Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of the RA Cohort
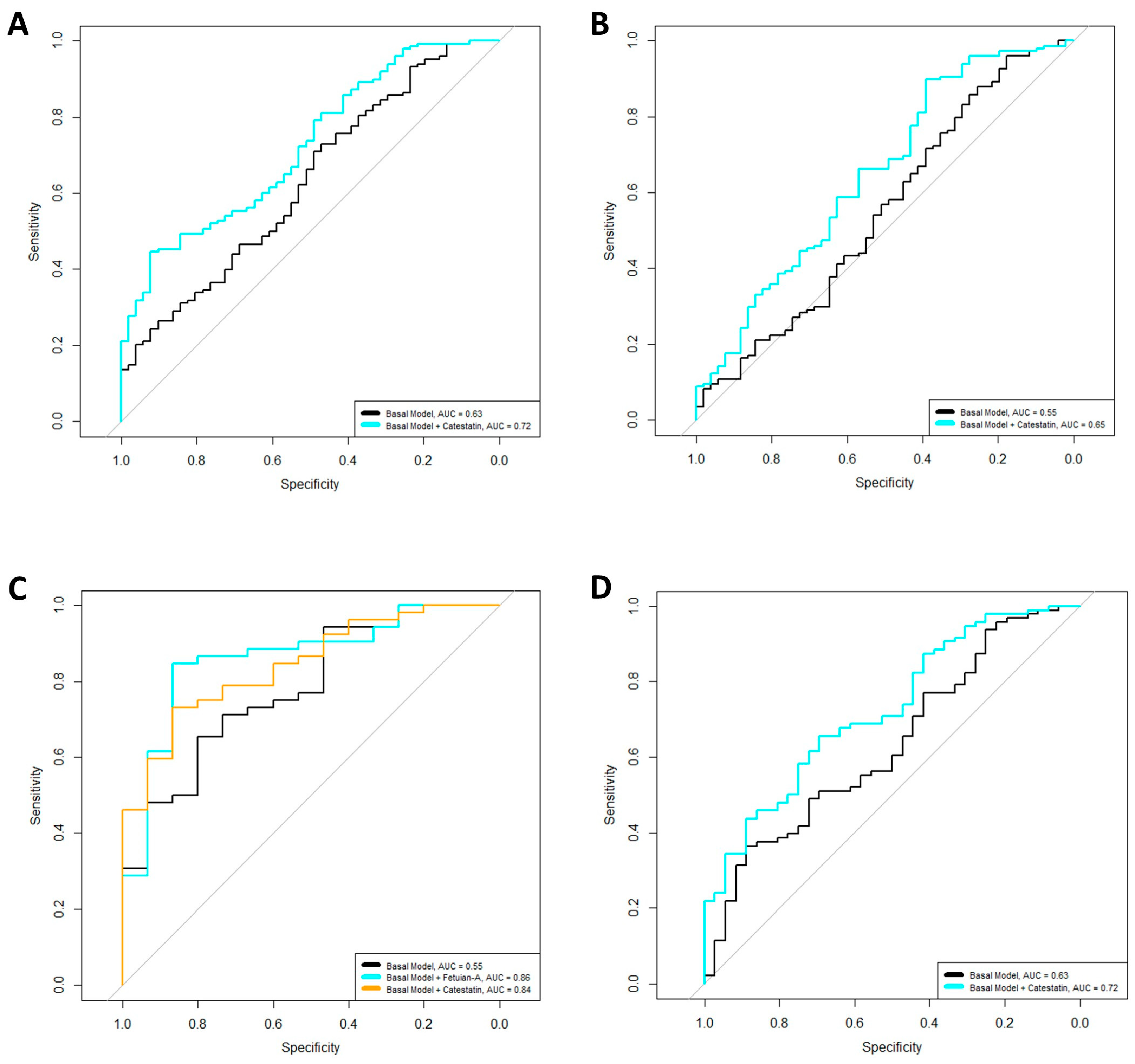
2.2. Associations of Biomarkers with RF and ACPA Positivity
2.3. Associations of Biomarkers and Inflammatory Markers of RA
3. Discussion
4. Methods
4.1. Patients
4.2. Clinical Evaluation
4.3. Laboratory Measurements
4.4. Ultrasound Evaluation of Carotid Intima-Media Thickness (cIMT) and Carotid Plaque Presence (cPP)
4.5. Biomarker Quantification
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharma-cologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet Lond. Engl. 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E.; Crowson, C.S.; Kremers, H.M.; Doran, M.F.; Turesson, C.; O’Fallon, W.M.; Matteson, E.L. Survival in rheumatoid arthritis: A population-based analysis of trends over 40 years. Arthritis Rheum. 2003, 48, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Care Res. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef] [PubMed]
- Cush, J.J. Rheumatoid Arthritis: Early Diagnosis and Treatment. Rheum. Dis. Clin. N. Am. 2022, 48, 537–547. [Google Scholar] [CrossRef]
- Ajeganova, S.; Humphreys, J.H.; Verheul, M.K.; van Steenbergen, H.W.; Nies, J.A.B.v.; Hafström, I.; Svensson, B.; Huizinga, T.W.J.; A Trouw, L.; Verstappen, S.M.M.; et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: A longitudinal study in three European cohorts. Ann. Rheum. Dis. 2016, 75, 1924–1932. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Tanasescu, C.; Jurcut, C.; Jurcut, R.; Ginghina, C. Vascular disease in rheumatoid arthritis: From subclinical lesions to cardiovascular risk. Eur. J. Intern. Med. 2009, 20, 348–354. [Google Scholar] [CrossRef]
- Castañeda, S.; Martín-Martínez, M.A.; González-Juanatey, C.; Llorca, J.; García-Yébenes, M.J.; Pérez-Vicente, S.; Sánchez-Costa, J.T.; Díaz-Gonzalez, F.; González-Gay, M.A.; CARMA Project Collaborative Group. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: Baseline data of the CARMA Project. Semin. Arthritis Rheum. 2015, 44, 618–626. [Google Scholar] [CrossRef]
- Day, A.L.; Singh, J.A. Cardiovascular Disease Risk in Older Adults and Elderly Patients with Rheumatoid Arthritis: What Role Can Disease-Modifying Antirheumatic Drugs Play in Cardiovascular Risk Reduction? Drugs Aging. 2019, 36, 493–510. [Google Scholar] [CrossRef]
- Stevens, R.J.; Douglas, K.M.; Saratzis, A.N.; Kitas, G.D. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev. Mol. Med. 2005, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Firestein, G.S.; McInnes, I.B. The pathogenesis of rheumatoid arthritis. Immunity 2022, 55, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- van den Oord, S.C.; Sijbrands, E.J.; ten Kate, G.L.; van Klaveren, D.; van Domburg, R.T.; van der Steen, A.F.; Schinkel, A.F. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis 2013, 228, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Ambrosino, P.; Lupoli, R.; Di Minno, A.; Tasso, M.; Peluso, R.; Di Minno, M.N.D. Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb. Haemost. 2015, 113, 916–930. [Google Scholar] [CrossRef]
