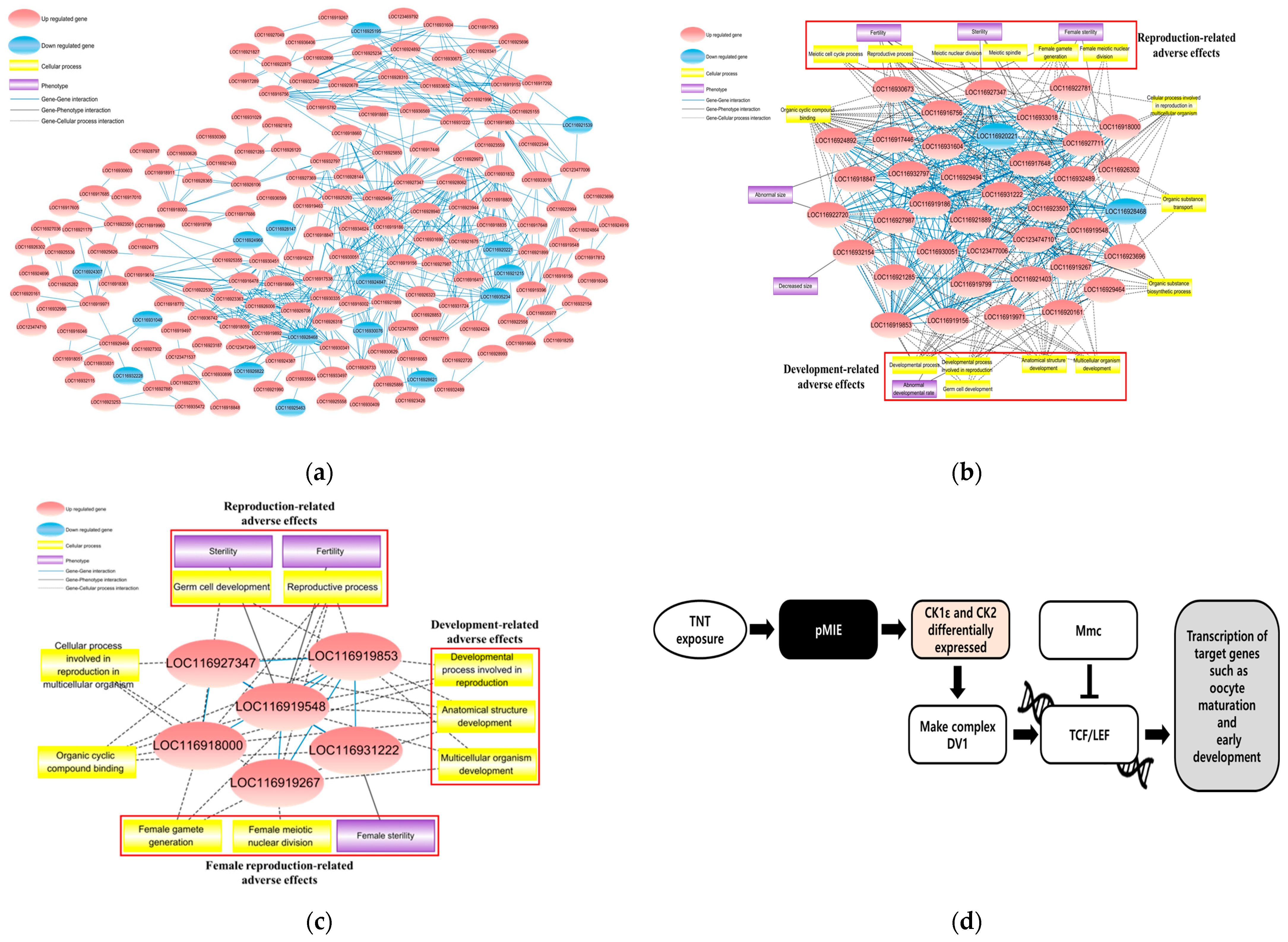
2.3.1. Biological Network Analyses
2.3.2. Putative Adverse Outcome Pathway (AOP) Development for TNT
3. Discussion
4. Materials and Methods
4.1. Data Collection and Differentially Expressed Gene Screening
4.2. Functional Enrichment Analysis
4.3. Network Analysis
4.3.1. Protein–Protein Interaction Analysis
4.3.2. Gene–Phenotype Association Analysis
4.3.3. Biological Network Analysis and Endocrine-Focused Biological Network Analysis
4.4. Putative Adverse Outcome Pathway (AOP) Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, Y.; Li, Z.; Yang, Y.; Jiao, Y.; Qu, J.; Wang, Y.; Zhang, Y. Effects of Effects of Common Environmental Endocrine-Disrupting Chemicals on Zebrafish Behavior. Water Res. 2022, 208, 117826. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Guo, F.; Liu, K.; Ding, R.; Wang, Y. The Effect of Endocrine-Disrupting Chemicals on Placental Development. Front. Endocrinol. 2023, 14, 1059854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Sarode, S.C. Various Forms of Silicon Electronic Waste and Predisposition to Cancer. J. Cancer Prev. 2023, 28, 1–2. [Google Scholar] [CrossRef]
- Jasrotia, R.; Langer, S.; Dhar, M. Endocrine Disrupting Chemicals in Aquatic Ecosystem: An Emerging Threat to Wildlife and Human Health. In Proceedings of the Zoological Society; Springer: New Delhi, India, 2021; pp. 634–647. [Google Scholar]
- Caserta, D.; Mantovani, A.; Marci, R.; Fazi, A.; Ciardo, F.; La Rocca, C.; Maranghi, F.; Moscarini, M. Environment and Women’s Reproductive Health. Hum. Reprod. Update 2011, 17, 418–433. [Google Scholar] [CrossRef]
- Yamindago, A.; Jo, Y.; Won, H.; Yum, S. Sublethal Effects of Acetaminophen Exposure on Benthic Aquatic Animal (Hydra magnipapillata). Mol. Cell. Toxicol. 2023, 1–10. [Google Scholar] [CrossRef]
- Muller, A.K.; Markert, N.; Leser, K.; Kampfer, D.; Crawford, S.E.; Schaffer, A.; Segner, H.; Hollert, H. Assessing Endocrine Disruption in Freshwater Fish Species from a “Hotspot” for Estrogenic Activity in Sediment. Environ. Pollut. 2020, 257, 113636. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Tizaoui, C.; Yang, Y.; Ali, J.; Zhang, W. Endocrine Disrupting Chemicals in Water and Recent Advances on Their Detection Using Electrochemical Biosensors. Sens. Diagn. 2023, 2, 46–77. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, D. Effect of Cadmium Chloride on Secretion of 17β-Estradiol by the Ovarian Follicles of Common Carp, Cyprinus Carpio. Gen. Comp. Endocrinol. 2013, 181, 107–114. [Google Scholar] [CrossRef]
- Lee, Y.M.; Oleszkiewicz, J.A.; Cicek, N.; Londry, K. Endocrine Disrupting Compounds (Edc) in Municipal Wastewater Treatment: A Need for Mass Balance. Environ. Technol. 2004, 25, 635–645. [Google Scholar] [CrossRef]
- Lima, D.R.; Bezerra, M.L.; Neves, E.B.; Moreira, F.R. Impact of Ammunition and Military Explosives on Human Health and the Environment. Rev. Environ. Health 2011, 26, 101–110. [Google Scholar] [CrossRef]
