Abstract
Among the most deadly malignancies that strike women worldwide, ovarian cancer is still one of the most common. The primary factor affecting a patient’s survival is early lesion discovery. Unfortunately, because ovarian cancer is a sneaky illness that usually manifests as nonspecific symptoms only in advanced stages, its early detection and screening are challenging. A lot of research is being conducted on effective methods of diagnosing and treating ovarian cancer. Recently, non-coding RNAs (ncRNAs) have gained great popularity, which are considered to be the main regulators of many cellular processes, especially those occurring in cancer. LncRNAs are also being studied for their therapeutic use in the treatment of ovarian cancer and their use in diagnostics and as indicators of poor prognosis. In this article, we reviewed lncRNAs described in the literature that may play an important role in ovarian cancer.
Keywords:
long non-coding RNA; ovarian cancer; NEAT 1; SOX21-AS1; H19; LOXL1-AS1; MAFG-AS1; GAS5; LINC0015; pseudogenes 1. Introduction
The term “ovarian cancer” (OC) refers to a wide spectrum of cancers developing from cells of the ovary, fallopian tube or peritoneum, with various histological, clinicopathological origins and molecular features [1]. Epithelial ovarian cancer is a highly variable illness that may be classified into five primary histotypes based on genetic differences and clinical characteristics [2]. One of the deadliest malignancies to affect women globally is still ovarian cancer [3], whose survival rates are mostly reliant on early identification [4]. The sneaky nature of ovarian cancer makes its early identification and screening hard [5]. Patients typically have non-specific symptoms, which are noted in advanced disease [6]. The most common symptom reported by patients with high-risk epithelial ovarian cancer is abdominal or pelvic pain. As the size of the tumor increases, so do the proportion of symptomatic women and the quantity of symptoms [7]. Ovarian epithelial tumor indicators, or cancer antigen 125 (CA125) and human epididymis protein 4 (HE4), are helpful in diagnosing, tracking efficacy, and keeping an eye out for relapses. However, the cancer stage, histological type, age, and menopausal state can also affect the levels of these markers. Additionally, they can occasionally be raised in a number of benign and malignant disorders affecting the female reproductive system. Although carbohydrate antigen 19-9 (CA199) and carcinoembryonic antigen (CEA) can also be raised, they are not specific to OC as they are frequently elevated in gastrointestinal cancers. Although ovarian tumors can be identified by imaging, a precise diagnosis and differentiation of benign from malignant lesions cannot be made. Imaging tests are also unable to detect an alteration until it has grown to a sufficient size [8]. On the other hand, multigene tests can be utilized to detect breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) mutation carriers and individuals at high risk of OC, which makes it easier to select therapeutic drugs and estimate their prognosis [9].
Surgery, radiation, targeted therapy, hormone therapy, immunological therapy, and polyadenosine diphosphate ribose polymerase inhibitor maintenance therapy are all used to treat OC patients [10,11]. Most patients with ovarian cancer experience clinical remission following their initial therapy; nevertheless, 70% of patients return and rapidly become resistant to platinum, and the five-year survival rate is only 46% [12]. Although there has been progress in the treatment of ovarian cancer, the disease is still lethal. Of the histological types, high-grade serous ovarian cancer (HGSOC) is the most frequent (>80%). Five-year survival rates for ovarian cancer patients are 13% and 27%, respectively, with approximately 80% of patients presenting in advanced stages III and IV [13,14].
