Characterization of the Immune-Modulating Properties of Different β-Glucans on Myeloid Dendritic Cells
Abstract
:1. Introduction
2. Results
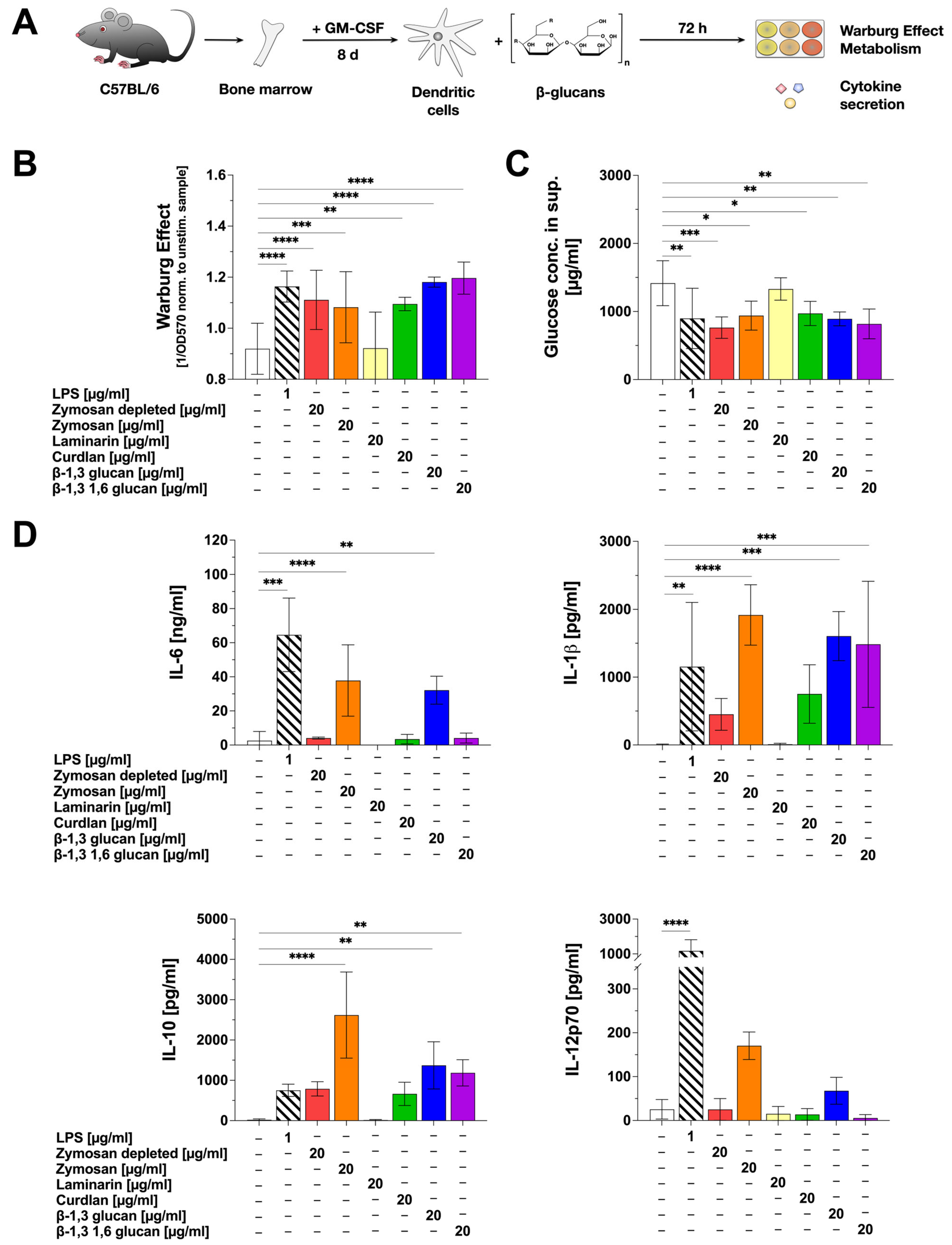
2.1. β-Glucans Differentially Activate mDCs In Vitro
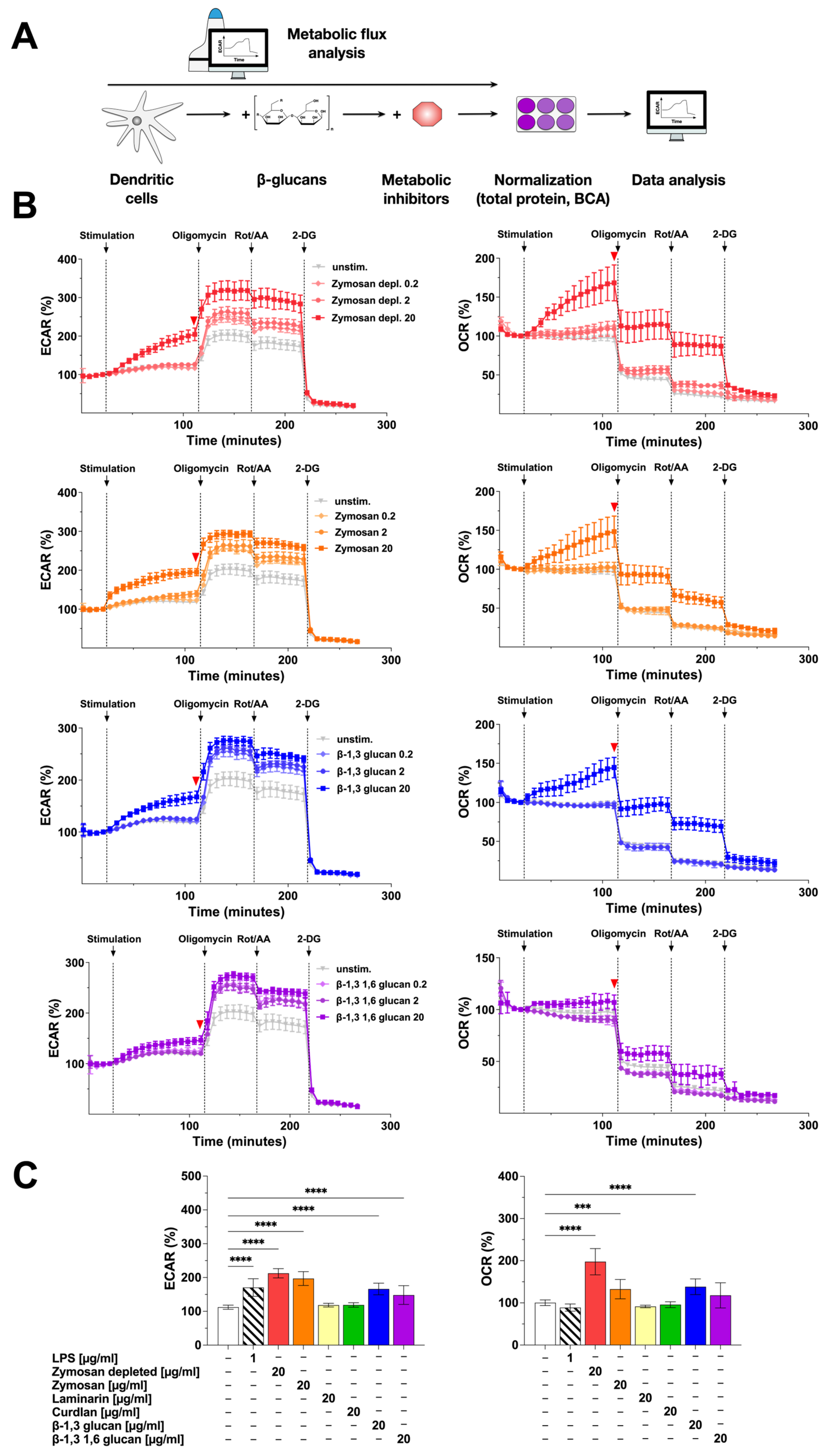
2.2. Stimulation of mDCs with β-Glucans Results in Increased Metabolic Activity
2.3. β-Glucans Differently Activate TLR2 and Dectin-1
2.4. Stimulation with β-Glucans Increases mDC Surface Expression of Both TLR2 and Dectin-1
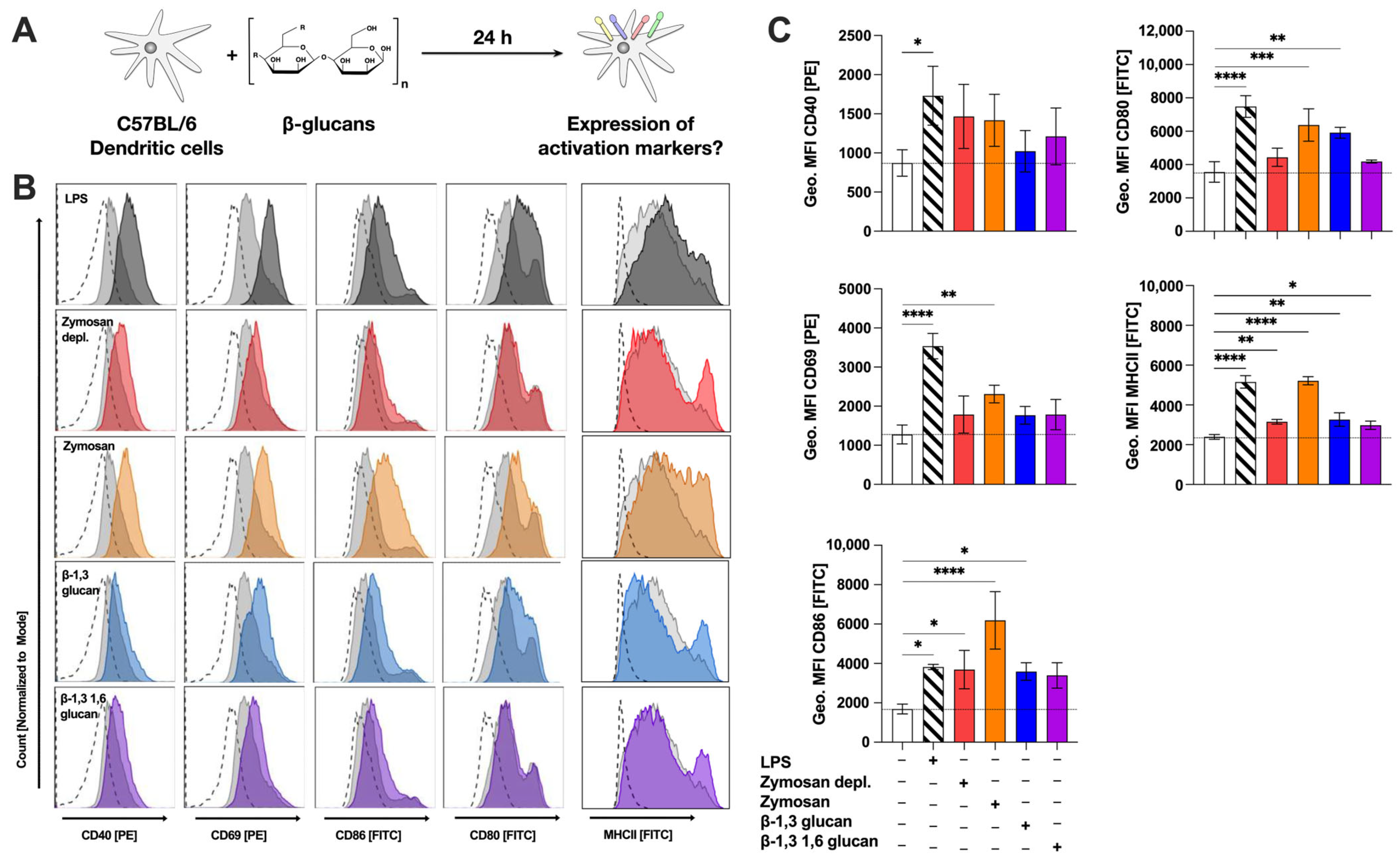
2.5. β-Glucans Upregulate the Expression of Surface Activation- and Co-Stimulatory Markers on mDCs
2.6. β-Glucan-Induced Cytokine Secretion in Part Depends on Syk-Activation
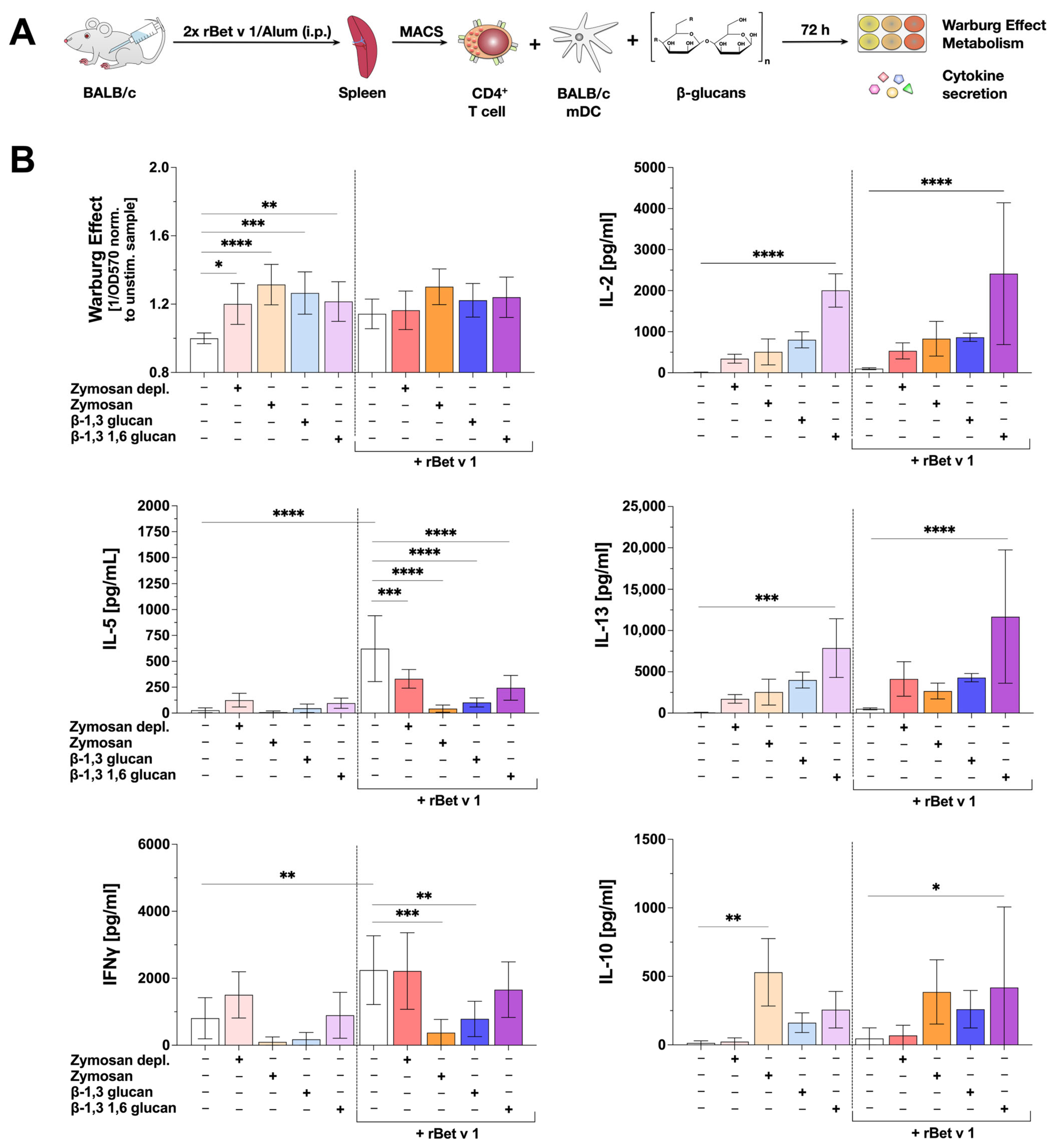
2.7. β-Glucan-Stimulated mDCs Can Suppress IL-5 Production from Bet v 1-Specific CD4+ T Cells
3. Discussion
3.1. β-Glucans Differ in Their Capacity to Trigger mDC-Derived Cytokine Secretion
3.2. Zymosan Depl., Zymosan, β-1,3 Glucan, and β-1,3 1,6 Glucan Strongly Activate mDC Metabolism
3.3. The Strong mDC Activation by Zymosan May Be the Result of Dual TLR2- and Dectin-1-Activation
3.4. β-Glucans Suppress IL-5 Secretion from Bet v 1-Specific Th2-Biased CD4+ T Cells but Have Different Effects on Bet v 1-Induced IFNγ Secretion
3.5. Some β-Glucans Are Interesting Adjuvant Candidates for AIT
4. Material and Methods
4.1. β-Glucans
4.2. Endotoxin Determination
4.3. SDS-PAGE and Protein Determination
4.4. DC Preparation and Stimulation
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Detection of the Warburg Effect and Glucose Consumption in Cell Culture Supernatants
4.7. Extracellular Flux Assays
4.8. Flow Cytometry
4.9. Reporter Assays to Determine Receptor Activation
4.10. Inhibition Experiments
4.11. mDC: T Cell Co-Cultures
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-DG | 2-deoxyglucose |
| ATP | adenosine triphosphate |
| Alum | aluminum hydroxide |
| BCA | bicinchoninic acid |
| CD[X] | cluster of differentiation [X] |
