Parvovirus B19 Infection Is Associated with the Formation of Neutrophil Extracellular Traps and Thrombosis: A Possible Linkage of the VP1 Unique Region
Abstract
:1. Introduction
2. Results
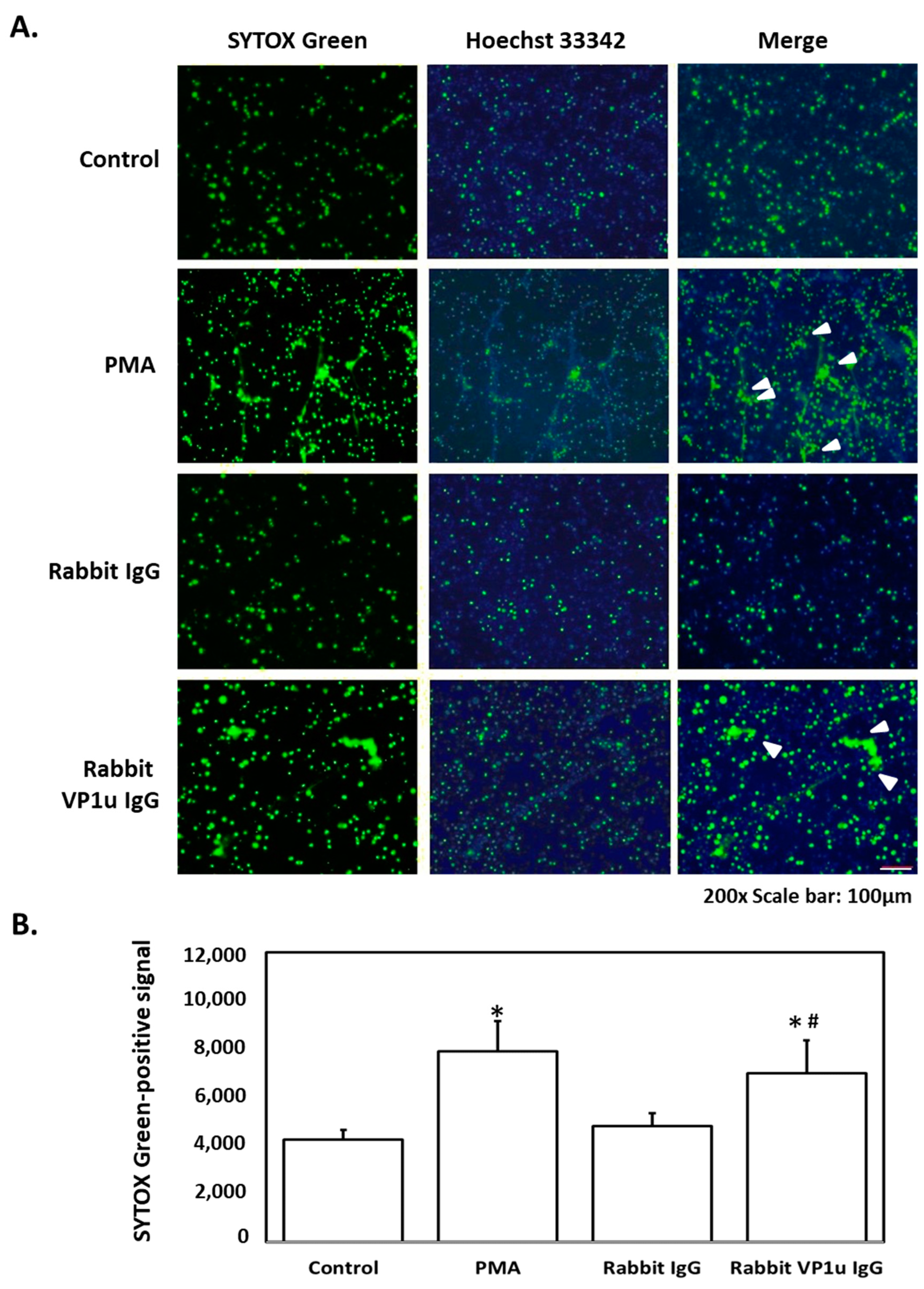
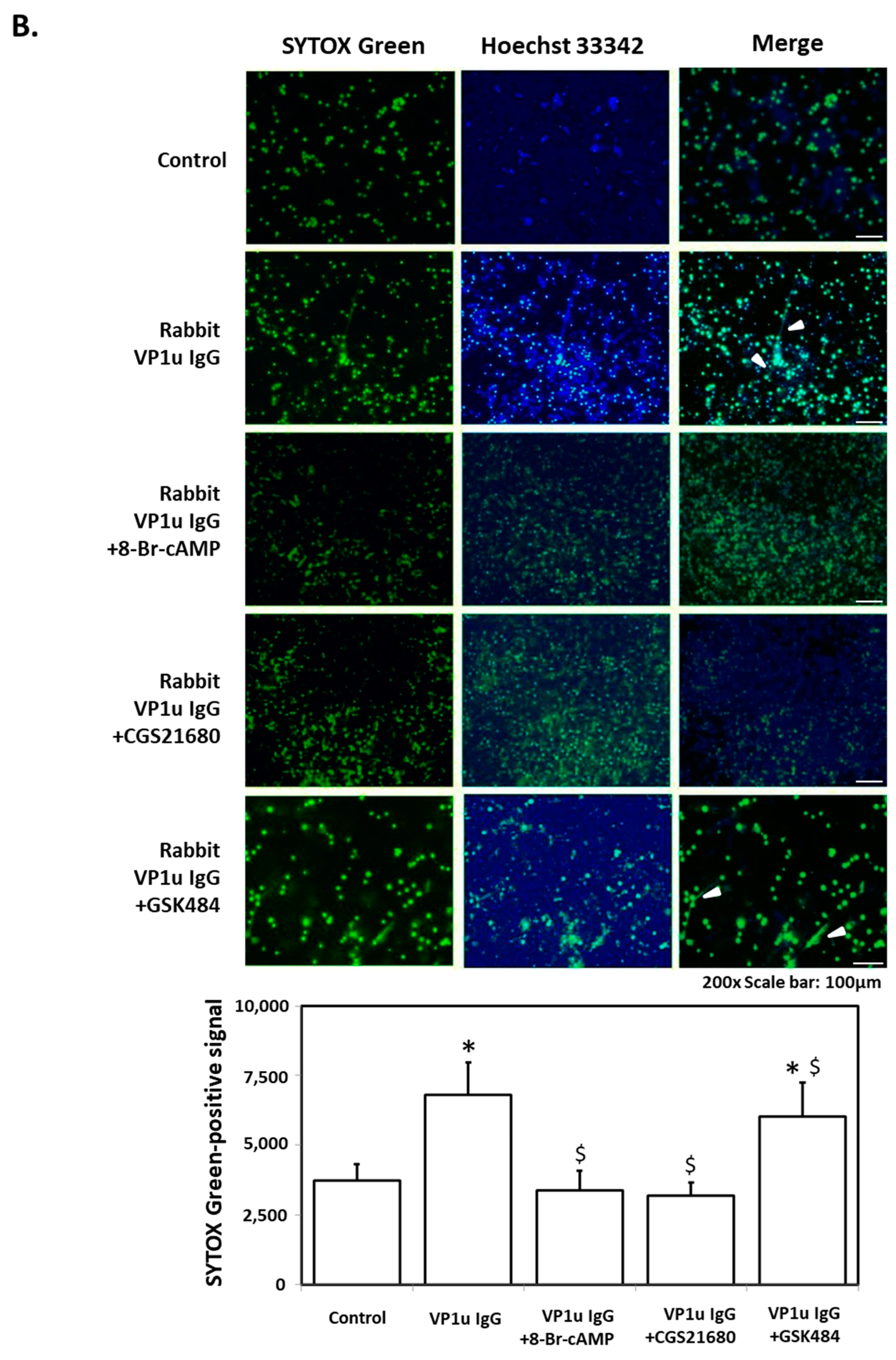
2.1. B19V-VP1u IgG Stimulates NETs Formation in dHL-60 Neutrophils
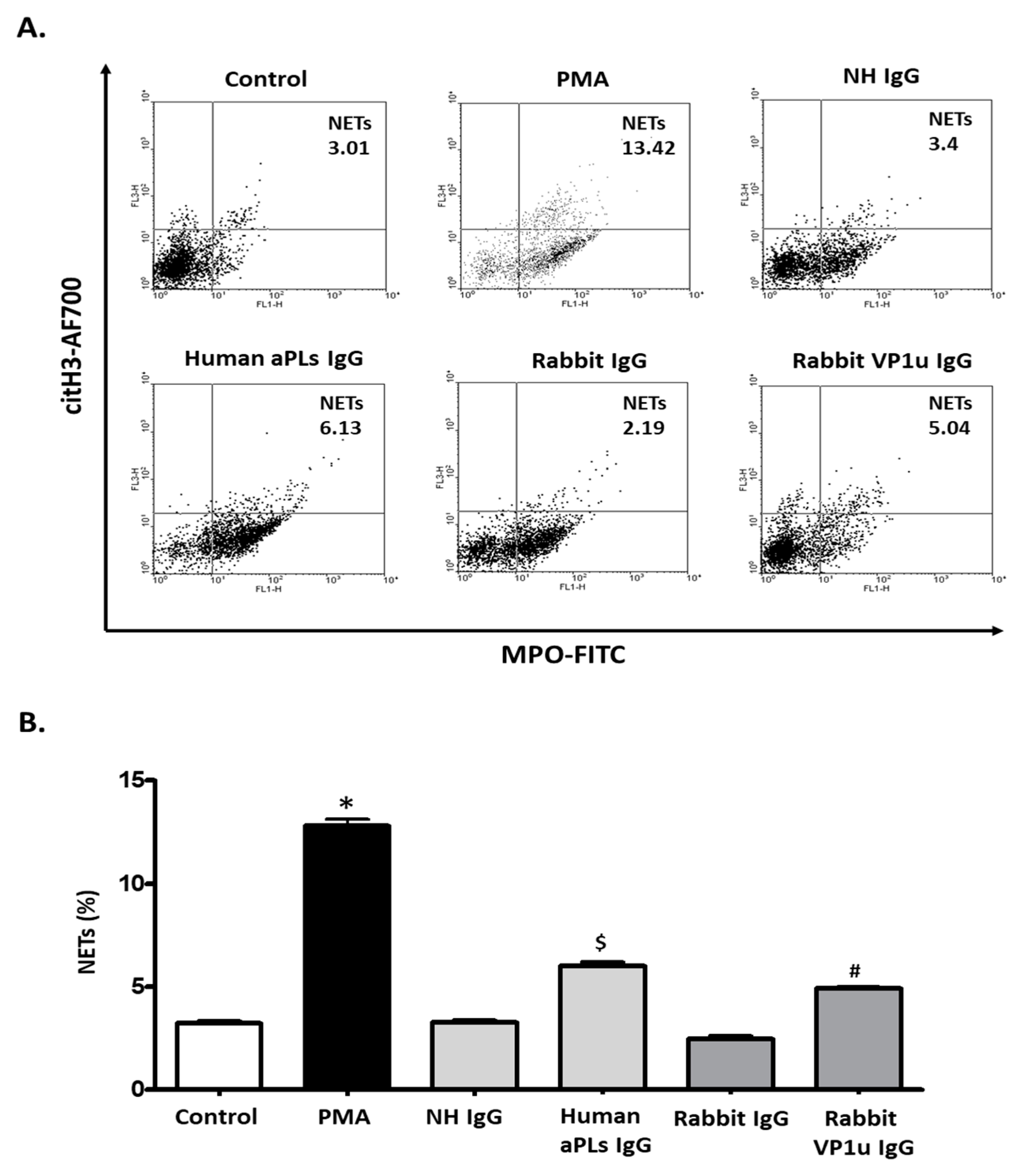
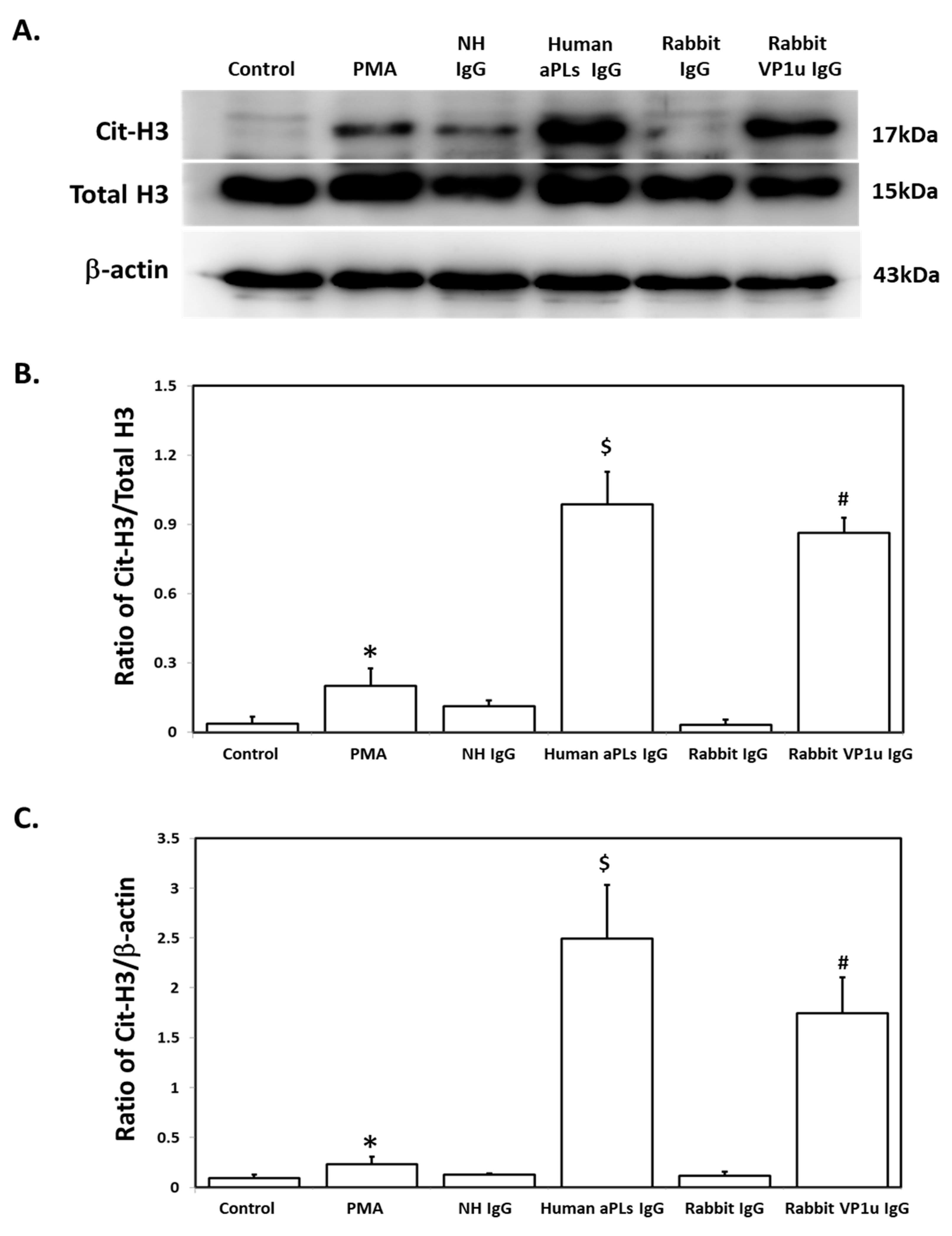
2.2. B19V-VP1u IgG Stimulates the Expressions of Cit-H3 and MPO in dHL-60 Neutrophils
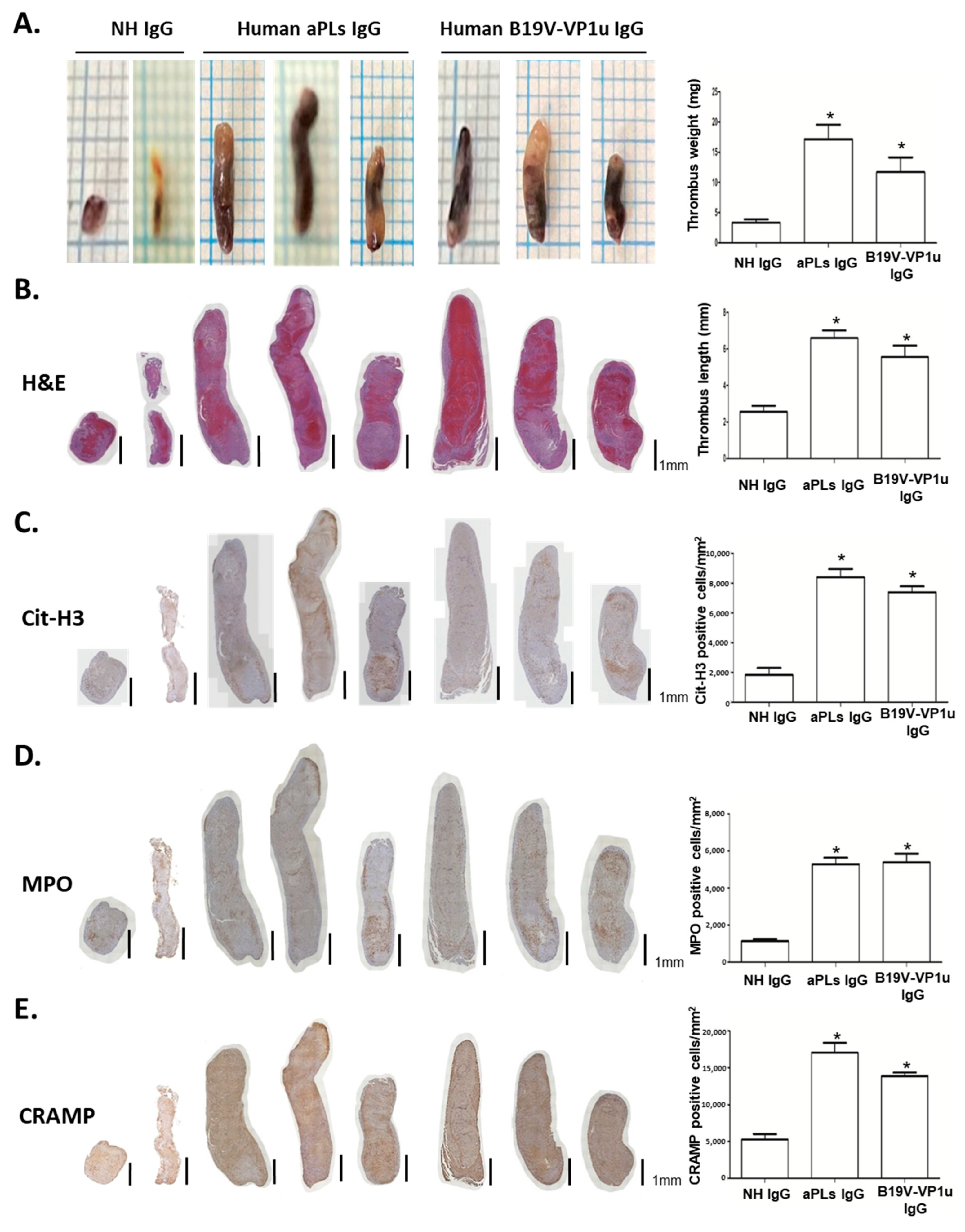
2.3. B19V-VP1u IgG Increases Thrombus Formation in Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Purification of Immunoglobulin G (IgG)
4.3. Immunofluorescence Staining for NETosis
4.4. Flow Cytometry
4.5. Immunoblotting
4.6. Determination of Reactive Oxygen Species (ROS) Production
4.7. Animal Model and Ethical Approval
4.8. Human Serum Sample and Ethical Approval
4.9. In Vivo Venous Thrombosis
4.10. Thrombus Sectioning, HE Stain, and Immunohistochemistry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Svenungsson, E.; Antovic, A. The antiphospholipid syndrome—Often overlooked cause of vascular occlusions? J. Intern. Med. 2020, 287, 349–372. [Google Scholar] [CrossRef]
- Duarte-García, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019, 71, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.C.; Leong, K.H.; Chiu, L.T.; Chou, P.Y.; Wu, L.C.; Chou, C.Y.; Kuo, C.F.; Tsai, S.Y. The trends in the incidence and thrombosis-related comorbidities of antiphospholipid syndrome: A 14-year nationwide population-based study. Thromb. J. 2022, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Fortin, P.R. Connective tissue diseases: Mitochondria drive NETosis and inflammation in SLE. Nat. Rev. Rheumatol. 