The Genome-Wide Identification of the Dihydroflavonol 4-Reductase (DFR) Gene Family and Its Expression Analysis in Different Fruit Coloring Stages of Strawberry
Abstract
:1. Introduction
2. Results
2.1. Sequence Characteristics of the Strawberry DFR Gene Family
2.2. Subcellular Localization and Secondary Structure Analysis of the Strawberry DFR Gene Family
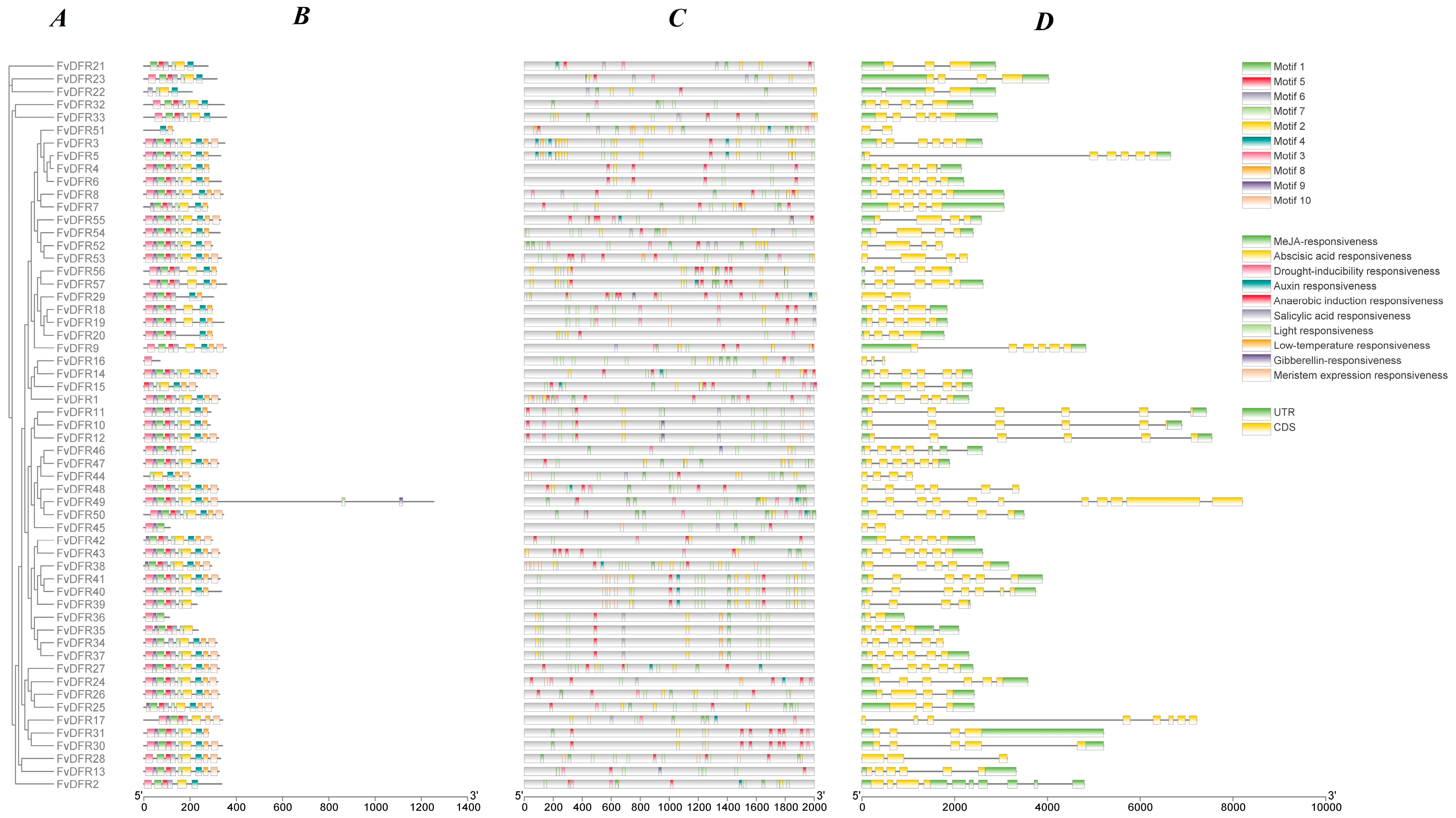
2.3. Analysis of Gene Structure, Motif, and cis-Acting Elements of Strawberry DFR Gene Family
2.4. Phylogenetic Tree Construction of the Strawberry DFR Family
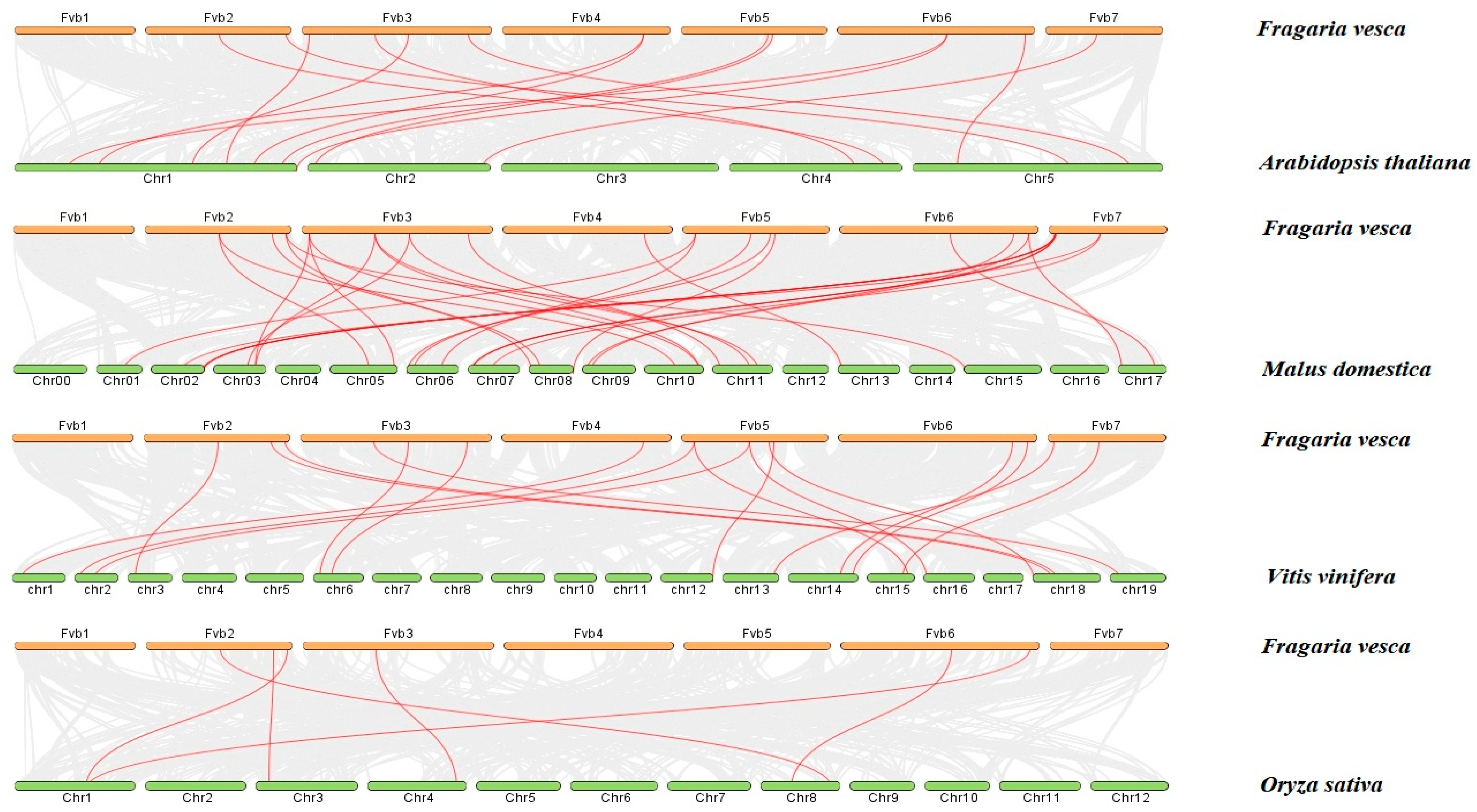
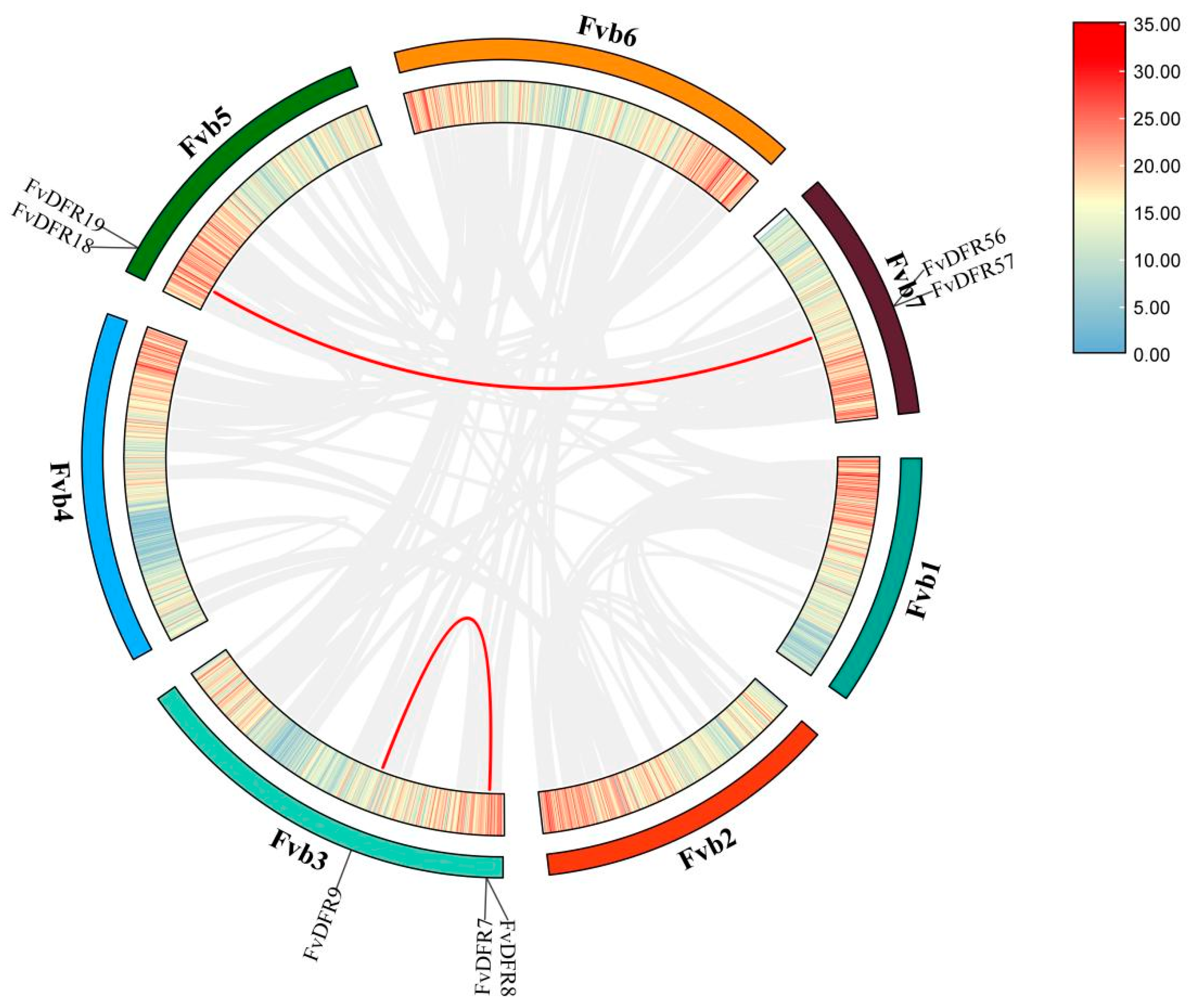
2.5. Collinearity Analysis of the Strawberry DFR Family
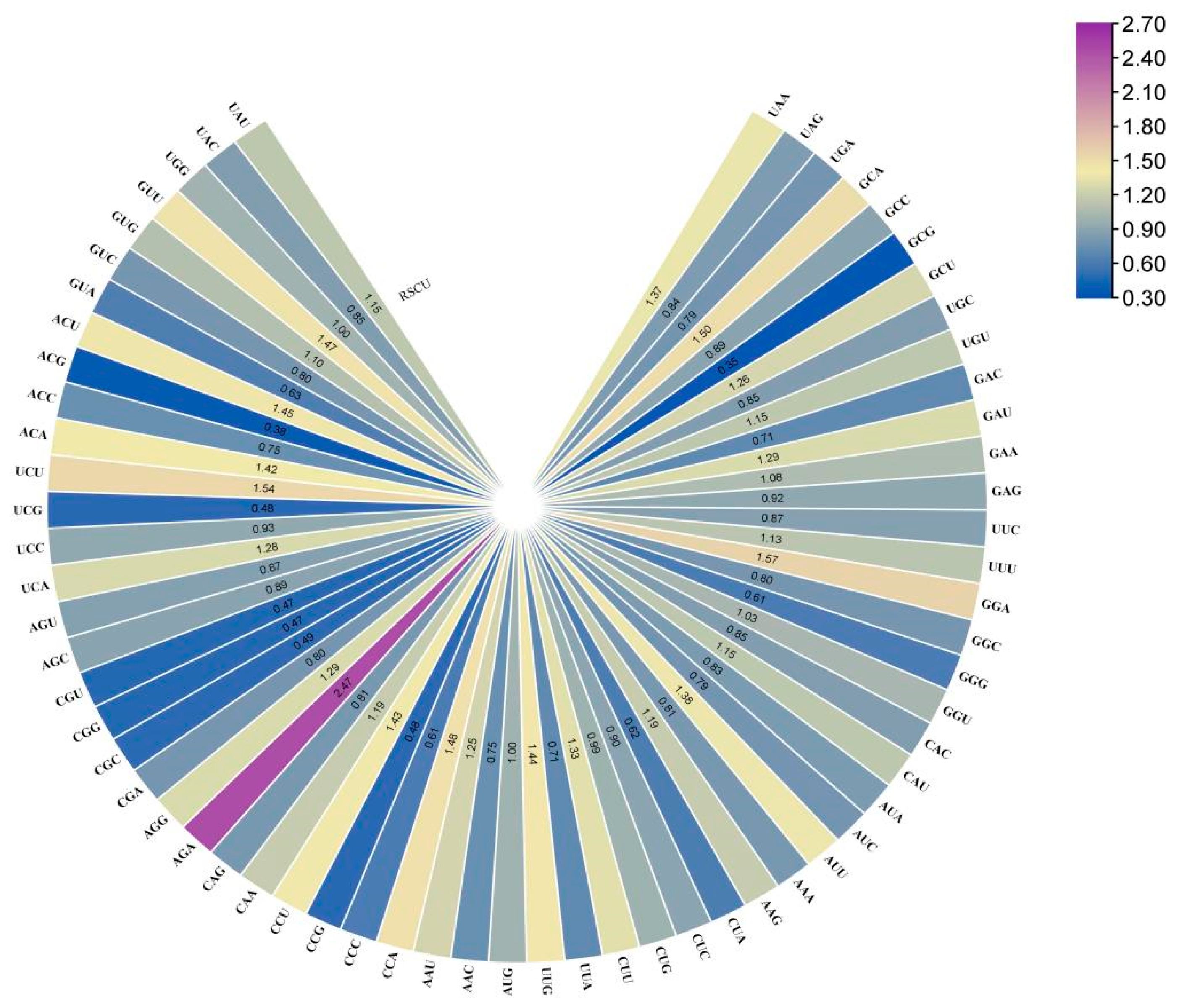
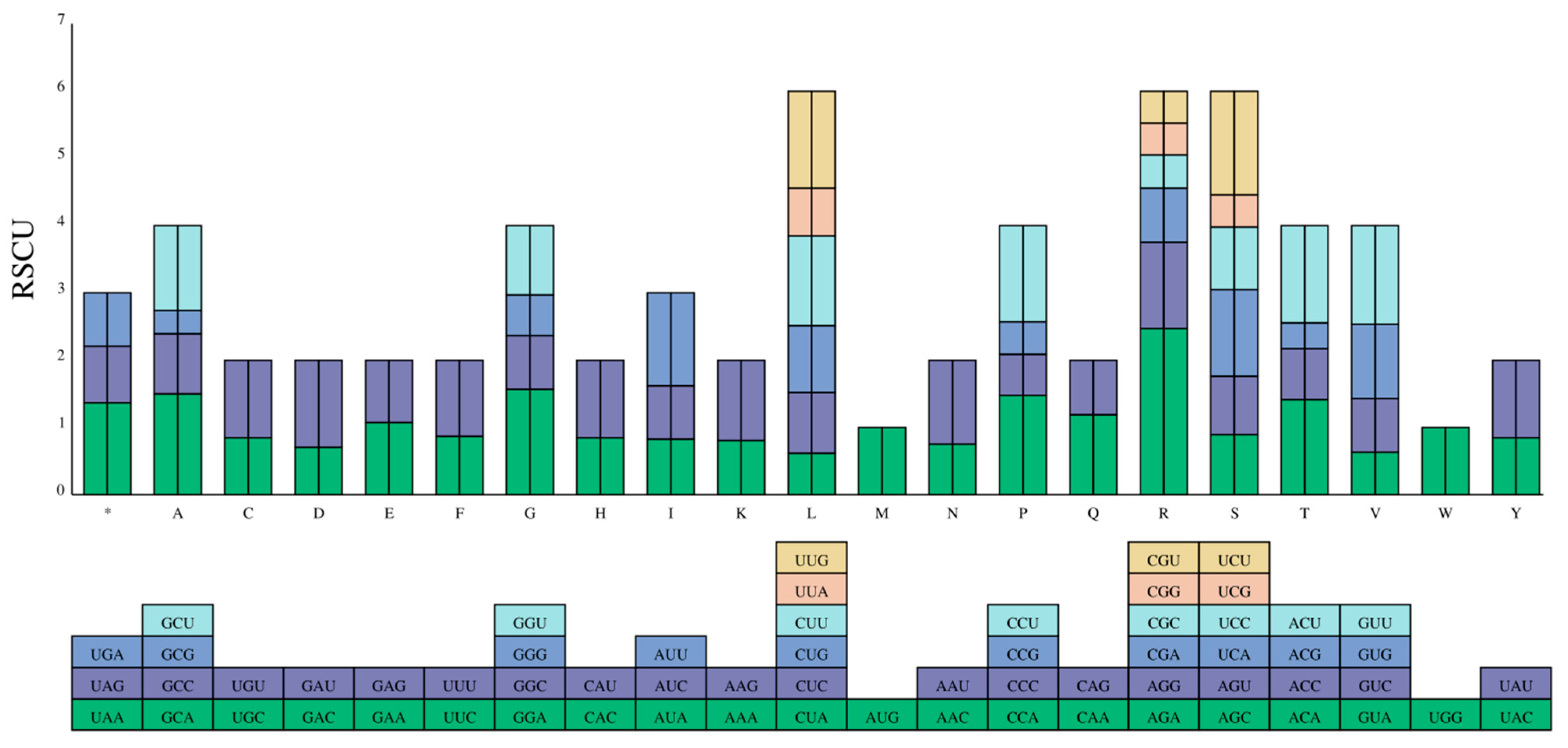
2.6. Codon Preference Analysis of the Strawberry DFR Family
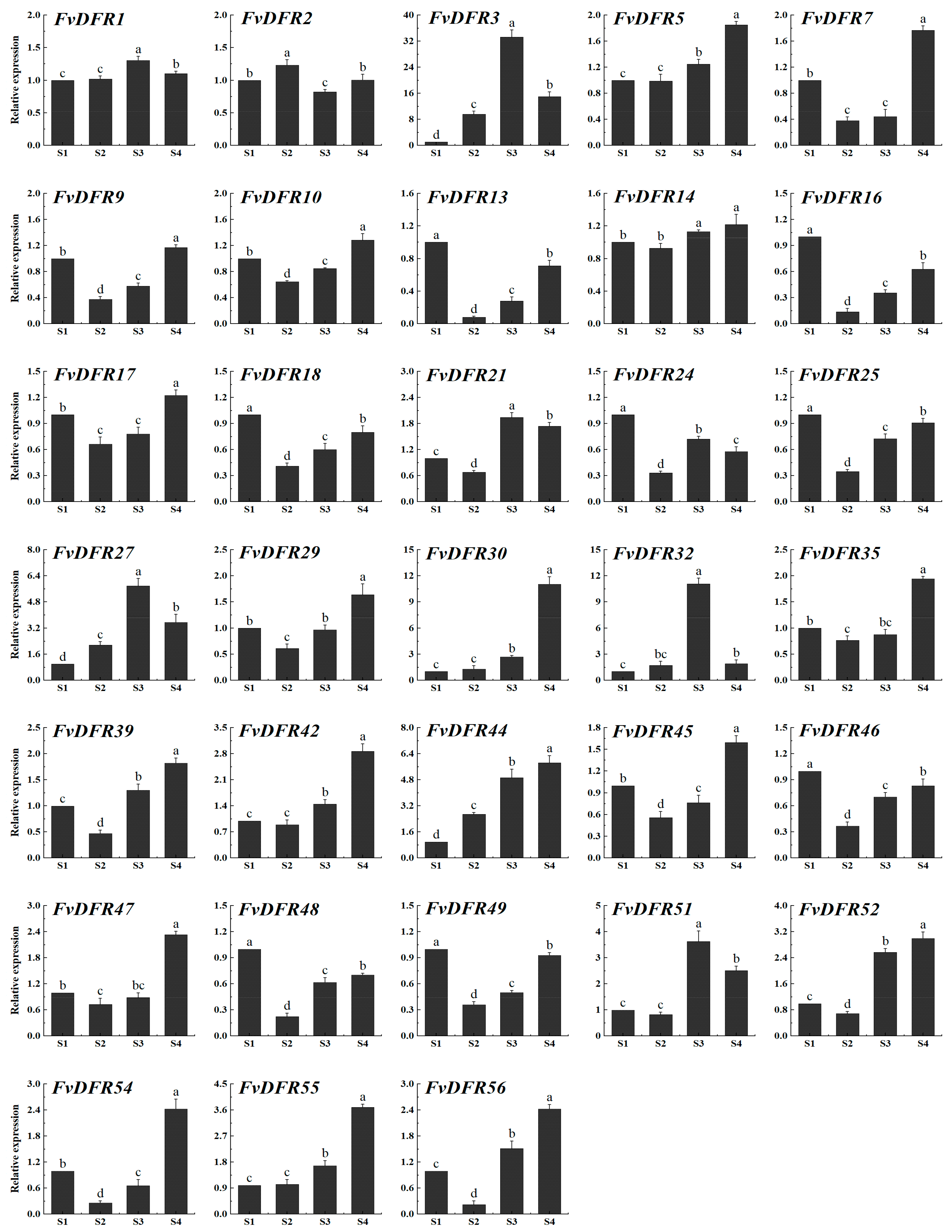
2.7. Analysis of the Expression Patterns of the DFR Gene Family in Strawberry
2.8. Quantitative Fluorescence Analysis of Strawberry DFR Gene Family and Determination of Anthocyanin Content in Strawberry Fruit
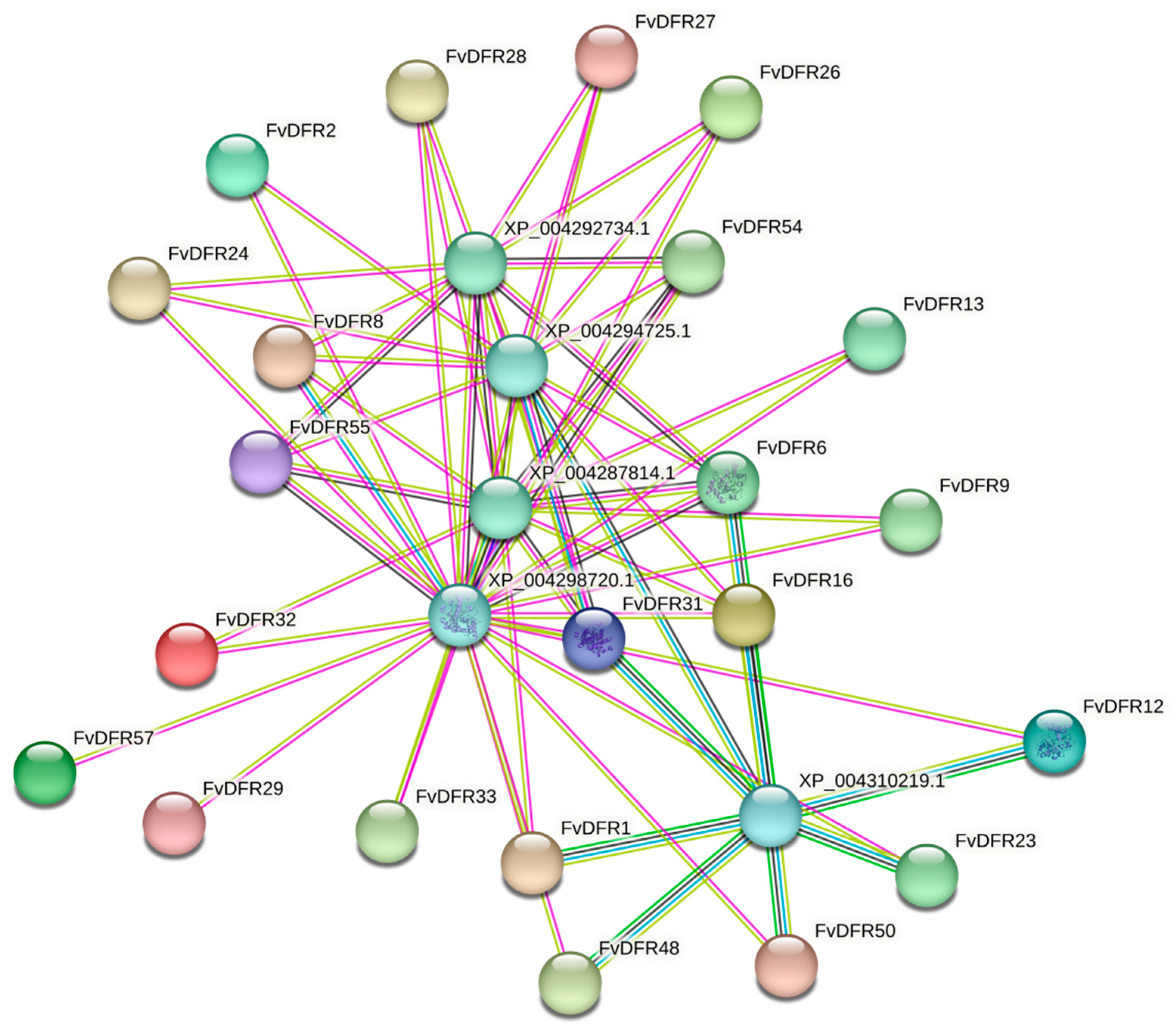
2.9. Protein Interaction Network Analysis and Prediction of Strawberry DFR Gene Family
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of the Strawberry DFR Gene Family
4.3. Chromosome Localization, Subcellular Localization, and Secondary Structure Analysis of the Strawberry DFR Gene Family
4.4. Analysis of Gene Structure, Motif, and cis-Acting Elements of Strawberry DFR Gene Family
4.5. Phylogenetic Tree Construction and Collinearity Analysis of the Strawberry DFR Family
4.6. Protein Interaction Network Analysis and Codon Preference Analysis of Strawberry DFR Gene Family
4.7. Tissue-Specific Expression Profile of the Strawberry DFR Gene Family
4.8. Determination of Anthocyanin Content in Strawberry Fruits in Different Time Periods
4.9. Quantitative RT-PCR (qRT-PCR) Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhao, T.; Zhang, Z.W.; Meng, J.F. Chemistry: Reduction of dihydrokaempferol by Vitis vinfera dihydroflavonol 4-reductase to produce orange pelargonidin-type anthocyanins. J. Agric. Food 2018, 66, 3524–3532. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, C.; Shepherd, N.S.; Pereira, A.; Schwarz-sommer, Z.; Bertram, I.; Robertson, D.S.; Peterson, P.A.; Saedler, H. Molecular cloning of the al locus in Zea mays using the transposable elements En and Mu1. Embo J. 1985, 4, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, Q.; Zhang, J. Dihydroflavonol 4-Reductase Gene (DFR) and Flower Color Modulation. Plant Physiol. J. 2005, 41, 715–719. [Google Scholar]
- Yu, T.; Ni, X.; Gao, L.; Han, G.; Zhu, C.; Sheng, Y. Advances in Study of Dihydroflavonol 4-reductase (DFR) Genes of Higher Plants. Bull. Bot. Res. 2018, 38, 632–640. [Google Scholar]
- Tian, J.; Han, Z.; Zhang, J.; Hu, Y.; Song, T.; Yao, Y. The Balance of Expression of Dihydroflavonol 4-reductase and Flavonol Synthase Regulates Flavonoid Biosynthesis and Red Foliage Coloration in Crabapples. Sci. Rep. 2015, 5, 12228. [Google Scholar] [CrossRef]