| CLR | C-type lectin |
| ECAR | extracellular acidification rate |
| ELISA | enzyme-linked immunosorbent assay |
| ETC | electron transport chain |
| FMO | fluorescence-minus-one |
| GM-CSF | granulocyte-macrophage-colony stimulating factor |
| HEK | human embryonic kidney |
| IL-[X] | interleukin-[X] |
| IFNγ | interferon gamma |
| i.p. | intraperitoneal |
| LAL | limulus amebocyte lysate |
| LPS | lipopolysaccharide |
| mDC | myeloid dendritic cell |
| Geo. MFI | geometric mean fluorescence intensity |
| OCR | oxygen consumption rate |
| OD | optical density |
| PBS | phosphate-buffered saline |
| PRR | pattern recognition receptor |
| ROS | reactive oxygen species |
| Rot/AA | rotenone/antimycin A |
| SEAP | secreted embryonic alkaline phosphatase |
| Th1/2 | T helper cell type 1/2 |
| TLR | “Toll”-like receptor |
References
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as Biological Response Modifiers: A Review of Structure-Functional Activity Relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Lee, C.; Verma, R.; Byun, S.; Jeun, E.-J.; Kim, G.-C.; Lee, S.; Kang, H.-J.; Kim, C.J.; Sharma, G.; Lahiri, A.; et al. Structural Specificities of Cell Surface β-Glucan Polysaccharides Determine Commensal Yeast Mediated Immuno-Modulatory Activities. Nat. Commun. 2021, 12, 3611. [Google Scholar] [CrossRef]
- Song, J.; Chen, H.; Wei, Y.; Liu, J. Synthesis of Carboxymethylated β-Glucan from Naked Barley Bran and Its Antibacterial Activity and Mechanism against Staphylococcus Aureus. Carbohydr. Polym. 2020, 242, 116418. [Google Scholar] [CrossRef] [PubMed]
- Marakalala, M.J.; Williams, D.L.; Hoving, J.C.; Engstad, R.; Netea, M.G.; Brown, G.D. Dectin-1 Plays a Redundant Role in the Immunomodulatory Activities of β-Glucan-Rich Ligands in vivo. Microbes Infect. 2013, 15, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. β1,3-Glucan: Silver Bullet or Hot Air? Open Glycosci. 2010, 3, 1–6. [Google Scholar]