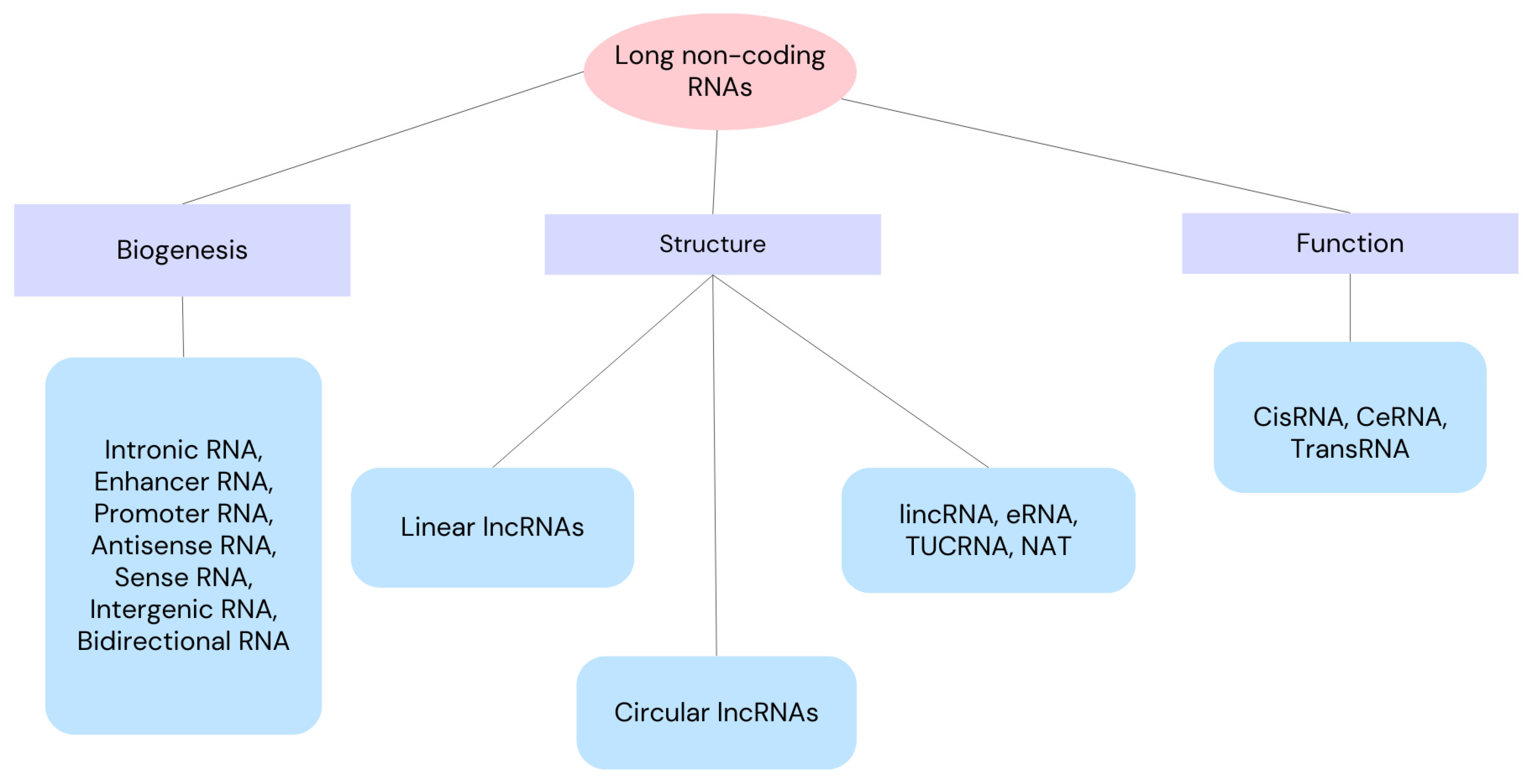
Non-coding RNAs (ncRNAs) are regarded as master regulators of a number of cellular processes, including ferroptosis’ molecular regulation. As a result, ncRNA-based therapies may be a good option for cancer treatment [15]. Small non-coding RNA (sncRNA) has fewer than 200 nucleotides, while long non-coding RNA (lncRNA) has more than 200 nucleotides. Both types of ncRNA have the capacity to encode proteins, either fully or partially [16,17,18]. Since lncRNA is important for translation, post-transcriptional transcription, gene transcription, and epigenetic modification [19], aberrant expression or lncRNA dysfunction can result in a number of disorders [20]. We can divide lncRNAs according to their function, biogenesis and structure as depicted in Figure 1. Through a variety of manners, lncRNAs can affect cell proliferation, apoptosis, migration, and invasion, which can impact the development of tumors [21,22]. Through the recruitment of regulatory components to the locus and the regulation of their functions, lncRNAs can operate as sponges, absorbing micro-RNAs (miRNAs) or regulating surrounding genes in cis. Additionally, proteins can be bound by lncRNAs to enhance their stability, function, and avoid destruction. Thus, lncRNA–protein interactions are crucial for the development of cancer and its spread [23,24,25]. In this review, we present an up-to-date literature review as of September 2024, including clinical trials. The purpose of this review is to determine the role of long non-coding RNAs in ovarian cancer cells. Here, we review NEAT 1, SOX21-AS1, H19, 74 LOXL1-AS1, MAFG-AS1, GAS5 and LINC0015 long non-coding RNAs because of their involvement in ovarian cancers.