- Petit, P.; Granier, T.; d’Estaintot, B.L.; Manigand, C.; Bathany, K.; Schmitter, J.M.; Lauvergeat, V.; Hamdi, S.; Gallois, B. Crystal structure of grape dihydroflavonol 4-reductase, a key enzyme in flavonoid biosynthesis. J. Mol. Biol. 2007, 368, 1345–1357. [Google Scholar] [CrossRef]
- Matsui, K.; Hisano, T.; Yasui, Y.; Mori, M.; Walker, A.R.; Morishita, T.; Kats, K. Isolation and characterization of genes encoding leucoanthocyanidin reductase (FeLAR) and anthocyanidin reductase (FeANR)in buckwheat (Fagopyrum esculentum). J. Plant Physiol. 2016, 205, 41–47. [Google Scholar] [CrossRef]
- Ni, J.; Ruan, R.; Wang, L.; Jiang, Z.; Gu, X.; Chen, L.; Xu, M. Functional and correlation analyses of dihydroflavonol-4-reductase genes indicate their roles in regulating anthocyanin changes in Ginkgo biloba. Ind. Crops Prod. 2020, 152, 112546. [Google Scholar] [CrossRef]
- Dzhanfezova, T.; Barba-Espín, G.; Müller, R.; Joernsgaard, B.; Hegelund, J.M.; Madsen, B.; Larsen, D.H.; Vega, M.M.; Toldam-Andersen, T.B. Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria × ananassa Duch) genetic resource collection. Food Biosci. 2020, 36, 100620. [Google Scholar] [CrossRef]
- Harakava, R. Genes encoding enzymes of the lignin in biosynthesis pathway in Eucalyptus. Genet. Mol. Biol. 2005, 28, 601–607. [Google Scholar] [CrossRef]
- Zou, H. Progress of studies on anti-inflammation, immunodulatory and anti-senility effects of total flavones. J. Anhui Health Vocat. Tech. Coll. 2003, 2, 48–50. [Google Scholar]
- Zhu, Y.; Xue, H.; Zhou, Q.; Zong, J.; Gao, Y. Cloning and Function Study of LaDFR1 Gene and Promoter from Lycoris albiflora. J. Agric. Biotechnol. 2003, 31, 1396–1404. [Google Scholar]
- Qin, S.; Liu, Y.; Cui, B.; Cheng, J.; Liu, S.; Liu, H. Isolation and functional diversification of dihydroflavonol 4-Reductase gene HvDFR from Hosta ventricosa indicate its role in driving anthocyanin accumulation. Plant Signal Behav. 2022, 17, 2010389. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, Y.; Lou, Q. Cloning and Expression Analysis of Dihydroflavonol 4-reductase Gene (DFR) from Grape Hyacinth (Muscari armeniacum). J. Agric. Biotechnol. 2014, 22, 529–540. [Google Scholar]
- Xu, L.; Feng, Q.; Yang, L.; Xu, Q. Cloning and expression of dfr in Ribes L. during fruit maturation. Chin. J. Biotechnol. 2020, 36, 620–1628. [Google Scholar]
- Duan, W.; Sun, P.; Li, J. Expression of genes involved in the anthocyanin biosynthesis pathway in white and red fruits of Fragaria pentaphylla and genetic variation in the dihydroflavonol-4-reductase gene. Biochem. Syst. Ecol. 2017, 72, 40–46. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Z.; Gao, A. Cloning and Expression Analysis of DFR Gene from Mango (Mangiferaindica). Mol. Plant Breed. 2015, 13, 816–821. [Google Scholar]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Yang, T.J.; Hur, Y.; Nou, I.S. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene 2014, 550, 46–55. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Lam, P.Y.; Dini-Andreote, F.; Dai, L.; Wei, Z. Multifaceted roles of flavonoids mediating plant-microbe interactions. Microbiome 2022, 10, 233. [Google Scholar] [CrossRef]