2016, 12, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Corsiero, E.; Pratesi, F.; Prediletto, E.; Bombardieri, M.; Migliorini, P. NETosis as Source of Autoantigens in Rheumatoid Arthritis. Front. Immunol. 2016, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, D.; Segelmark, M. Neutrophil Extracellular Traps in ANCA-Associated Vasculitis. Front. Immunol. 2016, 7, 256. [Google Scholar] [CrossRef]
- Wirestam, L.; Arve, S.; Linge, P.; Bengtsson, A.A. Neutrophils-Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Front. Immunol. 2019, 10, 2734. [Google Scholar] [CrossRef]
- Petri, M. Antiphospholipid syndrome. Transl. Res. 2020, 225, 70–81. [Google Scholar] [CrossRef]
- Ali, R.A.; Gandhi, A.A.; Meng, H.; Yalavarthi, S.; Vreede, A.P.; Estes, S.K.; Palmer, O.R.; Bockenstedt, P.L.; Pinsky, D.J.; Greve, J.M.; et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019, 10, 1916. [Google Scholar] [CrossRef]
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017, 69, 655–667. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Gudjonsson, J.E.; Kahlenberg, J.M.; Joseph McCune, W.; Bockenstedt, P.L.; Karp, D.R.; Knight, J.S. Anti-Neutrophil Extracellular Trap Antibodies and Impaired Neutrophil Extracellular Trap Degradation in Antiphospholipid Syndrome. Arthritis Rheumatol. 2020, 72, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Núñez-Álvarez, C.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, Y.; Li, F.; Mu, F.; Zha, C. Anti-β2GPI/β2GPI induces human neutrophils to generate NETs by relying on ROS. Cell Biochem. Funct. 2019, 37, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tambralli, A.; Gockman, K.; Knight, J.S. NETs in APS: Current Knowledge and Future Perspectives. Curr. Rheumatol. Rep. 2020, 22, 67. [Google Scholar] [CrossRef]