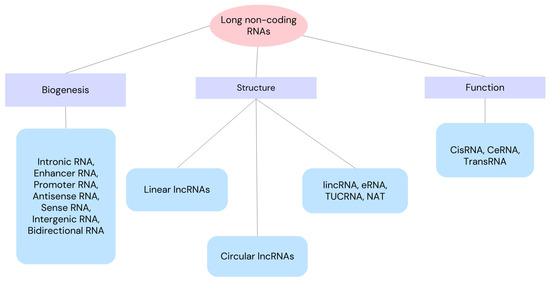
Figure 1.
Classification of long non-coding RNAs into classes and subclasses according to their function, biogenesis and structure. lincRNA—long intergenic non-coding RNA, eRNA—enhancer RNA, TUCRNA—transcribed ultra-conserved RNA, NAT—natural antisense transcript, CisRNA—cis-acting RNA, CeRNA—competitive endogenous RNA, TransRNA—trans-acting RNA.
2. Long Non-Coding RNA NEAT1
NEAT1, or nuclear-enriched autosomal transcript 1, is involved in the carcinogenesis of cancer [26]. Due to its potential involvement in the beginning and advancement of cancer, as well as the fact that its aberrant expression is associated with clinical aspects such as metastasis, treatment resistance, and patient survival, NEAT1 exhibits characteristics of a tumor driver [27,28]. In a variety of cancers, NEAT1 can enhance stem cell characteristics and mediate an oncogenic phenotype [29]. By focusing on different miRNAs, RNA-binding proteins, mature mRNAs, and non-protein-coding genes, lncRNAs control epithelial–mesenchymal transition (EMT) processes and tumor spread in malignancies of the reproductive system [30].
A high expression of NEAT1 is strongly connected with distant metastases, tumor stage, and a poor prognosis in ovarian cancer when considered as an independent factor [31]. NEAT1 promotes ovarian cancer cells’ EMT, invasion, and migration by mediating the expression of tight junction protein three (TJP3) and blocking the activity of miR-1321 [30]. NEAT1 increases the growth and invasion of cancer cells via binding to the human antigen R (HuR) protein and sponging miR-124-3p. HuR, a particular RNA-binding protein (RBP) plays important role in stabilizing and modulating the translation of many of its target mRNAs including p21, c-fos, VEGF, MKP-1, TNF-α, Bcl-2, Mcl-1, and p53. Higher HuR mRNA levels are also positively correlated with the International Federation of Gynecology and Obstetrics (FIGO) tumor stage and the occurrence of lymph node metastasis [32]. In individuals with ovarian cancer, high NEAT1 expression is associated with a worse prognosis and a lower survival rate. The expression of Rho-related protein kinase 1 (ROCK1), a gene linked to metastasis, can be targeted by NEAT1 to accelerate ovarian cell invasion and migration. Consequently, miR-382-3p inhibited the spread of tumors by focusing on ROCK1’s 3′-UTR [33].