- Liu, H.; Lou, Q.; Ma, J.; Su, B.; Gao, Z.; Liu, Y. Cloning and Functional Characterization of Dihydroflavonol 4-Reductase Gene Involved in Anthocyanidin Biosynthesis of Grape Hyacinth. Int. J. Mol. Sci. 2019, 20, 4743. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ma, J.; Cui, G.; Li, Y. Cloning and Functional Analysis of the Key Enzyme DFR Promoters in Turnip Anthocyanin Biosynthesis. Acta Hortic. Sin. 2014, 41, 1631–1641. [Google Scholar]
- Sun, Y.; Wang, J.; Qiu, Y.; Liu, T.; Song, J.; Li, X. Identification of ‘Xinlimei’ radish candidate genes associated with anthocyanin biosynthesis based on a transcriptome analysis. Gene 2018, 657, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zheng, W.; Hu, J.; Ma, J.; Sun, M.; Li, Y.; Liu, N.; Chen, T.; Wang, M.; Wang, L.; et al. Identification and Expression Analysis of DFR Gene Family in Brassica napus L. Plants 2023, 12, 2583. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, J.; Ma, Z.; Hou, Y.; Li, X.; Mao, J.; Chen, B. Molecular evolution and expression assessment of DFRs in apple. Chem. Biol. Technol. Agric. 2023, 10, 98. [Google Scholar] [CrossRef]
- Hu, Y. Bioinformatics analysis of the DFR gene of a garden ornamental pomegranate plant. South China Fruits 2016, 45, 95–100. [Google Scholar]
- Shi, Z.; Li, H.; Gao, M.; Guo, C.; Guo, D.; Bi, Y. Cloning of GmDFR gene from Soybean (Glycine max) and Identification of Its Function on Resistance to Iron Deficiency. J. Agric. Biotechnol. 2023, 30, 259–272. [Google Scholar]
- Ping, H.; Wang, J.; Zhang, H.; Zhang, P.; Wang, W.; Guo, X.; Du, C. Cloning and Bioinformatics Characteristics Analysis of DFR Gene in Paeonia delavay. Mol. Plant Breed. 2023, 21, 5620–5630. [Google Scholar]
- Feng, C.; Zhou, N.; Sun, S.; Zhou, J.; Sun, W. Cloning and Analysis of DFR3 Gene from Ophiorrhiza japonicus (OjDFR3) and Isolation and Purification of Its Coding Protein. Acta Bot. Boreali-Occident. Sin. 2021, 41, 926–932. [Google Scholar]
- Li, Y.; Li, W.; Wang, K.; Liu, Q.; Jiang, X.; Liu, Q. Cloning and expression analysis of DFR gene in Aconitum carmichaeli. Plant Physiol. J. 2017, 53, 2222–2228. [Google Scholar]
- Wang, H.; Qu, H.; Zhou, T.; Xu, Q. Cloning and Expression Analysis of Anthocyanin Biosynthesis-Associated DFR and MYB Genes in Calyx of Eggplant. Sci. Agric. Sin. 2017, 50, 2781–2792. [Google Scholar]
- Lei, T.; Huang, J.; Ruan, H.; Qian, W.; Fang, Z.; Gu, C.; Zhang, N.; Liang, Y.; Wang, Z.; Gao, L.; et al. Competition between FLS and DFR regulates the distribution of flavonols and proanthocyanidins in Rubus chingii Hu. Front. Plant Sci. 2023, 14, 1134993. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, L.; Li, D.; Chai, Z.; Zhang, X.; Huang, W. Expression and Molecular Evolution of Genes Related to Anthocyanin Biosynthesis in Blueberries. J. Food Sci. 2022, 43, 184–191. [Google Scholar]