- Gonzalez-Juanatey, C.; Llorca, J.; Martin, J.; Gonzalez-Gay, M.A. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2009, 38, 366–371. [Google Scholar] [CrossRef]
- Rundek, T.; Arif, H.; Boden-Albala, B.; Elkind, M.S.; Paik, M.C.; Sacco, R.L. Carotid plaque, a subclinical precursor of vascular events: The Northern Manhattan Study. Neurology 2008, 70, 1200–1207. [Google Scholar] [CrossRef]
- Corrales, A.; Vegas-Revenga, N.; Rueda-Gotor, J.; Portilla, V.; Atienza-Mateo, B.; Blanco, R.; Castañeda, S.; Ferraz-Amaro, I.; Llorca, J.; González-Gay, M.A. Carotid plaques as predictors of cardiovascular events in patients with Rheumatoid Arthritis. Results from a 5-year-prospective follow-up study. Semin. Arthritis Rheum. 2020, 50, 1333–1338. [Google Scholar] [CrossRef]
- Ahmad, S.; Kumar, R. An update of new/potential cardiovascular markers: A narrative review. Mol. Biol. Rep. 2024, 51, 179. [Google Scholar] [CrossRef]
- Wang, H.; Sama, A.E. Anti-inflammatory role of fetuin-A in injury and infection. Curr. Mol. Med. 2012, 12, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Hanssen, E.; McMahon, L.P.; Holt, S.G. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS ONE 2013, 8, e60904. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ran, L.; Jiang, J.; Chen, Y.; Ji, H.; Quan, X. Association between fetuin-A and prognosis of CAD: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13091. [Google Scholar] [CrossRef]
- Lim, P.; Moutereau, S.; Simon, T.; Gallet, R.; Probst, V.; Ferrieres, J.; Gueret, P.; Danchin, N. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am. J. Cardiol. 2013, 111, 31–37. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Mularczyk, M.; Marycz, K.; Bourebaba, L. Catestatin peptide of chromogranin A as a potential new target for several risk factors management in the course of metabolic syndrome. Biomed. Pharmacother. 2021, 134, 111113. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, J.; Ding, W.H.; Han, P.; Yang, Y.; Qi, L.T.; Zhang, B.W. Plasma catestatin level in patients with acute myocardial infarction and its correlation with ventricular remodelling. Postgrad. Med. J. 2013, 89, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Fan, Y.; Wang, Z.; Huang, F.; Li, Z.; Sun, Z.; Hua, S.; Jin, W.; Chen, Y. Catestatin Protects Against Diastolic Dysfunction by Attenuating Mitochondrial Reactive Oxygen Species Generation. J. Am. Heart Assoc. 2023, 12, e029470. [Google Scholar] [CrossRef]