By blocking caspase-3 action, NEAT1 overexpression can also promote cell division and decrease apoptosis in ovarian cancer cells. NEAT1 has the ability to upregulate miR-34a-5p and induce the expression of B-cell lymphoma-2 (BCL2). Elevated NEAT1 expression can also speed up a cell’s entry into the S phase and shorten the G0/G1 phase. Consequently, NEAT1 knockdown enhances apoptosis and decreases cell growth [34]. Abnormal NEAT1 expression was discovered in HGSOC by Yong et al. [35]. As an oncogene, LIN28B can bind to NEAT1 and stabilize its expression. This means that NEAT1 can control the growth, proliferation, adhesion, and development of cancer cells by blocking miR-506. Consequently, NEAT1 and LIN28B together might be potent HGSOC biomarkers. In ovarian cancer cells, a high expression of NEAT1 can increase basic leucine zipper and W2 domain-containing protein (BZW1) and inhibit miR-4500. NEAT1 knockdown causes apoptosis and prevents the colony formation, migration, glycolysis, and proliferation of ovarian cancer cells [36].
NEAT1 and FGF9 are overexpressed in ovarian cancer cells. NEAT1 targets VEGF, Ang-1, and MMP2 to induce angiogenesis. The overexpression of miR-365 or knockdown of NEAT1 or FGF9 reduces cell proliferation, colony formation, and angiogenesis. The effect of FGF9 knockdown can be reversed by the overexpression of NEAT1 or knockdown of miR-365 [37]. In ovarian cancer tissues and cell lines resistant to paclitaxel (PTX), NEAT1 also lowers miR-194. ZEB1 expression is stimulated by NEAT1 sponging miR-194, which also causes EMT and a drug-resistant phenotype. The knockdown of NEAT1 increases PTX-induced apoptosis in a cohort of PTX-resistant ovarian cancer cells in vitro, accelerating cell drug sensitivity. Treatment resistance is also decreased by NEAT1 knockdown in vivo [38]. NEAT1 expression is upregulated and let-7g is downregulated in ovarian cancer cells. Let-7g is competitively bound by NEAT1, which lowers the expression of let-7g. Let-7g decreases the proliferation, migration, and invasion of cancer cells while also increasing the synthesis of adipose triglyceride lipase (ATGL) and inhibiting the expression of mesoderm-specific transcript (MEST). Conversely, NEAT1 silencing inhibits the growth of xenograft tumors [39].
3. Long Non-Coding RNA SOX21-AS1
Chromosome 13q32.1 contains the LncRNA SOX21 antisense RNA1 (SOX21-AS1), which is involved in the initiation of some malignancies. By epigenetically suppressing p21 expression, SOX21-AS1 is linked to the advancement of hepatocellular carcinoma and may be utilized to forecast the prognosis of patients [40].
The development and spread of cancer may be primarily caused by aberrant amounts of lncRNA. The long non-coding RNA SOX21-AS1 has an up-stream role and could represent a novel oncogene in a number of cancers, such as melanoma, osteosarcoma, breast, lung, and ovarian cancer. Mostly functioning as a competitive endogenous RNA (ceRNA), SRY-box transcription factor 21 antisense divergent transcript 1 (SOX21-AS1) suppresses the amount of its target microRNAs (miRNAs), causing their targets to be upregulated. In addition, SOX21-AS1 participates in the signaling pathways of transforming growth factor-β (TGF-β), Wnt, and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT). Affected patients’ clinicopathological characteristics and lncRNA are also connected. The overexpression of SOX21-AS1 has been linked to processes connected to cancer, including cell cycle arrest, invasion, migration, apoptosis, and epithelial–mesenchymal transition (EMT). Given SOX21-AS1’s association with poor prognosis and shorter patient survival, it may be used as a prognostic biomarker and diagnostic biomarker in cancer. Furthermore, SOX21-AS1 makes ovarian cancer cells more resistant to the chemotherapeutic drug cisplatin [41].