- Martirosyan, A.; Aminov, R.; Manukyan, G. Environmental Triggers of Autoreactive Responses: Induction of Antiphospholipid Antibody Formation. Front. Immunol. 2019, 10, 1609. [Google Scholar] [CrossRef]
- Molinaro, R.; Yu, M.; Sausen, G.; Bichsel, C.A.; Corbo, C.; Folco, E.J.; Lee, G.Y.; Liu, Y.; Tesmenitsky, Y.; Shvartz, E.; et al. Targeted delivery of protein arginine deiminase-4 inhibitors to limit arterial intimal NETosis and preserve endothelial integrity. Cardiovasc. Res. 2021, 117, 2652–2663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.; Liu, Z. Impact of Neutrophil Extracellular Traps on Thrombosis Formation: New Findings and Future Perspective. Front. Cell Infect. Microbiol. 2022, 12, 910908. [Google Scholar] [CrossRef]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef]
- Ros, C.; Bieri, J.; Leisi, R. The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications. Viruses 2020, 12, 1463. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Brown, K.E. Human parvovirus B19. Clin Microbiol Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef]
- Chou, T.N.; Hsu, T.C.; Chen, R.M.; Lin, L.I.; Tsay, G.J. Parvovirus B19 infection associated with the production of anti-neutrophil cytoplasmic antibody (ANCA) and anticardiolipin antibody (aCL). Lupus 2000, 9, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Tzang, B.S.; Tsay, G.J.; Lee, Y.J.; Li, C.; Sun, Y.S.; Hsu, T.C. The association of VP1 unique region protein in acute parvovirus B19 infection and antiphospholipid antibody production. Clin. Chim. Acta 2007, 378, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chiu, C.C.; Cheng, J.; Lin, C.Y.; Shi, Y.F.; Tsai, C.C.; Tzang, B.S.; Hsu, T.C. Antigenicity analysis of human parvovirus B19-VP1u protein in the induction of anti-phospholipid syndrome. Virulence 2018, 9, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Tzang, B.S.; Chen, Y.M.; Lan, J.L.; Tsai, C.C.; Hsu, T.C. The association of anti-parvovirus B19-VP1 unique region antibodies with antiphospholipid antibodies in patients with antiphospholipid syndrome. Clin. Chim. Acta 2010, 411, 1084–1089. [Google Scholar] [CrossRef]