- Katic, J.; Jurisic, Z.; Kumric, M.; Borovac, J.A.; Anic, A.; Breskovic, T.; Supe-Domic, D.; Bozic, J. Serum Catestatin Concentrations Are Increased in Patients with Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2023, 10, 85. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yang, C.; Su, X.; Yang, W.; Dai, Y.; Han, H.; Jiang, J.; Lu, L.; Wang, H.; et al. Decreased circulating catestatin levels are associated with coronary artery disease: The emerging anti-inflammatory role. Atherosclerosis 2019, 281, 78–88. [Google Scholar] [CrossRef]
- Borovac, J.A.; Glavas, D.; Grabovac, Z.S.; Domic, D.S.; Stanisic, L.; D’Amario, D.; Kwok, C.S.; Bozic, J. Circulating sST2 and catestatin levels in patients with acute worsening of heart failure: A report from the CATSTAT-HF study. ESC Heart Fail. 2020, 7, 2818–2828. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Banach, J.; Budzyński, J.; Wołowiec, A.; Kozakiewicz, M.; Bieliński, M.; Jaśniak, A.; Olejarczyk, A.; Grześk, G. Prognostic Value of Plasma Catestatin Concentration in Patients with Heart Failure with Reduced Ejection Fraction in Two-Year Follow-Up. J. Clin. Med. 2023, 12, 4208. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-I.; Park, K.U.; Chun, E.J.; Choi, S.I.; Cho, Y.-S.; Youn, T.-J.; Cho, G.-Y.; Chae, I.-H.; Song, J.; Choi, D.-J.; et al. A novel biomarker of coronary atherosclerosis: Serum DKK1 concentration correlates with coronary artery calcification and atherosclerotic plaques. J. Korean Med. Sci. 2011, 26, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Otterdal, K.; Lekva, T.; Halvorsen, B.; Gabrielsen, A.; Sandberg, W.J.; Paulsson-Berne, G.; Pedersen, T.M.; Folkersen, L.; Gullestad, L.; et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arter. Thromb. Vasc. Biol. 2009, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- He, X.-W.; Wang, E.; Bao, Y.-Y.; Wang, F.; Zhu, M.; Hu, X.-F.; Jin, X.-P. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis 2016, 253, 22–28. [Google Scholar] [CrossRef]
- Seifert-Held, T.; Pekar, T.; Gattringer, T.; Simmet, N.E.; Scharnagl, H.; Stojakovic, T.; Fazekas, F.; Storch, M.K. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis 2011, 218, 233–237. [Google Scholar] [CrossRef]
- Goliasch, G.; Wiesbauer, F.; Kastl, S.P.; Katsaros, K.M.; Blessberger, H.; Maurer, G.; Schillinger, M.; Huber, K.; Wojta, J.; Speidl, W.S. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 2012, 222, 251–256. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, D.; Zhong, C.; Wang, A.; Xie, X.; Xu, T.; Chen, C.-S.; Peng, Y.; Peng, H.; Li, Q.; et al. Serum Dkk-1 (Dickkopf-1) Is a Potential Biomarker in the Prediction of Clinical Outcomes Among Patients with Acute Ischemic Stroke. Arter. Thromb. Vasc. Biol. 2019, 39, 285–293. [Google Scholar] [CrossRef]