- Czop, J.K. The Role of Beta-Glucan Receptors on Blood and Tissue Leukocytes in Phagocytosis and Metabolic Activation. Pathol. Immunopathol. Res. 1986, 5, 286–296. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal Beta-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A Signalling Non-TLR Pattern-Recognition Receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef]
- Kanjan, P.; Sahasrabudhe, N.; de Haan, B.; de Vos, P. Immune Effects of β-Glucan Are Determined by Combined Effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods 2017, 37, 433–440. [Google Scholar] [CrossRef]
- Chan, G.C.-F.; Chan, W.K.; Sze, D.M.-Y. The Effects of Beta-Glucan on Human Immune and Cancer Cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P. β-Glucan as a New Tool in Vaccine Development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Yang, L.; Qiu, Y.; Pang, J.; Hu, X.; Dong, Z.; Liu, Z.; Jin, X. Adjuvanticity of β -Glucan for Vaccine Against Trichinella Spiralis. Front. Cell Dev. Biol. 2021, 9, 701708. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Schülke, S.; Nishio, S.; Guo, Y.; Rainer, H.; Maren, K.; Cheng, T.-Y.; Nochi, T.; Vieths, S.; et al. β-1,3-Glucan, but Not β-1,3/1,6-Glucan, Exacerbates Experimental Food Allergy, While Both Increase IgA Induction. Allergy 2023, 79, 503–506. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-Glucan Recognition by the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Kankkunen, P.; Teirilä, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-Beta-Glucans Activate Both Dectin-1 and NLRP3 Inflammasome in Human Macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef]
- Bera, H.; Guo, X.; Abbasi, Y.F.; Mahanty, A.; Nayak, A.K.; Saha, S.; Baqui, M.N. Chapter 10—Curdlan-Based Nanomaterials in Drug Delivery Applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Bera, H., Hossain, C.M., Saha, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 253–273. ISBN 978-0-12-820874-8. [Google Scholar]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.-O. Laminarin Promotes Anti-Cancer Immunity by the Maturation of Dendritic Cells. Oncotarget 2017, 8, 38554–38567. [Google Scholar] [CrossRef]