- Tan, F.; Xiao, K.; Liu, X.; Huang, Q.; Zhang, A.; Wu, X. Formation and Gene Expression of Pigments in Eggplant Pericarp. Fujian J. Agric. Sci. 2023, 38, 158–165. [Google Scholar]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Jalal, A.; Ali, Q.; Manghwar, H.; Zhu, D. Identification, Phylogeny, Divergence, Structure, and Expression Analysis of A20/AN1 Zinc Finger Domain Containing Stress-Associated Proteins (SAPs) Genes in Jatropha curcas L. Genes 2022, 13, 1766. [Google Scholar] [CrossRef]
- Xiong, E.; Zheng, C.Y.; Wu, X.L.; Wang, W. Protein subcellular location: The gap between prediction and experimentation. Plant Mol. Biol. Rep. 2015, 34, 52–61. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Wang, S.; Zhang, X.; Wang, Y.; Shen, Y.; Yuan, Z. Genome-wide identification, gene cloning, subcellular location and expression analysis of SPL gene family in P. granatum L. BMC Plant Biol. 2021, 21, 400. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Jalal, A.; Sun, J.; Chen, Y.; Fan, C.; Liu, J.; Wang, C. Evolutionary Analysis and Functional Identification of Clock-Associated PSEUDO-RESPONSE REGULATOR (PRRs) Genes in the Flowering Regulation of Roses. Int. J. Mol. Sci. 2022, 23, 7335. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lu, J.; Liu, J.; Jalal, A.; Wang, C. Genome-wide identification and functional analysis of JmjC domain-containing genes in flower development of Rosa chinensis. Plant Mol Biol. 2020, 102, 417–430. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | GenBank No. | Number of Amino Acids | Molecular Weight/Da | pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity |
|---|---|---|---|---|---|---|---|
| FvDFR1 | FvH4_2g17230.t1.v4.0.a2 | 329 | 36,554.02 | 6.27 | 39.77 | 97.48 | −0.145 |
| FvDFR2 | FvH4_2g34590.t1.v4.0.a2 | 336 | 37,093.61 | 7.74 | 31.35 | 95.42 | −0.026 |
| FvDFR3 | FvH4_2g39520.t1.v4.0.a2 | 349 | 38,956.7 | 6.03 | 36.65 | 82.64 | −0.223 |
| FvDFR4 | FvH4_2g39520.t2.v4.0.a2 | 281 | 31,308.21 | 6.75 | 35.3 | 90.57 | −0.067 |
| FvDFR5 | FvH4_2g39520.t5.v4.0.a2 | 331 | 37,150.87 | 6.28 | 38.34 | 86.31 | −0.162 |
| FvDFR6 | FvH4_2g39520.t3.v4.0.a2 | 333 | 37,202.99 | 6.43 | 37.21 | 87.57 | −0.126 |
| FvDFR7 | FvH4_3g02980.t2.v4.0.a2 | 275 | 29,731.37 | 5.43 | 23.07 | 99.96 | 0.194 |
| FvDFR8 | FvH4_3g02980.t1.v4.0.a2 | 341 | 36,861.42 | 6.25 | 28.09 | 93.49 | −0.012 |
| FvDFR9 | FvH4_3g21610.t1.v4.0.a2 | 354 | 37,780.97 | 5.1 | 44.75 | 97.23 | 0.063 |
| FvDFR10 | FvH4_3g28560.t2.v4.0.a2 | 285 | 31,371.08 | 7.62 | 28.01 | 92.67 | −0.081 |
| FvDFR11 | FvH4_3g28560.t1.v4.0.a2 | 289 | 31,962.82 | 8.39 | 27.1 | 91.38 | −0.076 |
| FvDFR12 | FvH4_3g28560.t3.v4.0.a2 | 321 | 35,294.56 | 6.19 | 25.32 | 92.27 | −0.062 |
| FvDFR13 | FvH4_3g39460.t1.v4.0.a2 | 324 | 35,438.78 | 5.68 | 28.15 | 96.85 | −0.01 |
| FvDFR14 | FvH4_4g27430.t1.v4.0.a2 | 319 | 35,199.2 | 5.41 | 37.96 | 88.65 | −0.087 |
| FvDFR15 | FvH4_4g27430.t2.v4.0.a2 | 229 | 25,523.15 | 5.87 | 44.24 | 83.93 | −0.141 |
| FvDFR16 | FvH4_4g27470.t1.v4.0.a2 | 69 | 7686.98 | 9.43 | 24.09 | 93.33 | −0.013 |