- Tzang, B.S.; Tsai, C.C.; Chiu, C.C.; Shi, J.Y.; Hsu, T.C. Up-regulation of adhesion molecule expression and induction of TNF-alpha on vascular endothelial cells by antibody against human parvovirus B19 VP1 unique region protein. Clin. Chim. Acta 2008, 395, 77–83. [Google Scholar] [CrossRef]
- Su, C.C.; Hsu, T.C.; Hsiao, C.H.; Chiu, C.C.; Tzang, B.S. Effects of antibodies against human parvovirus B19 on angiogenic signaling. Mol. Med. Rep. 2020, 21, 1320–1327. [Google Scholar] [CrossRef]
- Hung, K.C.; Huang, Z.Y.; Yow, J.L.; Hsu, T.C.; Tzang, B.S. Effect of N-terminal region of human parvovirus B19-VP1 unique region on cardiac injury in naïve mice. Mol. Med. Rep. 2021, 24, 759. [Google Scholar] [CrossRef]
- Tzang, B.S.; Lee, Y.J.; Yang, T.P.; Tsay, G.J.; Shi, J.U.; Tsai, C.C.; Hsu, T.C. Induction of antiphospholipid antibodies and antiphospholipid syndrome-like autoimmunity in naive mice with antibody against human Parvovirus B19 VP1 unique region protein. Clin. Chim. Acta 2007, 382, 31–36. [Google Scholar] [CrossRef]
- Payne, H.; Brill, A. Stenosis of the Inferior Vena Cava: A Murine Model of Deep Vein Thrombosis. J. Vis. Exp. 2017, 130, 56697. [Google Scholar]
- Abdel-Wahab, N.; Lopez-Olivo, M.A.; Pinto-Patarroyo, G.P.; Suarez-Almazor, M.E. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016, 25, 1520–1531. [Google Scholar] [CrossRef]
- Mandrioli, J.; Portolani, M.; Cortelli, P.; Sola, P. Middle cerebral artery thrombosis in course of parvovirus B19 infection in a young adult: A new risk factor for stroke? J. Neurovirol. 2004, 10, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; St Onge, J. Parvovirus leading to thrombotic microangiopathy in a healthy adult. BMJ Case Rep. 2016, 2016, bcr2015213492. [Google Scholar] [CrossRef]