4. Long Non-Coding RNA H19
The first lncRNA that Brannan found was H19. This gene, which is paternally imprinted and situated close to the telomeric region of chromosome 11p15.5, is frequently implicated in the development of tumors [42,43]. Although H19 is re-expressed in tumor tissues, it is not expressed in postnatal tissues [44]. A number of malignancies, including ovarian, breast, stomach, and esophageal cancers, have been linked to disturbed H19 levels [45,46,47]. LncRNA H19 is believed to play an important role in the development of ovarian cancer by regulating various pathways, as evidenced by increased expression levels in cisplatin-resistant A2780-DR cells. Studies conducted in vitro and in vivo have demonstrated that H19 knockdown results in a decreased expression of NRF2-targeted proteins like G6PD, GSR, NQO1, GCLM, GSTP1, and GCLC and the enhanced susceptibility of A2780-DR cells to cisplatin. H19 modifies glutathione metabolism, which also leads to treatment resistance [48]. H19 is essential for the epithelial-to-mesenchymal transition (EMT) that is brought about by the release of spongiform miR-370-3p [49]. Warburg effect regulation is accomplished via H19’s alteration of glycolytic metabolism. By sponging miR-324-5p, H19 silencing lowers lactate generation, PKM2 expression, and glucose consumption [50].
In the ovarian cell line A2780/CP, which is resistant to cisplatin, valproic acid lowers H19 expression. The A2780/CP cell line’s susceptibility to cisplatin and rate of apoptosis both significantly increase upon the subsequent silencing of H19 [51]. On the other hand, H19 expression was shown to be considerably higher in the cisplatin-resistant ovarian cancer cell line OVCAR3/DDP than in the OVCAR3 cell line. Slug, twist, and snail were among the EMT markers that OVCAR3/DDP cells overexpressed, while E-cadherin was downregulated. In OVCAR3/DDP cells, subsequent H19 knockdown resulted in the promotion of E-cadherin expression and the inhibition of migration and EMT-positive markers [52]. Ginsenoside Rg3 inhibits OC cell proliferation, migration and invasion by partially inhibiting the expression of lncRNA H19. H19 suppression was identified as the cause of the ginsenoside Rg3 treatment’s reduction in N-cadherin expression and increase in E-cadherin levels [53].
5. Long Non-Coding RNA Lysyl Oxidase-like 1 Antisense RNA 1 (LOXL1-AS1)
Lysyl oxidase-like 1 antisense RNA 1 (LOXL1-AS1) is am lncRNA found on human chromosome 15q24.1. It plays a role in the development and progression of a number of cancers, including gastric cancer, bladder cancer, prostate cancer, ovarian cancer, cervical cancer, breast cancer, glioma, thymus cancer, liver cancer [54] and pancreatic cancer [55].
Patients with ovarian cancer exhibit a notably elevated expression level of LOXL1-AS1 in comparison to healthy individuals. The expression level of LOXL1-AS1 is associated with distant metastases and an advanced FIGO stage. Some findings suggested that patients with ovarian cancer who had higher levels of circulating LOXL1-AS1 may have experienced a worse overall survival rate [56]. Since LOXL1-AS1 expression in OC cells is substantially higher than in normal ovarian cells, downregulating it will cause OC cells to proliferate less rapidly and undergo apoptosis by specifically regulating miR-761 [57]. By the miR-18b-5p/VMA21 axis, LOXL1-AS1 stimulates the proliferation, migration, and invasion of OC cells [58]. LOXL1-AS1 is a putative target for OC therapy as it is a possibly carcinogenic lncRNA in OC.