- Ueland, T.; Åkerblom, A.; Ghukasyan, T.; Michelsen, A.E.; Becker, R.C.; Bertilsson, M.; Himmelmann, A.; James, S.K.; Siegbahn, A.; Storey, R.F.; et al. Admission Levels of DKK1 (Dickkopf-1) Are Associated with Future Cardiovascular Death in Patients with Acute Coronary Syndromes. Arter. Thromb. Vasc. Biol. 2019, 39, 294–302. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Wang, A.; Zhong, C.; Zhu, Z.; Xu, T.; Peng, Y.; Peng, H.; Chen, C.-S.; Wang, J.; Ju, Z.; Li, Q.; et al. Serum Galectin-3 and Poor Outcomes Among Patients with Acute Ischemic Stroke. Stroke 2018, 49, 211–214. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Z.; Wang, R.; Zheng, Y.; Li, H.; Yang, L. Galectin-3 Is a Potential Mediator for Atherosclerosis. J. Immunol. Res. 2020, 2020, 5284728. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, L.; Xue, Y.; Zeng, T.; Shi, Y.; Lin, Y.; Liu, L. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease. Clin. Chim. Acta 2019, 497, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Huang, W.; Wang, R.; Chen, C.; Chen, Y.; Wang, Y.; Tan, X. Elevated circulating IL-32 presents a poor prognostic outcome in patients with heart failure after myocardial infarction. Int. J. Cardiol. 2017, 243, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, M.; Vieira, T.S.; Martins, M.J.; Lucas, R.; Costa, L.; Pereira, J.G.; Ventura, F.; Martins, E. Myocardial Perfusion in Rheumatoid Arthritis Patients: Associations with Traditional Risk Factors and Novel Biomarkers. BioMed. Res. Int. 2017, 2017, 6509754. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gkaliagkousi, E.; Gavriilaki, E.; Triantafyllou, A.; Dolgyras, P.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Association of galectin-3 with markers of myocardial function, atherosclerosis, and vascular fibrosis in patients with rheumatoid arthritis. Clin. Cardiol. 2019, 42, 62–68. [Google Scholar] [CrossRef]
- Nussdorf, A.; Park, E.; Amigues, I.; Geraldino-Pardilla, L.; Bokhari, S.; Giles, J.T.; Bathon, J.M. Associations of galectin-3 levels with measures of vascular disease in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2024, 65, 152357. [Google Scholar] [CrossRef]
- Simac, P.; Perkovic, D.; Bozic, I.; Matijas, M.; Gugo, K.; Martinovic, D.; Bozic, J. Serum catestatin levels in patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 3812. [Google Scholar] [CrossRef]
- Zhu, D.; Xie, H.; Wang, X.; Liang, Y.; Yu, H.; Gao, W. Correlation of plasma catestatin level and the prognosis of patients with acute myocardial infarction. PLoS ONE 2015, 10, e0122993. [Google Scholar] [CrossRef]