- Leffler, P.; Brannas, E.; Ragnvaldsson, D.; Wingfors, H.; Berglind, R. Toxicity and Accumulation of Trinitrotoluene (Tnt) and Its Metabolites in Atlantic Salmon Alevins Exposed to an Industrially Polluted Water. J. Toxicol. Environ. Health A 2014, 77, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Letzel, S.; Goen, T.; Bader, M.; Angerer, J.; Kraus, T. Exposure to Nitroaromatic Explosives and Health Effects during Disposal of Military Waste. Occup. Environ. Med. 2003, 60, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Zare, M.; Ganjali, M.R.; Norouzi, P.; Tavana, B. A New Molecularly Imprinted Polymer (Mip)-Based Electrochemical Sensor for Monitoring 2,4,6-Trinitrotoluene (Tnt) in Natural Waters and Soil Samples. Biosens. Bioelectron. 2010, 25, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Khromykh, N.; Marenkov, O.; Sharamok, T.; Anishchenko, A.; Yesipova, N.; Nesterenko, O.; Kurchenko, V.; Mylostyvyi, R. Simulating 2, 4, 6-Trinitrotoluene (Tnt) Elimination in a Pond Inhabited by Freshwater Algae of the Rhizoclonium Genus. Regul. Mech. Biosyst. 2023, 14, 365–369. [Google Scholar] [CrossRef]
- Ek, H.; Dave, G.; Nilsson, E.; Sturve, J.; Birgersson, G. Fate and Effects of 2,4,6-Trinitrotoluene (Tnt) from Dumped Ammunition in a Field Study with Fish and Invertebrates. Arch. Environ. Contam. Toxicol. 2006, 51, 244–252. [Google Scholar] [CrossRef]
- Türker, L.; Çelik Bayar, Ç. A Dft Study on Estrone–Tnt Interaction. Z. Für Anorg. Und Allg. Chem. 2013, 639, 1871–1875. [Google Scholar] [CrossRef]
- Lin, D.; Chen, Y.; Liang, L.; Huang, Z.; Guo, Y.; Cai, P.; Wang, W. Effects of Exposure to the Explosive and Environmental Pollutant 2,4,6-Trinitrotoluene on Ovarian Follicle Development in Rats. Environ. Sci. Pollut. Res. Int. 2023, 30, 96412–96423. [Google Scholar] [CrossRef]
- Ruqa, W.A.; Pennacchia, F.; Rusi, E.; Zoccali, F.; Bruno, G.; Talarico, G.; Barbato, C.; Minni, A. Smelling Tnt: Trends of the Terminal Nerve. Int. J. Mol. Sci. 2024, 25, 3920. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Sun, L.; Zhao, Y.; Pei, C.; Nie, Y.; Liu, X.; Wu, L.; Xu, A. Transgenerational Reproductive Toxicity of 2,4,6-Trinitrotoluene (Tnt) and Its Metabolite 4-Adnt in Caenorhabditis Elegans. Environ. Toxicol. Pharmacol. 2022, 92, 103865. [Google Scholar] [CrossRef]
- Adomako-Bonsu, A.G.; Jacobsen, J.; Maser, E. Metabolic Activation of 2,4,6-Trinitrotoluene; a Case for Ros-Induced Cell Damage. Redox Biol. 2024, 72, 103082. [Google Scholar] [CrossRef]
- Kovačević, G.; Tramontana Ljubičić, P.; Petrinec, D.; Sirovina, D.; Novosel, M.; Želježić, D. How Daphnia magna Defends Itself against Predators: Mechanisms and Adaptations in a Freshwater Microcosm. Water 2024, 16, 398. [Google Scholar] [CrossRef]