6. Long Non-Coding RNA MAFG-AS1
Chromosome 17 is the location of the transcription factor MAF BZIP G-antisense RNA 1 (MAFG-AS1). In addition to suppressing 16 miRNAs and directly influencing the expression of 22 protein-coding genes, MAFG-AS1 is elevated in 15 human malignancies [59]. Tumor size, clinical stage, distant metastases, overall survival, and disease-free survival are all highly associated with MAFG-AS1 levels. Through cell proliferation, invasion, glycolysis, metastasis, and drug sensitivity, MAFG-AS1 contributes to the development of disease.
The mechanism underlying the interaction between MAFG-AS1, miR-339-5p, NFKB1, and IGF1 was identified by characterizing their binding affinities by Bai et al. In order to examine the impact of MAFG-AS1 overexpression or knockdown on OC cell invasion, migration, and EMT, they also silenced NFKB1 and IGF1. Nude mice were used for xenografts in order to validate the in vitro findings. In OC tissues and cells, MAFG-AS1 was shown to have a considerably high expression pattern. It was also shown that MAFG-AS1 recruited NFKB1 to enhance the expression pattern of IGF1, and that it bound to miR-339-5p to raise the expression pattern of NFKB1. Therefore, it can be said that suppressing IGF1 or NFKB1 would reverse the effects of MAFG-AS1 overexpression, which increases the EMT, invasion, and migration of OC cells. Consequently, MAFG-AS1 might offer an opportunity for treatment against OC [60,61].
7. Long Non-Coding RNA GAS5
LncRNA growth arrest specific 5 (GAS5) is the source of multiple non-coding short nucleolar RNAs (snoRNAs) [62]. Numerous malignancies have been shown to have altered GAS5 levels. GAS5 has the potential to control cisplatin resistance in cervical cancer by modulating microRNA-21 (miR-21) [63]. Reduced lncRNA GAS5 levels in OC are frequently linked to a poor prognosis [64]; regrettably, further research is needed to understand the biological mechanism of GAS5 in OC. The effect of altered GAS5 on ovarian cancer cell phenotypes was investigated in vitro and in vivo. The results showed that GAS5 is decreased in cancer tissues, especially in tumors with a larger size, deeper invasiveness and higher tumor stage. Patients with lower levels of GAS5 expression had worse disease-free survival (p < 0.0001) and overall survival (p = 0.0016) than patients with high GAS5 expression. In contrast, GAS5 overexpression inhibits ovarian cancer cell proliferation in vitro and in vivo. GAS5 influences ovarian cancer cell proliferation by regulating the expression of cyclin D1, p21, and apoptosis protease-activating factor 1 (APAF1). LncRNA GAS5 may therefore be a potential indicator of poor prognosis in ovarian cancer and a therapeutic target [65].
By decreasing the expression of miR-96-5p, GAS5 overexpression can drastically impair the ability of ovarian cancer cells to proliferate and invade. GAS5 may also have an impact on the signaling pathway that involves PTEN, protein kinase B (AKT), and mTOR [66]. In ovarian cancer, a higher tumor volume and an FIGO stage (III–IV) are associated with low GAS5 expression and high miR-196a-5p expression [67]. GAS5 downregulation causes ovarian cancer cells to proliferate more quickly, experience a lower rate of apoptosis, and grow larger tumors in rats. GAS5 has the ability to bind and control miR-196a-5p directly. miR-196a-5p expression is elevated in ovarian cancer tissues and cell lines, indicating that it may favor ovarian cancer [68]. In ovarian cancer cells, high expression levels of GAS5 promote apoptosis.