- Smith, A.J.; Graves, B.; Child, R.; Rice, P.J.; Ma, Z.; Lowman, D.W.; Ensley, H.E.; Ryter, K.T.; Evans, J.T.; Williams, D.L. Immunoregulatory Activity of the Natural Product Laminarin Varies Widely as a Result of Its Physical Properties. J. Immunol. 2018, 200, 788–799. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.H.; Magee, A.S.; Danielson, M.E.; et al. Activation of the Innate Immune Receptor Dectin-1 upon Formation of a “Phagocytic Synapse”. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Rothfuchs, A.G.; Bafica, A.; Feng, C.G.; Egen, J.G.; Williams, D.L.; Brown, G.D.; Sher, A. Dectin-1 Interaction with Mycobacterium Tuberculosis Leads to Enhanced IL-12p40 Production by Splenic Dendritic Cells. J. Immunol. 2007, 179, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T.; et al. Yeast Zymosan, a Stimulus for TLR2 and Dectin-1, Induces Regulatory Antigen-Presenting Cells and Immunological Tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 Mediates Macrophage Recognition of Candida Albicans Yeast but Not Filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef]
- Gersuk, G.M.; Underhill, D.M.; Zhu, L.; Marr, K.A. Dectin-1 and TLRs Permit Macrophages to Distinguish between Different Aspergillus Fumigatus Cellular States. J. Immunol. 2006, 176, 3717–3724. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Li, X.; Yan, Q.; Ma, J.; Jiang, Z. Curdlan (Alcaligenes faecalis) (1→3)-β-d-Glucan Oligosaccharides Drive M1 Phenotype Polarization in Murine Bone Marrow-Derived Macrophages via Activation of MAPKs and NF-κB Pathways. Molecules 2019, 24, 4251. [Google Scholar] [CrossRef]
- Schülke, S.; Waibler, Z.; Mende, M.-S.; Zoccatelli, G.; Vieths, S.; Toda, M.; Scheurer, S. Fusion Protein of TLR5-Ligand and Allergen Potentiates Activation and IL-10 Secretion in Murine Myeloid DC. Mol. Immunol. 2010, 48, 341–350. [Google Scholar] [CrossRef]