- Zhu, D.; Xie, H.; Wang, X.; Liang, Y.; Yu, H.; Gao, W. Catestatin-A Novel Predictor of Left Ventricular Remodeling After Acute Myocardial Infarction. Sci. Rep. 2017, 7, 44168. [Google Scholar] [CrossRef]
- Pei, Z.; Ma, D.; Ji, L.; Zhang, J.; Su, J.; Xue, W.; Chen, X.; Wang, W. Usefulness of catestatin to predict malignant arrhythmia in patients with acute myocardial infarction. Peptides 2014, 55, 131–135. [Google Scholar] [CrossRef]
- Adlan, A.M.; Lip, G.Y.; Paton, J.F.; Kitas, G.D.; Fisher, J.P. Autonomic function and rheumatoid arthritis—A systematic review. Semin. Arthritis Rheum. 2014, 44, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Derksen, V.F.; Ajeganova, S.; Trouw, L.A.; van der Helm-van Mil, A.H.; Hafström, I.; Huizinga, T.W.; Toes, R.E.; Svensson, B.; van der Woude, D. Rheumatoid arthritis phenotype at presentation differs depending on the number of autoantibodies present. Ann. Rheum. Dis. 2017, 76, 716–720. [Google Scholar] [CrossRef] [PubMed]
- López-Longo, F.J.; Oliver-Miñarro, D.; de la Torre, I.; de Rábago, E.G.; Sánchez-Ramón, S.; Rodríguez-Mahou, M.; Paravisini, A.; Monteagudo, I.; González, C.; GarCía-Castro, M.; et al. Association between anti–cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009, 61, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Majka, D.S.; Vu, T.T.; Pope, R.M.; Teodorescu, M.; Karlson, E.W.; Liu, K.; Chang, R.W. Association of Rheumatoid Factors with Subclinical and Clinical Atherosclerosis in African American Women: The Multiethnic Study of Atherosclerosis. Arthritis Care Res. 2017, 69, 166–174. [Google Scholar] [CrossRef]
- Cambridge, G.; Acharya, J.; Cooper, J.A.; Edwards, J.C.; Humphries, S.E. Antibodies to citrullinated peptides and risk of coronary heart disease. Atherosclerosis 2013, 228, 243–246. [Google Scholar] [CrossRef]
- Sato, H.; Kazama, J.J.; Wada, Y.; Kuroda, T.; Narita, I.; Gejyo, F.; Gao, P.; Yamashita, H. Decreased levels of circulating alpha2-Heremans-Schmid glycoprotein/Fetuin-A (AHSG) in patients with rheumatoid arthritis. Intern. Med. 2007, 46, 1685–1692. [Google Scholar] [CrossRef]
- Memoli, B.; De Bartolo, L.; Favia, P.; Morelli, S.; Lopez, L.C.; Procino, A.; Barbieri, G.; Curcio, E.; Giorno, L.; Esposito, P.; et al. Fetuin-A gene expression, synthesis and release in primary human hepatocytes cultured in a galactosylated membrane bioreactor. Biomaterials 2007, 28, 4836–4844. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef]
- Saroha, A.; Kumar, S.; Chatterjee, B.P.; Das, H.R. Jacalin bound plasma O-glycoproteome and reduced sialylation of alpha 2-HS glycoprotein (A2HSG) in rheumatoid arthritis patients. PLoS ONE 2012, 7, e46374. [Google Scholar] [CrossRef]