Ma et al. found that ovarian cancer tissues and cell lines exhibit significantly higher expression levels of microRNA (miR)-21 than adjacent non-cancerous tissues and normal ovarian epithelial cells but significantly lower expression levels of GAS5 and Sprouty homolog 2 (SPRY2). The luciferase experiment revealed that, in ovarian cancer-derived A2780 cells, miR-21 was directly targeted by GAS5, and that its target gene was SPRY2. The downregulation of miR-21 and upregulation of SPRY2 are observed in conjunction with a considerable inhibition of ovarian cancer cell growth caused by GAS5 overexpression. On the other hand, A2780 cell growth is significantly inhibited by miR-21 overexpression, along with a decrease in SPRY2 expression. Additionally, the overexpression of miR-21 enhances GAS5’s stimulating influence on SPRY2 expression while attenuating its suppressive effect on A2780 cell proliferation. On the other hand, SPRY2 knockdown restores GAS5’s inhibitory effect on A2780 cell proliferation. Thus, by first suppressing the expression of miR-21 and then upregulating the expression of SPRY2, GAS5 inhibits the proliferation of ovarian cancer cells. Thus, the GAS5/miR-21/SPRY2 signaling pathway could be a viable target for treatment in ovarian cancer [69].
The malignant tumor of the female reproductive system with the highest fatality rate is epithelial ovarian cancer (EOC). Tolerance to early-stage chemotherapeutic medications, such as cisplatin, is a significant factor contributing to a poor prognosis in EOC. Long et al. investigated the in vitro and in vivo effects of lncRNA GAS5 on human ovarian cancer cell lines HEY, A2780, A2780/DDP, HO8910, HO8910PM, SKOV3, and SKOV3/DDP and a normal human ovarian epithelial cell line. They found an extremely low expression of lncRNA GAS5 in epithelial ovarian cancer (EOC) samples, which was associated with prognosis. While GAS5 overexpression greatly improved the sensitivity of OC cells to cisplatin in vivo and in vitro, it also caused G0/G1 OC cell arrest and increased apoptosis. Low GAS5 expression was also seen in cisplatin-resistant OC cell lines. By attracting the E2F4 transcription factor to the promoter of PARP1, GAS5 may control the transcription factor’s activity, which in turn affects the MAPK pathway’s activity. Because of the 5’TOP structure, the transcription inhibitor rapamycin can control GAS5 in OC cells [70].
8. Long Non-Coding RNA LINC00152
Long intergenic non-coding RNA 00152 (lncRNA LINC00152) is an 828-bp lncRNA located on chromosome 2p11.2. Many malignancies, including colorectal, liver, gastric, breast, ovarian, lung, pleural, and glioblastoma, express it aberrantly [71,72,73,74].
When compared to normal tissue, epithelial ovarian cancer tissue exhibits a considerable increase in LINC00152 expression. In SKOV3 cells, LINC00152 controls both cell cycle and proliferation [75]. LINC00152 mediates cell proliferation, thereby affecting MCL-1 expression and mitochondrial apoptosis pathways via MCL-1, and acts as a competitive endogenous RNA (ceRNA) of miR-125b, which may represent a novel molecular mechanism for reversing cell proliferation in ovarian cancer [76]. Furthermore, the cisplatin sensitivity of epithelial ovarian cancer cells is enhanced by LINC00152 knockdown, which lowers MDR1, MRP1, and GST expression levels and enhances apoptosis [77]. Additionally, it is thought that LINC00152 can attach to B-cell lymphoma 6 (BCL6)’s Ser333/Ser343 and stabilize it against ubiquitination to encourage the growth and invasion of ovarian tumors [78].
9. Pseudogenes
Pseudogenes, or faulty copies of genes produced during genome evolution, are referred to as “junk DNA” [79]. Only two-thirds of the nearly 18,000 pseudogenes found in the human genome are transcribed [80]. It is possible for pseudogenes to control both transcriptional and post-transcriptional aspects of gene expression. In ovarian cancer, the dysregulation of many pseudogenes has been reported [81]. Nuclear proteins known as high-mobility group A (HMGA) aid in the construction of nucleoprotein complexes related to transcription, gene replication, and chromatin architecture. Proto-oncogenic competitive endogenous RNAs are HMGA1P6 and HMGA1P7 [82]. High-mobility group AT-hook 1 pseudogene 6, or HMGA1P6, is overexpressed in HGSOC and exhibits an inverse relationship with patient survival. By functioning as a competitive endogenous RNA (ceRNA) and causing an increase in the production of HMGA1 and HMGA2, HMGA1P6 mechanically increases the malignancy of ovarian cancer cells. In ovarian cancer, the MYC oncogene transcriptionally activates HMGA1P6 [83]. The overexpression of HMGA1/2 is found to be inversely associated with miRNAs [84].