- Schülke, S.; Flaczyk, A.; Vogel, L.; Gaudenzio, N.; Angers, I.; Löschner, B.; Wolfheimer, S.; Spreitzer, I.; Qureshi, S.; Tsai, M.; et al. MPLA Shows Attenuated Pro-Inflammatory Properties and Diminished Capacity to Activate Mast Cells in Comparison with LPS. Allergy 2015, 70, 1259–1268. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1β Production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Park, H.-G.; Kim, Y.O.; Kang, J.S.; Kim, H.M.; Hong, J.T.; Kim, Y.; Han, S.-B. Induction of Dendritic Cell Maturation by β-Glucan Isolated from Sparassis crispa. Int. Immunopharmacol. 2010, 10, 1284–1294. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Heinsbroek, S.E.M.; Taylor, P.R.; Rosas, M.; Willment, J.A.; Williams, D.L.; Gordon, S.; Brown, G.D. Expression of Functionally Different Dectin-1 Isoforms by Murine Macrophages. J. Immunol. 2006, 176, 5513–5518. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Huang, T.-C.; Hung, H.-J.; Yu, J.-H.; Chung, W.-B.; Chaung, H.-C. Investigation of Beta-Glucans Binding to Human/Mouse Dectin-1 and Associated Immunomodulatory Effects on Two Monocyte/Macrophage Cell Lines. Biotechnol. Prog. 2010, 26, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR Signaling Pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: A Central Player in Innate Immune Signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll-like Receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef]
- Ikeda, Y.; Adachi, Y.; Ishii, T.; Miura, N.; Tamura, H.; Ohno, N. Dissociation of Toll-like Receptor 2-Mediated Innate Immune Response to Zymosan by Organic Solvent-Treatment without Loss of Dectin-1 Reactivity. Biol. Pharm. Bull. 2008, 31, 13–18. [Google Scholar] [CrossRef]