- Sağ, S.; Güzel, D.; Sağ, M.S.; Tekeoğlu, I.; Kamanli, A.; Nas, K.; Doğanay, S. The Evaluation of Serum Tumor Necrosis Factor-Like Weak Inducer of Apoptosis, Interleukin-6, Fetuin-A, Homeostatic Model Assessment-Insulin Resistance, and Insulin Levels in Rheumatoid Arthritis Patients in Clinical Remission. Arch. Rheumatol. 2018, 34, 71–78. [Google Scholar] [CrossRef]
- Harman, H.; Tekeoğlu, İ.; Gürol, G.; Sağ, M.S.; Karakeçe, E.; Çİftçİ, İ.H.; Kamanlı, A.; Nas, K. Comparison of fetuin-A and transforming growth factor beta 1 levels in patients with spondyloarthropathies and rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Tekeoğlu, İ.; Harman, H.; Sağ, S.; Altındiş, M.; Kamanlı, A.; Nas, K. Levels of serum pentraxin 3, IL-6, fetuin A and insulin in patients with rheumatoid arthritis. Cytokine 2016, 83, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, M.; Bi, Y.; Song, A.; Huang, Y.; Liu, Y.; Wu, Y.; Chen, Y.; Wang, W.; Li, X.; et al. Serum fetuin-A is correlated with metabolic syndrome in middle-aged and elderly Chinese. Atherosclerosis 2011, 216, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Shlipak, M.G.; Brandenburg, V.M.; Ali, S.; Ketteler, M.; Whooley, M.A. Association between human fetuin-A and the metabolic syndrome: Data from the Heart and Soul Study. Circulation 2006, 113, 1760–1767. [Google Scholar] [CrossRef]
- Ix, J.H.; Barrett-Connor, E.; Wassel, C.L.; Cummins, K.; Bergstrom, J.; Daniels, L.B.; Laughlin, G.A. The associations of fetuin-A with subclinical cardiovascular disease in community-dwelling persons: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2011, 58, 2372–2379. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Liu, Y.-Y.; Ye, H.; Guo, J.-P.; Li, R.; Liu, X.; Li, Z.-G. Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J. Rheumatol. 2011, 38, 821–827. [Google Scholar] [CrossRef]
- Ohshima, S.; Kuchen, S.; Seemayer, C.A.; Kyburz, D.; Hirt, A.; Klinzing, S.; Michel, B.A.; Gay, R.E.; Liu, F.; Gay, S.; et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2788–2795. [Google Scholar] [CrossRef]
- Mendez-Huergo, S.P.; Hockl, P.F.; Stupirski, J.C.; Maller, S.M.; Morosi, L.G.; Pinto, N.A.; Berón, A.M.; Musuruana, J.L.; Nasswetter, G.G.; Cavallasca, J.A.; et al. Clinical Relevance of Galectin-1 and Galectin-3 in Rheumatoid Arthritis Patients: Differential Regulation and Correlation with Disease Activity. Front. Immunol. 2018, 9, 3057. [Google Scholar] [CrossRef] [PubMed]
- Taverner, D.; Llop, D.; Rosales, R.; Ferré, R.; Masana, L.; Vallvé, J.-C.; Paredes, S. Plasma expression of microRNA-425-5p and microRNA-451a as biomarkers of cardiovascular disease in rheumatoid arthritis patients. Sci. Rep. 2021, 11, 15670. [Google Scholar] [CrossRef]