- Flossdorf, S.; Schiwy-Bochat, K.H.; Teifel, D.; Fries, J.W.U.; Rothschild, M.A. Sudden death of a young adult with coronary artery vasculitis, coronary aneurysms, parvovirus B19 infection and Kawasaki disease. Forensic Sci. Med. Pathol. 2020, 16, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Furkan Demir, B.; Karahan Meteris, A.; Yirgin, G.; Comoglu, M.; Katipoglu, B.; Yılmaz, N.; Ates, I. Parvovirus-induced thrombocytopenia. Hematol. Oncol. Stem Cell Ther. 2019, 12, 226–227. [Google Scholar] [CrossRef]
- Ali, E.A.; Rasheed, M.; Al-Sadi, A.; Awadelkarim, A.M.; Saad, E.A.; Yassin, M.A. Immune Thrombocytopenic Purpura and Paradoxical Thrombosis: A Systematic Review of Case Reports. Cureus 2022, 14, e30279. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, C.; Zhang, S.; Hou, X.; Chen, Z.; Zhang, J.; Zhang, Y.; Sun, J.; Wang, Y. Why thromboembolism occurs in some patients with thrombocytopenia and treatment strategies. Thromb. Res. 2020, 196, 500–509. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Bonaventura, A.; Liberale, L.; Carbone, F.; Vecchié, A.; Diaz-Cañestro, C.; Camici, G.G.; Montecucco, F.; Dallegri, F. The Pathophysiological Role of Neutrophil Extracellular Traps in Inflammatory Diseases. Thromb. Haemost. 2018, 118, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Hasler, P.; Giaglis, S.; Hahn, S. Neutrophil extracellular traps in health and disease. Swiss Med. Wkly. 2016, 146, w14352. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads toneutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 8674–8679. [Google Scholar] [CrossRef]