- Patidar, A.; Mahanty, T.; Raybarman, C.; Sarode, A.Y.; Basak, S.; Saha, B.; Bhattacharjee, S. Barley Beta-Glucan and Zymosan Induce Dectin-1 and Toll-like Receptor 2 Co-Localization and Anti-Leishmanial Immune Response in Leishmania Donovani-Infected BALB/c Mice. Scand. J. Immunol. 2020, 92, e12952. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Herre, J.; Brown, G.D.; Gordon, S. The Potential for Toll-like Receptors to Collaborate with Other Innate Immune Receptors. Immunology 2004, 112, 521–530. [Google Scholar] [CrossRef]
- Walachowski, S.; Tabouret, G.; Foucras, G. Triggering Dectin-1-Pathway Alone Is Not Sufficient to Induce Cytokine Production by Murine Macrophages. PLoS ONE 2016, 11, e0148464. [Google Scholar] [CrossRef]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of Immune Suppression by Myeloid-Derived Suppressor Cells: The Role of Interleukin-10 as a Key Immunoregulatory Cytokine. Open Biol. 2020, 10, 200111. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Huang, A.; Nie, J.; Tan, J.; Xing, S.; Qu, Y.; Jiang, K. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front. Immunol. 2021, 12, 683332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Matsunaga, K.; Shirai, T.; Hirai, K.; Gon, Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 2670. [Google Scholar] [CrossRef]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate Immune Response in Th1- and Th2-Dominant Mouse Strains. Shock 2004, 22, 460–466. [Google Scholar] [CrossRef]
- Liu, T.; Matsuguchi, T.; Tsuboi, N.; Yajima, T.; Yoshikai, Y. Differences in Expression of Toll-like Receptors and Their Reactivities in Dendritic Cells in BALB/c and C57BL/6 Mice. Infect. Immun. 2002, 70, 6638–6645. [Google Scholar] [CrossRef]
- Ning, Y.; Xu, D.; Zhang, X.; Bai, Y.; Ding, J.; Feng, T.; Wang, S.; Xu, N.; Qian, K.; Wang, Y.; et al. β-Glucan Restores Tumor-Educated Dendritic Cell Maturation to Enhance Antitumor Immune Responses. Int. J. Cancer 2016, 138, 2713–2723. [Google Scholar] [CrossRef]
- Li, B.; Cai, Y.; Qi, C.; Hansen, R.; Ding, C.; Mitchell, T.C.; Yan, J. Orally Administered Particulate Beta-Glucan Modulates Tumor-Capturing Dendritic Cells and Improves Antitumor T-Cell Responses in Cancer. Clin. Cancer Res. 2010, 16, 5153–5164. [Google Scholar] [CrossRef]