- Llop, D.; Ibarretxe, D.; Plana, N.; Rosales, R.; Taverner, D.; Masana, L.; Vallvé, J.C.; Paredes, S. A panel of plasma microRNAs improves the assessment of surrogate markers of cardiovascular disease in rheumatoid arthritis patients. Rheumatology 2023, 62, 1677–1686. [Google Scholar] [CrossRef]

| RA (n = 199) | Female (n = 132) | Male (n = 67) | p | |
|---|---|---|---|---|
| Characteristics of the groups | ||||
| Sex—female (%, n) | 66%, 132 | |||
| Age (years, SD) | 57.8 (12.4) | 57.3 (12.53) | 58.65 (12.17) | 0.47 |
| Body mass index (kg/m2, IQR) | 26.8 (23.2–31.2) | 26.5 (22.7–31.6) | 27.85 (25.7–30.1) | 0.13 |
| Waist circumference (cm, SD) | 91.88 (15.11) | 88.04 (15.11) | 99.45 (12.17) | <0.001 |
| SBP (mmHg, IQR) | 135 (120–150) | 133.5 (120–148.5) | 139 (128–156.5) | 0.04 |
| DBP (mmHg, IQR) | 80 (71.50–89) | 80 (70.75–88) | 85 (75–90) | 0.02 |
| LDL cholesterol (mg/dL, IQR) | 115 (99–135) | 115 (97–135.5) | 118 (100–134.5) | 0.64 |
| HDL cholesterol (mg/dL, IQR) | 66 (53.50–75) | 69 (61–80.25) | 54 (43–66) | <0.001 |
| Triglycerides (mg/dL, IQR) | 92 (69–127.5) | 88.5 (65.75–125.25) | 94 (75–131) | 0.31 |
| Glucose (mg/dL, IQR) | 89 (82–99) | 88 (81–97) | 93 (84–102) | 0.07 |
| Current smoker (%, n) | 27%, 54 | 28%, 37 | 25.37%, 17 | 0.82 |
| Hypertension (%, n) | 59.29%, 118 | 53.03%, 70 | 71.64%, 48 | 0.01 |
| Diabetes mellitus (%, n) | 11.55%, 23 | 10.6%, 14 | 13.43%, 9 | 0.72 |
| Dyslipidaemia (%, n) | 40.70%, 81 | 39.39%, 52 | 43.28%, 29 | 0.71 |
| Disease features | ||||
| Disease duration (years, IQR) | 8 (3–13) | 8.5 (3–13.25) | 6 (2–11.50) | 0.33 |
| DAS28-ESR (median, IQR) | 3.43 (2.6–4.26) | 3.59 (2.77–4.62) | 3 (2.41–3.70) | <0.001 |
| DAS28-ESR ≥ 3.2 (%, n) | 56%, 112 | 65%, 86 | 39%, 26 | <0.001 |
| DAS28-ESR < 3.2 (%, n) | 44%, 87 | 35%, 46 | 61%, 41 | <0.001 |
| HAQ (median, IQR) | 0.25 (0–0.75) | 0.5 (0.125–0.875) | 0 (0–0.25) | <0.001 |
| Rheumatoid factor + (%, n) | 74.37%, 148 | 72.72%, 96 | 77.61%, 52 | 0.57 |
| ACPAs + (%, n) | 73.86%, 147 | 74.24%, 98 | 73.13%, 49 | 1 |
| ESR (mm/h, IQR) | 31 (18.50–50.50) | 31 (18.75–54) | 29 (18.50–46.50) | 0.30 |
| CRP (mg/dL, IQR) | 0.5 (0.2–0.9) | 0.4 (0.2–0.9) | 0.4 (0.2–0.95) | 0.68 |
| Fibrinogen (mg/dL, SD) | 445.64 (96.53) | 442.21 (95.50) | 452.40 (98.91) | 0.49 |
| cIMT (mm, IQR) | 636 (571.8–709.8) | 610.2 (565.5–694.2) | 667.5 (608.5–744.5) | 0.003 |
| Treatments (%, n) | ||||
| RA | ||||
| csDMARDs | 74.87%, 149 | 71.21%, 94 | 82.09%, 55 | 0.13 |
| Biological agent | 21.6%, 43 | 24.24%, 32 | 16.41%, 11 | 0.27 |
| NSAIDs | 57.28%, 114 | 58.33%, 77 | 55.22%, 37 | 0.79 |
| Glucocorticoids (Mean dose: 2.91 mg) | 51%, 102 | 52.27%, 69 | 49.25%, 33 | 0.80 |
| Hypertension | ||||
| RAAS inhibitors | 24.62%, 49 | 24.24%, 32 | 25.37%, 17 | 0.98 |
| Other | 26.13%, 52 | 22.72%, 30 | 32.83%, 22 | 0.17 |
| Lipid-lowering | ||||