| FvDFR17 | FvH4_5g01060.t1.v4.0.a2 | 340 | 36,937.43 | 8.6 | 38.59 | 89.15 | −0.207 |
| FvDFR18 | FvH4_5g04220.t2.v4.0.a2 | 296 | 32,710.5 | 5.63 | 42.37 | 87.94 | −0.033 |
| FvDFR19 | FvH4_5g04220.t1.v4.0.a2 | 345 | 38,216.82 | 5.68 | 42.72 | 86.17 | −0.105 |
| FvDFR20 | FvH4_5g04260.t1.v4.0.a2 | 295 | 32,503.36 | 5.49 | 41.21 | 90.88 | 0.003 |
| FvDFR21 | FvH4_5g22420.t2.v4.0.a2 | 276 | 30,947.39 | 5.2 | 33.71 | 87.93 | −0.113 |
| FvDFR22 | FvH4_5g22420.t3.v4.0.a2 | 208 | 23,072.49 | 6.42 | 26.94 | 91.39 | −0.075 |
| FvDFR23 | FvH4_5g22420.t1.v4.0.a2 | 315 | 34,963.73 | 5.8 | 37.03 | 83.87 | −0.182 |
| FvDFR24 | FvH4_5g26020.t1.v4.0.a2 | 319 | 35,559.9 | 6.9 | 28.47 | 95.61 | −0.213 |
| FvDFR25 | FvH4_5g26030.t2.v4.0.a2 | 298 | 33,155.86 | 5.26 | 34.07 | 89.9 | −0.208 |
| FvDFR26 | FvH4_5g26030.t1.v4.0.a2 | 326 | 36,038.11 | 5.47 | 31.69 | 87.85 | −0.206 |
| FvDFR27 | FvH4_5g26090.t1.v4.0.a2 | 325 | 36,066.5 | 7.47 | 35.87 | 87.51 | −0.231 |
| FvDFR28 | FvH4_5g27181.t1.v4.0.a2 | 331 | 35,783.64 | 5.76 | 38.84 | 87.79 | −0.063 |
| FvDFR29 | FvH4_5g33950.t1.v4.0.a2 | 300 | 33,330.22 | 6.26 | 41.39 | 84.2 | −0.172 |
| FvDFR30 | FvH4_6g28680.t1.v4.0.a2 | 339 | 37,193.48 | 6.17 | 34.23 | 92.57 | −0.206 |
| FvDFR31 | FvH4_6g28680.t2.v4.0.a2 | 279 | 30,321.63 | 5.78 | 32.07 | 95.02 | −0.045 |
| FvDFR32 | FvH4_6g45521.t1.v4.0.a2 | 347 | 38,638.31 | 5.92 | 35.14 | 86.83 | −0.178 |
| FvDFR33 | FvH4_6g50850.t1.v4.0.a2 | 356 | 39,502.16 | 7.53 | 38.3 | 90.08 | −0.196 |
| FvDFR34 | FvH4_7g01511.t3.v4.0.a2 | 315 | 34,545.75 | 5.1 | 22.84 | 98.7 | 0.003 |
| FvDFR35 | FvH4_7g01511.t2.v4.0.a2 | 233 | 25,752.74 | 5.24 | 29.24 | 96.61 | 0.083 |
| FvDFR36 | FvH4_7g01511.t4.v4.0.a2 | 109 | 11,760.52 | 5.63 | 14.46 | 100.09 | 0.117 |
| FvDFR37 | FvH4_7g01511.t1.v4.0.a2 | 325 | 35,637.98 | 5.03 | 23.63 | 97.75 | 0.01 |
| FvDFR38 | FvH4_7g01520.t3.v4.0.a2 | 292 | 32,217 | 5.3 | 36.75 | 100.17 | −0.047 |
| FvDFR39 | FvH4_7g01520.t2.v4.0.a2 | 229 | 24,937.86 | 6.9 | 23.09 | 105.94 | 0.128 |
| FvDFR40 | FvH4_7g01520.t4.v4.0.a2 | 334 | 37,337.49 | 8.75 | 29.31 | 108.86 | 0.095 |
| FvDFR41 | FvH4_7g01520.t1.v4.0.a2 | 328 | 35,944.43 | 5.93 | 30.99 | 102.53 | 0.013 |
| FvDFR42 | FvH4_7g01560.t2.v4.0.a2 | 297 | 32,874.99 | 5.87 | 33.82 | 98.11 | 0.01 |
| FvDFR43 | FvH4_7g01560.t1.v4.0.a2 | 328 | 35,963.57 | 6.51 | 30.53 | 99.24 | 0.03 |
| FvDFR44 | FvH4_7g01561.t1.v4.0.a2 | 200 | 22,086.52 | 5.13 | 27.98 | 102.4 | 0.049 |
| FvDFR45 | FvH4_7g01562.t1.v4.0.a2 | 113 | 12,170.15 | 8.4 | 30.5 | 103.45 | 0.249 |
| FvDFR46 | FvH4_7g01563.t1.v4.0.a2 | 223 | 24,257.09 | 6.43 | 27.89 | 98.74 | 0.07 |
| FvDFR47 | FvH4_7g01564.t1.v4.0.a2 | 323 | 35,549.88 | 5.28 | 25.65 | 96.22 | 0.021 |
| FvDFR48 | FvH4_7g01580.t3.v4.0.a2 | 322 | 35,390.69 | 6.14 | 29.12 | 95.68 | −0.039 |
| FvDFR49 | FvH4_7g01580.t2.v4.0.a2 | 1253 | 136,295.17 | 5.85 | 32.8 | 101.35 | 0.056 |
| FvDFR50 | FvH4_7g01580.t1.v4.0.a2 | 344 | 37,808.72 | 8.61 | 27.73 | 96.34 | −0.071 |
| FvDFR51 | FvH4_7g11750.t1.v4.0.a2 | 126 | 14,089.26 | 4.69 | 33.68 | 104.37 | 0.185 |