- Ren, J.; Yang, F.; Ding, N.; Mo, J.; Guo, J. Transcriptomic Responses to Cytotoxic Drug Cisplatin in Water Flea Daphnia magna. Environ. Toxicol. Pharmacol. 2022, 95, 103964. [Google Scholar] [CrossRef] [PubMed]
- EPA. Ecological Effects Test Guidelines. Gammarid Acute Toxic. Test OPPTS 1996, 850, 1300. [Google Scholar]
- OECD. Test No. 211: Daphnia magna Reproduction Test; OECD: Paris, France, 2012. [Google Scholar]
- Nguyen, N.D.; Matsuura, T.; Kato, Y.; Watanabe, H. Dnmt3.1 Controls Trade-Offs between Growth, Reproduction, and Life Span under Starved Conditions in Daphnia magna. Sci. Rep. 2021, 11, 7326. [Google Scholar] [CrossRef]
- Pietropoli, E.; Pauletto, M.; Tolosi, R.; Iori, S.; Lopparelli, R.M.; Montanucci, L.; Giantin, M.; Dacasto, M.; De Liguoro, M. An in Vivo Whole-Transcriptomic Approach to Assess Developmental and Reproductive Impairments Caused by Flumequine in Daphnia magna. Int. J. Mol. Sci. 2023, 24, 9396. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Na, H.W.; Jang, Y.; Kim, S.J.; Kee, N.G.; Shin, D.Y.; Choi, H.; Kim, H.J.; Seo, Y.R. Integrative Analysis to Explore the Biological Association between Environmental Skin Diseases and Ambient Particulate Matter. Sci. Rep. 2022, 12, 9750. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.M.; Cho, E.M.; Seo, Y.R. Adverse Human Health Effects of Chromium by Exposure Route: A Comprehensive Review Based on Toxicogenomic Approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef]
- Fu, X.; Chang, J.; Jiao, D.; Zhu, M.; Ma, Y. Slit3 Knockdown Inhibited Tgf-Β-Induced Hepatic Stellate Cells Activation by down-Regulating Yap Signal. Mol. Cell. Toxicol. 2024, 20, 251–258. [Google Scholar] [CrossRef]
- Hu, N.; Huang, C.; He, Y.; Li, S.; Yuan, J.; Zhong, G.; Chen, Y. A Novel Immune-Related Lncrna Prognostic Signature for Cutaneous Melanoma. Mol. Cell. Toxicol. 2024, 20, 377–387. [Google Scholar] [CrossRef]
- Lzaod, S.; Dutta, T. Recent Advances in the Development of Oxidoreductase-Based Biosensors for Detection of Phenolic Antioxidants in Food and Beverages. ACS Omega 2022, 7, 47434–47448. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Surh, Y.J.; Lee, Y.S. Effects of Exhaustive Exercise on Inflammatory, Apoptotic, and Antioxidative Signaling Pathways in Human Peripheral Blood Mononuclear Cells. J. Cancer Prev. 2023, 28, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Feligioni, M.; Marcelli, S.; Knock, E.; Nadeem, U.; Arancio, O.; Fraser, P.E. Sumo Modulation of Protein Aggregation and Degradation. AIMS Mol. Sci. 2015, 2, 382–410. [Google Scholar] [CrossRef]
- Wright, C.M.; Whitaker, R.H.; Onuiri, J.E.; Blackburn, T.; McGarity, S.; Bjornsti, M.A.; Placzek, W.J. Ubc9 Mutant Reveals the Impact of Protein Dynamics on Substrate Selectivity and Sumo Chain Linkages. Biochemistry 2019, 58, 621–632. [Google Scholar] [CrossRef]
- Ihara, M.; Stein, P.; Schultz, R.M. Ube2i (Ubc9), a Sumo-Conjugating Enzyme, Localizes to Nuclear Speckles and Stimulates Transcription in Mouse Oocytes. Biol. Reprod. 2008, 79, 906–913. [Google Scholar] [CrossRef]