- Mauracher, L.M.; Posch, F.; Martinod, K.; Grilz, E.; Däullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D.D.; et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Loizou, S.; Cazabon, J.K.; Walport, M.J.; Tait, D.; So, A.K. Similarities of specificity and cofactor dependence in serum antiphospholipid antibodies from patients with human parvovirus B19 infection and from those with systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil extracellular traps: Mechanisms of formation and role in health and disease. Biochemistry 2014, 79, 1286–1296. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Allen, L.H.; Criss, A.K. Cell intrinsic functions of neutrophils and their manipulation by pathogens. Curr. Opin. Immunol. 2019, 60, 124–129. [Google Scholar] [CrossRef]
- Mollinedo, F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019, 40, 228–242. [Google Scholar] [CrossRef]
- Gordon, R.A.; Herter, J.M.; Rosetti, F.; Campbell, A.M.; Nishi, H.; Kashgarian, M.; Bastacky, S.I.; Marinov, A.; Nickerson, K.M.; Mayadas, T.N.; et al. Lupus and proliferative nephritis are PAD4 independent in murine models. JCI Insight 2017, 2, e92926. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.A.; Wigerblad, G.; Sahlström, P.; Garimella, M.G.; Chemin, K.; Steen, J.; Titcombe, P.J.; Marklein, B.; Zhou, D.; Stålesen, R.; et al. Differential ACPA Binding to Nuclear Antigens Reveals a PAD-Independent Pathway and a Distinct Subset of Acetylation Cross-Reactive Autoantibodies in Rheumatoid Arthritis. Front. Immunol. 2019, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Arvia, R.; Stincarelli, M.A.; Manaresi, E.; Gallinella, G.; Zakrzewska, K. Parvovirus B19 in Rheumatic Diseases. Microorganisms 2024, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, M.; Peláez, L.F.; Rojas, M.; Narváez-Sánchez, R.; Velásquez, J.A.; Escudero, C.; San Martín, S.; Cadavid, Á.P. Differences in Endothelial Activation and Dysfunction Induced by Antiphospholipid Antibodies among Groups of Patients With Thrombotic, Refractory, and Non-refractory Antiphospholipid Syndrome. Front. Physiol. 