- Bellinghausen, I.; Brand, P.; Böttcher, I.; Klostermann, B.; Knop, J.; Saloga, J. Production of Interleukin-13 by Human Dendritic Cells after Stimulation with Protein Allergens Is a Key Factor for Induction of T Helper 2 Cytokines and Is Associated with Activation of Signal Transducer and Activator of Transcription-6. Immunology 2003, 108, 167–176. [Google Scholar] [CrossRef]
- Crespo, H.; Guillén, H.; de Pablo-Maiso, L.; Gómez-Arrebola, C.; Rodríguez, G.; Glaria, I.; de Andrés, D.; Reina, R. Lentinula Edodes β-Glucan Enriched Diet Induces pro- and Anti-Inflammatory Macrophages in Rabbit. Food Nutr. Res. 2017, 61, 1412791. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ichinose, T.; Ren, Y.; Song, Y.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Nishikawa, M.; Takano, H.; Sun, G. PM2.5-Rich Dust Collected from the Air in Fukuoka, Kyushu, Japan, Can Exacerbate Murine Lung Eosinophilia. Inhal. Toxicol. 2015, 27, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Lilly, L.M.; Gessner, M.A.; Dunaway, C.W.; Metz, A.E.; Schwiebert, L.; Weaver, C.T.; Brown, G.D.; Steele, C. The β-Glucan Receptor Dectin-1 Promotes Lung Immunopathology during Fungal Allergy via IL-22. J. Immunol. 2012, 189, 3653–3660. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Urbancek, S.; Majtan, J.; Banovcin, P.; Hercogova, J. β-Glucan-Based Cream (Containing Pleuran Isolated from Pleurotus ostreatus) in Supportive Treatment of Mild-to-Moderate Atopic Dermatitis. J. Dermatol. Treat. 2016, 27, 351–354. [Google Scholar] [CrossRef]
- Yamada, J.; Hamuro, J.; Hatanaka, H.; Hamabata, K.; Kinoshita, S. Alleviation of Seasonal Allergic Symptoms with Superfine Beta-1,3-Glucan: A Randomized Study. J. Allergy Clin. Immunol. 2007, 119, 1119–1126. [Google Scholar] [CrossRef]
- Kirmaz, C.; Bayrak, P.; Yilmaz, O.; Yuksel, H. Effects of Glucan Treatment on the Th1/Th2 Balance in Patients with Allergic Rhinitis: A Double-Blind Placebo-Controlled Study. Eur. Cytokine Netw. 2005, 16, 128–134. [Google Scholar]