- Johnson, A.N.; Mokalled, M.H.; Valera, J.M.; Poss, K.D.; Olson, E.N. Post-Transcriptional Regulation of Myotube Elongation and Myogenesis by Hoi Polloi. Development 2013, 140, 3645–3656. [Google Scholar] [CrossRef]
- Ye, J.; She, X.; Liu, Z.; He, Z.; Gao, X.; Lu, L.; Liang, R.; Lin, Y. Eukaryotic Initiation Factor 4a-3: A Review of Its Physiological Role and Involvement in Oncogenesis. Front. Oncol. 2021, 11, 712045. [Google Scholar] [CrossRef]
- Liu, T.; Sun, H.; Zhu, D.; Dong, X.; Liu, F.; Liang, X.; Chen, C.; Shao, B.; Wang, M.; Wang, Y.; et al. Tra2a Promoted Paclitaxel Resistance and Tumor Progression in Triple-Negative Breast Cancers via Regulating Alternative Splicing. Mol. Cancer Ther. 2017, 16, 1377–1388. [Google Scholar] [CrossRef]
- Yasmin, R.; Gogoi, S.; Bora, J.; Chakraborty, A.; Dey, S.; Ghaziri, G.; Bhattacharjee, S.; Singh, L.H. Novel Insight into the Cellular and Molecular Signalling Pathways on Cancer Preventing Effects of Hibiscus Sabdariffa: A Review. J. Cancer Prev. 2023, 28, 77–92. [Google Scholar] [CrossRef]
- Bartkowiak, B.; Greenleaf, A.L. Expression, Purification, and Identification of Associated Proteins of the Full-Length Hcdk12/Cyclink Complex. J. Biol. Chem. 2015, 290, 1786–1795. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, X.; Deng, Y.; Yuan, P.; Xie, Y.; Wang, J. Tra2a Promotes Proliferation, Migration, Invasion and Epithelial Mesenchymal Transition of Glioma Cells. Brain Res. Bull. 2018, 143, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Della Torre, C.; Garrone, G.; D’amato, A.; Parenti, C.; Binelli, A. First Evidence of Protein Modulation by Polystyrene Microplastics in a Freshwater Biological Model. Environ. Pollut. 2019, 250, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. Kegg for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Zhang, H.; Rong, X.; Wang, C.; Liu, Y.; Lu, L.; Li, Y.; Zhao, C.; Zhou, J. Vbp1 Modulates Wnt/Beta-Catenin Signaling by Mediating the Stability of the Transcription Factors Tcf/Lefs. J. Biol. Chem. 2020, 295, 16826–16839. [Google Scholar] [CrossRef]
- Khalid, M.; Hodjat, M.; Abdollahi, M. Environmental Exposure to Heavy Metals Contributes to Diseases via Deregulated Wnt Signaling Pathways. Iran. J. Pharm. Res. 2021, 20, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Del Valle-Perez, B.; Arques, O.; Vinyoles, M.; de Herreros, A.G.; Dunach, M. Coordinated Action of Ck1 Isoforms in Canonical Wnt Signaling. Mol. Cell Biol. 2011, 31, 2877–2888. [Google Scholar] [CrossRef]
- Kirsten, D.; Meister, W.; Strauss, B. Value of Bronchoscopy in the Diagnosis and Therapy of Obstructive Bronchitis and Bronchial Asthma. Z. Arztl. Fortbild. 1987, 81, 1117–1119. [Google Scholar]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/Beta-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Stanley, J.K.; Perkins, E.J.; Habib, T.; Sims, J.G.; Chappell, P.; Escalon, B.L.; Wilbanks, M.; Garcia-Reyero, N. The Good, the Bad, and the Toxic: Approaching Hormesis in Daphnia magna Exposed to an Energetic Compound. Environ. Sci. Technol. 2013, 47, 9424–9433. [Google Scholar] [CrossRef]