| FvDFR52 | FvH4_7g11760.t1.v4.0.a2 | 296 | 32,516.22 | 6.32 | 35.39 | 85.61 | −0.176 |
| FvDFR53 | FvH4_7g11780.t1.v4.0.a2 | 334 | 36,985.41 | 6.08 | 39.18 | 85.81 | −0.126 |
| FvDFR54 | FvH4_7g11781.t1.v4.0.a2 | 328 | 36,100.34 | 5.64 | 39.55 | 92.1 | −0.018 |
| FvDFR55 | FvH4_7g11790.t1.v4.0.a2 | 331 | 36,612.02 | 5.25 | 32.18 | 87.13 | −0.05 |
| FvDFR56 | FvH4_7g16111.t2.v4.0.a2 | 313 | 34,918.03 | 6.51 | 46.13 | 88.43 | −0.105 |
| FvDFR57 | FvH4_7g16111.t1.v4.0.a2 | 357 | 39,939.81 | 6.32 | 45.53 | 87.37 | −0.128 |
| Protein | Cytoplasm | Chloroplast | Nucleus | Plasma Membrane | Mitochondria | Peroxisome |
|---|---|---|---|---|---|---|
| FvDFR1 | 3 | 4 | 3 | - | 1 | - |
| FvDFR2 | - | 11.5 | - | - | 2.5 | - |
| FvDFR3 | 4 | 4 | 1 | 2 | - | - |
| FvDFR4 | 1 | 9 | - | 2 | - | - |
| FvDFR5 | 4 | 4 | 2 | 2 | - | - |
| FvDFR6 | 1 | 8 | 1 | 2 | - | - |
| FvDFR7 | 4.5 | 1 | 1.5 | - | 1 | - |
| FvDFR8 | 2 | 7 | 1 | - | 1 | - |
| FvDFR9 | 10 | - | 2 | - | 1 | - |
| FvDFR10 | 2 | 8 | - | 1 | 1 | - |
| FvDFR11 | 1 | 10 | - | 1 | 1 | - |
| FvDFR12 | 1 | 9 | - | 1 | 1 | - |
| FvDFR13 | 3 | 9 | 1 | - | - | - |
| FvDFR14 | 8 | 2 | 1 | - | - | - |
| FvDFR15 | - | 10 | 1 | - | 3 | - |
| FvDFR16 | 11.5 | - | 1 | - | - | - |
| FvDFR17 | 8 | 1 | - | - | 1 | - |
| FvDFR18 | 2 | 10 | 2 | - | - | - |
| FvDFR19 | 1 | 11 | 2 | - | - | - |
| FvDFR20 | - | 12 | 1 | - | 1 | - |
| FvDFR21 | 10 | 2 | - | 1 | - | - |
| FvDFR22 | 4 | 9 | - | - | - | 1 |
| FvDFR23 | 9 | 2 | 2 | 1 | - | - |
| FvDFR24 | 2 | 7 | 1 | - | - | - |
| FvDFR25 | 8 | 3 | - | - | 1 | 2 |
| FvDFR26 | 9 | 3 | - | - | - | 2 |
| FvDFR27 | 11 | 1 | 1 | - | - | 1 |
| FvDFR28 | 4 | 8 | - | - | - | - |
| FvDFR29 | 3 | 2 | 7 | - | 1 | - |
| FvDFR30 | 2 | 11 | - | - | - | - |
| FvDFR31 | 1 | 10 | - | 1 | 1 | - |
| FvDFR32 | 11 | 1 | 1 | 1 | - | - |
| FvDFR33 | 1 | 4 | 2 | 2 | - | - |
| FvDFR34 | 11 | - | - | - | - | 2 |
| FvDFR35 | 9.5 | 3 | 1 | - | - | - |
| FvDFR36 | 12 | 1 | 1 | - | - | - |
| FvDFR37 | 9 | 1 | 1 | - | - | 2 |
| FvDFR38 | 4 | 2 | - | - | - | - |
| FvDFR39 | 1 | 9 | 1 | - | - | - |
| FvDFR40 | 3 | 10 | 1 | - | - | - |
| FvDFR41 | 2 | 8 | 1 | - | - | - |
| FvDFR42 | 6.5 | 1 | 1.5 | - | 2 | - |
| FvDFR43 | 6 | 7 | - | 1 | - | - |
| FvDFR44 | 10 | 2 | - | - | - | - |
| FvDFR45 | 2 | 10 | - | - | 1 | - |
| FvDFR46 | - | 14 | - | - | - | - |
| FvDFR47 | 3 | 9 | 1 | - | - | - |
| FvDFR48 | - | 9 | - | 2 | - | - |
| FvDFR49 | 1 | 2 | 1 | 9 | - | - |
| FvDFR50 | 1.5 | 4 | 3.5 | - | 3 | 1 |
| FvDFR51 | 7 | - | 1 | 1.5 | - | 1 |
| FvDFR52 | 5 | 5 | 1 | 2 | - | - |
| FvDFR53 | 3 | 4 | 2 | 1 | 2 | - |
| FvDFR54 | 7 | 1 | - | - | 1 | - |
| FvDFR55 | 2 | 4 | 1 | 2 | - | - |
| FvDFR56 | 1 | 5.5 | 3 | - | 4 | - |
| FvDFR57 | 1 | 3.5 | 3 | 1 | 5 | - |
| Protein | Alpha Helix/% | Extended Strand/% | Beta Turn/% | Random Coil/% |
|---|---|---|---|---|
| FvDFR1 | 42.86 | 13.07 | 7.29 | 36.78 |
| FvDFR2 | 45.24 | 13.69 | 7.14 | 33.93 |
| FvDFR3 | 38.97 | 14.33 | 7.16 | 39.54 |
| FvDFR4 | 42.35 | 16.37 | 6.76 | 34.52 |