2021, 12, 764702. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin Immunopathol. 2022, 44, 347–362. [Google Scholar] [CrossRef]
- Edwards, M.H.; Pierangeli, S.; Liu, X.; Barker, J.H.; Anderson, G.; Harris, E.N. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation 1997, 96, 4380–4384. [Google Scholar] [CrossRef]
- Pierangeli, S.S.; Girardi, G.; Vega-Ostertag, M.; Liu, X.; Espinola, R.G.; Salmon, J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005, 52, 2120–2124. [Google Scholar] [CrossRef]
- Esmon, C.T. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009, 23, 225–229. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, F.; Wang, Q.; Wang, K.; Pan, S.; Pan, Z.; Xu, S.; Li, L.; Zhao, D. Differentiation of HL-60 cells in serum-free hematopoietic cell media enhances the production of neutrophil extracellular traps. Exp. Ther. Med. 2021, 21, 353. [Google Scholar] [CrossRef]
- Monti, M.; Iommelli, F.; De Rosa, V.; Carriero, M.V.; Miceli, R.; Camerlingo, R.; Di Minno, G.; Del Vecchio, S. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. PLoS ONE 2017, 12, e0171362. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Tzang, B.S.; Chiang, S.Y.; Hsu, G.J.; Hsu, T.C. Increased expression of Matrix Metalloproteinase 9 in liver from NZB/W F1 mice received antibody against human parvovirus B19 VP1 unique region protein. J. Biomed. Sci. 2009, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Wang, J.; Guo, C.; Kleiman, K.; Meng, H.; Knight, J.S.; Eitzman, D.T. Proprotein convertase subtilisin/kexin type 9 (PCSK9) Deficiency is Protective Against Venous Thrombosis in Mice. Sci. Rep. 2017, 7, 14360. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Miyashita, T.; Yamamoto, Y.; Munesue, S.; Harashima, A.; Takayama, H.; Fushida, S.; Ohta, T. Citrullinated Histone H3: Early Biomarker of Neutrophil Extracellular Traps in Septic Liver Damage. J. Surg. Res. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Kiguchi, M.M.; Hager, E.S.; Winger, D.G.; Hirsch, S.A.; Chaer, R.A.; Dillavou, E.D. Factors that influence perforator thrombosis and predict healing with perforator sclerotherapy for venous ulceration without axial reflux. J. Vasc. Surg. 2014, 59, 1368–1376. [Google Scholar] [CrossRef]
- Sexton, T.; Smyth, S.S. Novel mediators and biomarkers of thrombosis. J. Thromb. Thrombolysis 2014, 37, 1–3. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzang, B.-S.; Chin, H.-Y.; Tzang, C.-C.; Chuang, P.-H.; Chen, D.-Y.; Hsu, T.-C. Parvovirus B19 Infection Is Associated with the Formation of Neutrophil Extracellular Traps and Thrombosis: A Possible Linkage of the VP1 Unique Region. Int. J. Mol. Sci. 2024, 25, 9917. https://doi.org/10.3390/ijms25189917
Tzang B-S, Chin H-Y, Tzang C-C, Chuang P-H, Chen D-Y, Hsu T-C. Parvovirus B19 Infection Is Associated with the Formation of Neutrophil Extracellular Traps and Thrombosis: A Possible Linkage of the VP1 Unique Region. International Journal of Molecular Sciences. 2024; 25(18):9917. https://doi.org/10.3390/ijms25189917
Chicago/Turabian StyleTzang, Bor-Show, Hao-Yang Chin, Chih-Chen Tzang, Pei-Hua Chuang, Der-Yuan Chen, and Tsai-Ching Hsu. 2024. "Parvovirus B19 Infection Is Associated with the Formation of Neutrophil Extracellular Traps and Thrombosis: A Possible Linkage of the VP1 Unique Region" International Journal of Molecular Sciences 25, no. 18: 9917. https://doi.org/10.3390/ijms25189917