- EPA. Ecological Effects Test Guidelines: Seed Germination/Root Elongation Toxicity Test. OPPTS 1996, 850, 4200. [Google Scholar]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for Rna-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The String Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Weerasinghe-Mudiyanselage, P.D.; Kim, B.; Kang, S.; Kim, J.-S.; Moon, C. Impact of Diesel Particulate Matter on the Olfactory Bulb of Mice: Insights from Behavioral, Histological, and Molecular Assessments. Mol. Cell. Toxicol. 2024, 20, 735–745. [Google Scholar] [CrossRef]
- Ismail, N.I.B.; Kato, Y.; Matsuura, T.; Watanabe, H. Generation of White-Eyed Daphnia magna Mutants Lacking Scarlet Function. PLoS ONE 2018, 13, e0205609. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. Blast+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
| GO Term Category | GO Term for Upregulated DEGs | GO Term for Downregulated DEGs | ||
|---|---|---|---|---|
| GO Term Description | FDR | GO Term Description | FDR | |
| Biological process | Cellular process | 5.42 × 10−90 | Cellular process | 9.71 × 10−17 |
| Organic substance metabolic process | 9.54 × 10−51 | Metabolic process | 4.73 × 10−11 | |
| Metabolic process | 9.54 × 10−51 | Organic substance metabolic process | 9.28 × 10−10 | |
| Nitrogen compound metabolicprocess | 2.1 × 10−49 | Organonitrogen compoundmetabolic process | 1.26 × 10−8 | |
| Primary metabolic process | 1.29 × 10−48 | Response to stimulus | 4.84 × 10−8 | |
| Macromolecule metabolic process | 1.37 × 10−46 | Primary metabolic process | 1.23 × 10−6 | |
| Cellular metabolic process | 1.15 × 10−44 | Nitrogen compound metabolic process | 1.40 × 10−5 | |
| Biological regulation | 3.36 × 10−43 | Regulation of biological quality | 3.83 × 10−5 | |
| Cellular component organization or biogenesis | 3.99 × 10−40 | Glutathione metabolic process | 6.61 × 10−5 | |
| Regulation of biological process | 6.02 × 10−38 | Response to ethanol | 1.10 × 10−4 | |
| Cellular Component | Intracellular anatomical structure | 1.86 × 10−104 | Cellular anatomical entity | 3.54 × 10−23 |
| Cellular anatomical entity | 1.15 × 10−99 | Cytoplasm | 6.45 × 10−8 | |
| Intracellular organelle | 1.63 × 10−69 | Sarcomere | 4.75 × 10−6 | |
| Organelle | 7.16 × 10−69 | Intracellular anatomical structure | 5.18 × 10−6 | |
| Intracellular membrane-boundedorganelle | 1.29 × 10−58 | Extracellular region | 6.61 × 10−6 | |
| Membrane-bounded organelle | 4.02 × 10−56 | Supramolecular fiber | 1.10 × 10−4 | |
| Protein-containing complex | 1.23 × 10−53 | Membrane | 1.10 × 10−4 | |