| FvDFR5 | 38.97 | 13.90 | 8.16 | 38.97 |
| FvDFR6 | 41.44 | 14.11 | 7.81 | 36.64 |
| FvDFR7 | 46.18 | 14.55 | 5.09 | 34.18 |
| FvDFR8 | 41.64 | 14.96 | 6.74 | 36.66 |
| FvDFR9 | 40.68 | 13.56 | 5.93 | 39.83 |
| FvDFR10 | 39.65 | 16.49 | 8.07 | 35.79 |
| FvDFR11 | 39.79 | 16.96 | 8.30 | 34.95 |
| FvDFR12 | 42.06 | 16.82 | 7.17 | 33.96 |
| FvDFR13 | 45.68 | 14.81 | 7.10 | 32.41 |
| FvDFR14 | 41.69 | 14.42 | 7.52 | 36.36 |
| FvDFR15 | 41.48 | 13.54 | 4.80 | 40.17 |
| FvDFR16 | 23.19 | 31.88 | 7.25 | 37.68 |
| FvDFR17 | 42.94 | 17.35 | 5.00 | 34.71 |
| FvDFR18 | 40.20 | 14.53 | 7.77 | 37.50 |
| FvDFR19 | 40.87 | 13.91 | 7.25 | 37.97 |
| FvDFR20 | 38.98 | 16.61 | 7.12 | 37.29 |
| FvDFR21 | 44.93 | 16.67 | 5.43 | 32.97 |
| FvDFR22 | 46.63 | 17.31 | 6.73 | 29.33 |
| FvDFR23 | 44.44 | 17.14 | 4.13 | 34.29 |
| FvDFR24 | 46.71 | 12.54 | 7.21 | 33.54 |
| FvDFR25 | 48.99 | 12.08 | 4.36 | 34.56 |
| FvDFR26 | 44.79 | 13.80 | 6.13 | 35.28 |
| FvDFR27 | 46.77 | 14.15 | 5.54 | 33.54 |
| FvDFR28 | 43.81 | 12.39 | 6.95 | 36.86 |
| FvDFR29 | 38.00 | 15.00 | 8.33 | 38.67 |
| FvDFR30 | 41.89 | 14.75 | 6.78 | 36.58 |
| FvDFR31 | 43.37 | 16.49 | 6.45 | 33.69 |
| FvDFR32 | 51.59 | 13.26 | 6.05 | 29.11 |
| FvDFR33 | 49.16 | 11.80 | 4.49 | 34.55 |
| FvDFR34 | 42.86 | 16.83 | 6.03 | 34.29 |
| FvDFR35 | 41.63 | 17.17 | 6.44 | 34.76 |
| FvDFR36 | 41.28 | 17.43 | 11.01 | 30.28 |
| FvDFR37 | 42.15 | 15.38 | 5.85 | 36.62 |
| FvDFR38 | 41.10 | 14.38 | 6.16 | 38.36 |
| FvDFR39 | 42.79 | 17.03 | 6.99 | 33.19 |
| FvDFR40 | 41.02 | 18.86 | 6.89 | 33.23 |
| FvDFR41 | 41.16 | 14.63 | 6.10 | 38.11 |
| FvDFR42 | 41.75 | 16.84 | 6.73 | 34.68 |
| FvDFR43 | 42.07 | 16.77 | 7.01 | 34.15 |
| FvDFR44 | 45.00 | 14.50 | 5.50 | 35.00 |
| FvDFR45 | 46.90 | 18.58 | 10.62 | 23.89 |
| FvDFR46 | 42.60 | 17.94 | 7.17 | 32.29 |
| FvDFR47 | 43.96 | 16.10 | 7.74 | 32.20 |
| FvDFR48 | 43.17 | 17.39 | 6.83 | 32.61 |
| FvDFR49 | 34.80 | 17.64 | 3.27 | 44.29 |
| FvDFR50 | 43.02 | 15.41 | 5.52 | 36.05 |
| FvDFR51 | 36.51 | 29.37 | 8.73 | 25.40 |
| FvDFR52 | 42.91 | 15.88 | 7.77 | 33.45 |
| FvDFR53 | 41.62 | 14.97 | 8.98 | 34.43 |
| FvDFR54 | 42.99 | 15.55 | 7.32 | 34.15 |
| FvDFR55 | 37.76 | 14.50 | 7.55 | 40.18 |
| FvDFR56 | 40.26 | 15.65 | 7.67 | 36.42 |
| FvDFR57 | 40.62 | 14.57 | 7.28 | 37.54 |
| Conserved Motif | Length/bp | Amino Acid-Conserved Sequence |
|---|---|---|
| Motif1 | 31 | RLHLFKADLLDEGSFDSAIDGCEGVFHTASP |
| Motif2 | 38 | SKTLAEQAAWKFAKENGJDLVTINPGLVIGPLLQPTLN |
| Motif3 | 32 | VVCVTGASGFIASWLVKLLLZRGYTVKATVRB |
| Motif4 | 26 | YRFVHVDDVASAHIFAFENPSASGRY |
| Motif5 | 21 | DPZAELIDPAVKGTLNVLKSC |
| Motif6 | 21 | KSPTVKRVVLTSSIAAVAYNG |
| Motif7 | 15 | WWSDPEFCEKLKLWY |
| Motif8 | 21 | HISEIVKILRELYPEYNIPEK |
| Motif9 | 15 | NDLKKTEHLLSLDGA |
| Motif10 | 29 | QVSKEKAQSLGVKFTPLEVTLKDTVESLK |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | CATTCATCACCACCGACTACA | GAAGGGTCTTCTCATCCTTGAC |
| FvDFR1 | CATGTCACTGGAACTGTCAGAGATC | TCAACTAAGTCAGCCTTCACCAATC |
| FvDFR2 | CACGCCGCCGCCTTAGTC | CTTGGTCTCCCTGATCGCTCTC |
| FvDFR3 | GCTGAGCAAGAGGCATGGAAG | TGAGACTCGGCGGCATAGC |