- Comer, J.; Bassette, M.; Burghart, R.; Loyd, M.; Ishiguro, S.; Azhagiya Singam, E.R.; Vergara-Jaque, A.; Nakashima, A.; Suzuki, K.; Geisbrecht, B.V.; et al. Beta-1,3 Oligoglucans Specifically Bind to Immune Receptor CD28 and May Enhance T Cell Activation. Int. J. Mol. Sci. 2021, 22, 3124. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying Intracellular Rates of Glycolytic and Oxidative ATP Production and Consumption Using Extracellular Flux Measurements. J. Biol. Chem. 2017, 292, 7189–7207. [Google Scholar] [CrossRef]
- Schülke, S.; Fiedler, A.-H.; Junker, A.-C.; Flaczyk, A.; Wolfheimer, S.; Wangorsch, A.; Heinz, A.; Beckert, H.; Nagl, B.; Bohle, B.; et al. Critical Role of Mammalian Target of Rapamycin for IL-10 Dendritic Cell Induction by a Flagellin A Conjugate in Preventing Allergic Sensitization. J. Allergy Clin. Immunol. 2018, 141, 1786–1798. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rainer, H.; Goretzki, A.; Lin, Y.-J.; Schiller, H.R.; Krause, M.; Döring, S.; Strecker, D.; Junker, A.-C.; Wolfheimer, S.; Toda, M.; et al. Characterization of the Immune-Modulating Properties of Different β-Glucans on Myeloid Dendritic Cells. Int. J. Mol. Sci. 2024, 25, 9914. https://doi.org/10.3390/ijms25189914
Rainer H, Goretzki A, Lin Y-J, Schiller HR, Krause M, Döring S, Strecker D, Junker A-C, Wolfheimer S, Toda M, et al. Characterization of the Immune-Modulating Properties of Different β-Glucans on Myeloid Dendritic Cells. International Journal of Molecular Sciences. 2024; 25(18):9914. https://doi.org/10.3390/ijms25189914
Chicago/Turabian StyleRainer, Hannah, Alexandra Goretzki, Yen-Ju Lin, Hannah Ruth Schiller, Maren Krause, Sascha Döring, Daniel Strecker, Ann-Christine Junker, Sonja Wolfheimer, Masako Toda, and et al. 2024. "Characterization of the Immune-Modulating Properties of Different β-Glucans on Myeloid Dendritic Cells" International Journal of Molecular Sciences 25, no. 18: 9914. https://doi.org/10.3390/ijms25189914