| Cytoplasm | 2.31 × 10−52 | Z disc | 2.30 × 10−3 | |
| Nucleus | 2.24 × 10−50 | Organelle | 2.90 × 10−3 | |
| Ribonucleoprotein complex | 6.20 × 10−34 | Intracellular organelle | 7.60 × 10−3 | |
| Molecular function | Binding | 1.69 × 10−65 | Catalytic activity | 5.48 × 10−12 |
| Organic cyclic compound binding | 2.30 × 10−46 | Ion binding | 1.93 × 10−7 | |
| Heterocyclic compound binding | 6.98 × 10−46 | Binding | 5.32 × 10−7 | |
| Protein binding | 1.77 × 10−27 | Cation binding | 5.22 × 10−5 | |
| Nucleic acid binding | 9.34 × 10−27 | Oxidoreductase activity | 2.10 × 10−4 | |
| RNA binding | 1.97 × 10−25 | Glutathione transferase activity | 2.50 × 10−4 | |
| Catalytic activity | 6.67 × 10−22 | Metal ion binding | 3.00 × 10−4 | |
| Ion binding | 7.46 × 10−22 | Transferase activity | 6.70 × 10−4 | |
| Carbohydrate derivative binding | 4.74 × 10−20 | Catalytic activity, acting on a protein | 2.50 × 10−3 | |
| Small molecule binding | 1.35 × 10−19 | Protein binding | 2.80 × 10−3 | |
| GO Term Category | GO Term Description | FDR |
|---|---|---|
| Biological process | Organic substance biosynthetic process | 5.44 × 10−9 |
| Developmental process | 2.60 × 10−4 | |
| Cellular process involved in reproduction in multicellular organism | 4.60 × 10−4 | |
| Anatomical structure development | 5.10 × 10−4 | |
| Reproductive process | 5.60 × 10−4 | |
| Female gamete generation | 2.50 × 10−3 | |
| Organic substance transport | 7.50 × 10−3 | |
| Developmental process involved in reproduction | 9.40 × 10−3 | |
| Multicellular organism development | 1.63 × 10−2 | |
| Meiotic cell cycle process | 1.79 × 10−2 | |
| Germ cell development | 3.46 × 10−2 | |
| Meiotic nuclear division | 3.88 × 10−2 | |
| Female meiotic nuclear division | 4.15 × 10−2 | |
| Cellular Component | Meiotic spindle | 1.05 × 10−2 |
| Molecular function | Organic cyclic compound binding | 1.41 × 10−43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, H.W.; Shin, D.Y.; Han, J.P.; Jang, Y.; Park, J.Y.; Yun, S.-G.; Cho, E.-M.; Seo, Y.R. The Chronic Toxicity of Endocrine-Disrupting Chemical to Daphnia magna: A Transcriptome and Network Analysis of TNT Exposure. Int. J. Mol. Sci. 2024, 25, 9895. https://doi.org/10.3390/ijms25189895
Lee J, Kim HW, Shin DY, Han JP, Jang Y, Park JY, Yun S-G, Cho E-M, Seo YR. The Chronic Toxicity of Endocrine-Disrupting Chemical to Daphnia magna: A Transcriptome and Network Analysis of TNT Exposure. International Journal of Molecular Sciences. 2024; 25(18):9895. https://doi.org/10.3390/ijms25189895
Chicago/Turabian StyleLee, Jun, Hyun Woo Kim, Dong Yeop Shin, Jun Pyo Han, Yujin Jang, Ju Yeon Park, Seok-Gyu Yun, Eun-Min Cho, and Young Rok Seo. 2024. "The Chronic Toxicity of Endocrine-Disrupting Chemical to Daphnia magna: A Transcriptome and Network Analysis of TNT Exposure" International Journal of Molecular Sciences 25, no. 18: 9895. https://doi.org/10.3390/ijms25189895