| FvDFR5 | GGACCCTGAGAACGAAGTGATAAAG | CGATGCTCTTCAATGGCGACAG |
| FvDFR7 | ACCACTGCCAAGCCACCTAC | AGAGAAGCACCAGCCATGAGAG |
| FvDFR9 | CCACACGGTTCATGCCACAG | AAACGCCATCACAGCCCTTAAC |
| FvDFR10 | CGCAACCCAAATGATCCAACAAAG | AACACATTCACAGCCCTCAACAC |
| FvDFR13 | CGTCTTCCACCTCGCCTCTC | GCCGCCGTCAGAACATTCAG |
| FvDFR14 | CGGAGAAAGATGCGTGGAGAATC | GTGCTTGTAGGTTGTGGTGCTAG |
| FvDFR16 | CGAGACCCAGCCATAGCCTTC | CCTTGGTGTGCATGAAACTTCCC |
| FvDFR17 | ATATTTGTGGTGGTAGGAGGAGGAG | TCGTCGGTGGCTATGTCTGTG |
| FvDFR18 | ACACAGTCCATGCCACTCTCAG | AGCAGGCTTGAAGTCATTAGGATTG |
| FvDFR21 | GCGTGGGCAATGGCAATGG | GATGGACAAGTCGGCGTTCAAC |
| FvDFR24 | AGGTGCTGGAGGCTTCGTTG | TCTCTGATGGTTCCGTGGACTATG |
| FvDFR25 | GCAATCCACCCTAATTTCCAGTAGC | TCACGAACATCCACCACAGTCC |
| FvDFR27 | AACTTGGTGCTGATGTCTCTTCTG | TGTATCTGCCTCCTGCTTCTGG |
| FvDFR29 | GTTGGACACCGCTTGATGCC | CCCAGTAAGTTGTGCCAGAATCC |
| FvDFR30 | GAGAGGAGTGGACTTGGTGGTG | GGATGATGCTGGCGTTGATGG |
| FvDFR32 | GGAAGACCACTGCGGAGATAGC | CATGTCCTTGCCACCTTTGAGATAG |
| FvDFR35 | ACAGAACACTTGCTCGCACTTG | CACCTTCACATCCATCAACAACAAG |
| FvDFR39 | AAACAGAACACTTGCTTTCGCTTG | AACACCATCACATCCATCAACCG |
| FvDFR42 | CGCACATCTCCGAGGCTCTG | GGTCAGAAGGAAGAATACGAACAGG |
| FvDFR44 | TCTTACAGCCAACTCTGAACACAAG | CGCAATCGCAACATCTCTAACATC |
| FvDFR45 | TGTGACAGGAGCATCTGGGTTC | CGGACAGTGGCTTTGACAGTATAAC |
| FvDFR46 | CACCTGAGACACCTGTAATAGTTCC | GCTGCCATAGAGGATGTGATAACC |
| FvDFR47 | CAGAACACCTGCTCTCACTTGAAG | AGAAGTACAGAGGATGCCGTATGG |
| FvDFR48 | TGAACTAATTGACCCTGCCTTGAAG | ACCACCCTCTTAATAGACGGAACC |
| FvDFR49 | GTTCAATCGGGTCTCCTTTGGTTC | TGGCAGCAGTCACTTCCTTCC |
| FvDFR51 | GCAAGTCAAAGCGGAATTTCAGATG | ATCGTCGCCACCACCAAGG |
| FvDFR52 | CTGAGAGCCTGCCTGAAGTCC | ATGTCTTTGTTGGTTCCGCTATGAG |
| FvDFR54 | TGCGAGCCACCGTTAGATCC | TCATTGAAGGTCTCAGGGTTGTTG |
| FvDFR55 | GAGGTTCTGGGTTCATTGGTTCC | CTTTCTTGTCTTCTGCTGGGTCTG |
| FvDFR56 | TTCAAAGACACTGGCAGAGAAAGAG | ATTCCCTCCCACAAGACCACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-Z.; Tian, X.-C.; Feng, Y.-Q.; Qiao, H.-L.; Wu, A.-Y.; Li, X.; Hou, Y.-J.; Ma, Z.-H. The Genome-Wide Identification of the Dihydroflavonol 4-Reductase (DFR) Gene Family and Its Expression Analysis in Different Fruit Coloring Stages of Strawberry. Int. J. Mol. Sci. 2024, 25, 9911. https://doi.org/10.3390/ijms25189911
Chen L-Z, Tian X-C, Feng Y-Q, Qiao H-L, Wu A-Y, Li X, Hou Y-J, Ma Z-H. The Genome-Wide Identification of the Dihydroflavonol 4-Reductase (DFR) Gene Family and Its Expression Analysis in Different Fruit Coloring Stages of Strawberry. International Journal of Molecular Sciences. 2024; 25(18):9911. https://doi.org/10.3390/ijms25189911
Chicago/Turabian StyleChen, Li-Zhen, Xue-Chun Tian, Yong-Qing Feng, Hui-Lan Qiao, Ai-Yuan Wu, Xin Li, Ying-Jun Hou, and Zong-Huan Ma. 2024. "The Genome-Wide Identification of the Dihydroflavonol 4-Reductase (DFR) Gene Family and Its Expression Analysis in Different Fruit Coloring Stages of Strawberry" International Journal of Molecular Sciences 25, no. 18: 9911. https://doi.org/10.3390/ijms25189911