| Statins | 16.08%, 32 | 15.15%, 20 | 17.91%, 12 | 0.77 |
| Other | 0.15%, 3% | 0.15%, 2 | 0.14%, 1 | 1 |
| Growth factors | ||||
| Fetuin A (µg/mL) | 273.2 (204.4–354) | 289.68 (215.2–355.3) | 249 (197.6–342.6) | 0.15 |
| DKK1 (ng/mL) | 7.30 (5.90–8.56) | 7.40 (5.90–8.54) | 7.05 (5.89–8.59) | 0.77 |
| Galectin-3 (ng/mL) | 0.85 (0.02–2.63) | 0.92 (0.02–3.26) | 0.27 (0.02–2.32) | 0.10 |
| IL-32 (pg/mL) | 281.76 (412.19) | 256.78 (341.41) | 330.99 (526.96) | 0.30 |
| Catestatin (ng/mL) | 7.57 (5.48–9.93) | 7.67 (5.75–10.08) | 7.04 (5.10–9.40) | 0.32 |
| OR | p | R2 (%) | AUC | AIC | |
|---|---|---|---|---|---|
| Overall cohort | |||||
| RF positivity | |||||
| Basal Model | 0.0001 | 5.41 | 0.63 | 234.25 | |
| +Catestatin | 2.45 | 0.0001 | 13.51 | 0.72 | 217.92 |
| ACPA positivity | |||||
| Basal Model | 0.01 | 3.68 | 0.55 | 240.18 | |
| +Catestatin | 1.48 | 0.04 | 5.71 | 0.65 | 237.57 |
| Male patients | |||||
| RF positivity | |||||
| Basal Model | 0.01 | 20.32 | 0.78 | 74.77 | |
| +Fetuin-A | 0.38 | 0.03 | 27.40 | 0.86 | 71.73 |
| Basal Model | |||||
| +Catestatin | 3.20 | 0.03 | 30.16 | 0.84 | 69.76 |
| Female patients | |||||
| RF positivity | |||||
| Basal Model | 0.001 | 5.24 | 0.63 | 164.58 | |
| +Catestatin | 2.40 | 0.001 | 13.19 | 0.72 | 154.28 |
| β | p | R2 (%) | AIC | |
|---|---|---|---|---|
| Overall cohort | ||||
| ESR | ||||
| Basal Model | 0.001 | 10.57 | 561.50 | |
| +Fetuin-A | −0.15 | 0.037 | 12.60 | 558.94 |
| Female patients | ||||
| ESR | ||||
| Basal Model | 0.0001 | 9.94 | 377.77 | |
| +Fetuin-A | −0.25 | 0.004 | 15.74 | 370.99 |
| DAS28-ESR | ||||
| Basal Model | 0.001 | 9.32 | 378.68 | |
| +Fetuin-A | −0.29 | 0.01 | 14.03 | 373.65 |
| Fibrinogen | ||||
| Basal Model | 0.001 | 5.32 | 384.38 | |
| +Fetuin-A | −0.22 | 0.01 | 9.82 | 379.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pàmies, A.; Llop, D.; Ibarretxe, D.; Rosales, R.; Girona, J.; Masana, L.; Vallvé, J.-C.; Paredes, S. Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2024, 25, 9910. https://doi.org/10.3390/ijms25189910
Pàmies A, Llop D, Ibarretxe D, Rosales R, Girona J, Masana L, Vallvé J-C, Paredes S. Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2024; 25(18):9910. https://doi.org/10.3390/ijms25189910
Chicago/Turabian StylePàmies, Anna, Dídac Llop, Daiana Ibarretxe, Roser Rosales, Josefa Girona, Lluís Masana, Joan-Carles Vallvé, and Silvia Paredes. 2024. "Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 25, no. 18: 9910. https://doi.org/10.3390/ijms25189910
APA StylePàmies, A., Llop, D., Ibarretxe, D., Rosales, R., Girona, J., Masana, L., Vallvé, J.-C., & Paredes, S. (2024). Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 25(18), 9910. https://doi.org/10.3390/ijms25189910