10. Circular RNAs
Circular RNAs (CircRNAs) are single-stranded RNAs covalently linked at the 5′ and 3′ ends that are generated by back-splicing from pre-mRNA [85]. CircRNAs most often act through sponging microRNAs (miRNAs) to influence target gene expression. They also interact with proteins to modulate their activity and regulate host gene transcription [86,87,88]. The dysregulation of circRNA causes the development of many diseases, especially cancer [89]. Many circRNAs are dysregulated in EOC and can be used as diagnostic and prognostic markers. We described selected circRNAs dysregulated in OC in Table 1, focusing on their level in OC and clinical significance.

Table 1.
Selected dysregulated circRNAs in OC.
11. Treatment of Ovarian Cancer with lncRNA-Modulating Drugs
A clinical trial focuses on the innovative combination of two targeted inhibitors, Palbociclib and Bevacizumab, using long non-coding RNAs (lncRNA) as biomarkers for colorectal, lung, breast, and ovarian cancers. Palbociclib is a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor that disrupts cell cycle progression, and Bevacizumab, on the other hand, is an inhibitor of vascular endothelial growth factor (VEGF), which promotes angiogenesis. The mechanisms of both drugs complement each other, and lncRNAs are used in predicting treatment response and prognosis [102]. LncRNA CTD-2589M5 is coexpressed with most multidrug resistance genes, namely ABCB1, ABCB4, ABCC3, and ABCG2, showing a role in multidrug resistance in ovarian cancer [103]. Information about the mechanisms of action and clinical significance of lncRNAs is listed in Table 2.

Table 2.
Summary of information on selected long non-coding RNAs in ovarian cancer.
12. Conclusions
- Some LncRNAs such as lncRNA NEAT1 and SOX21-AS1 can be used in the diagnosis of OC; however, further research and standardization are necessary.
- LncRNA SOx21-AS1, NEAT1 and GAS5 can also be used as indicators of poor prognosis in OC, but further research and standardization are also necessary.
- Treatment with lncRNA H19, MAFG-AS1 or GAS5 is a promising treatment for ovarian cancer, although further research is needed in this area. Unfortunately, the heterogeneity of epithelial ovarian cancer may constitute a limitation in the development of an effective treatment method using lncRNA.
- Resistance to drugs such as cisplatin remains the main problem in the treatment of OC. Although HGSOC is at least initially susceptible to platinum-based drugs, this is not the case for low-grade serous ovarian cancers or clear cell ovarian cancers. LncRNA NEAT1 and H19 may prove helpful in the fight against chemotherapy resistance, but further research is necessary.
A limitation of the study may be the fact that most studies refer to cell lines. The profile and activity of different lncRNAs differ significantly; therefore, it is necessary to better understand the mechanisms of action of each lncRNA.
Author Contributions
Conceptualization, A.G. and M.K.; methodology, A.G.; software, A.G.; validation, A.G. and M.K.; formal analysis, A.G.; investigation, A.G.; resources, A.G.; data curation, M.K.; writing—original draft preparation, A.G.; writing—review and editing, A.G.; visualization, A.G.; supervision, M.K.; project administration, A.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shih, I.-M.; Wang, Y.; Wang, T.-L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer. 2022, 151, 1535–1541. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B. Ovarian Cancer Biomarkers: Moving Forward in Early Detection. Adv. Exp. Med. Biol. 2020, 1219, 355–363. [Google Scholar] [PubMed]
- American Academy of Obstetrics and Gynecology. Committee Opinion No. 716 Summary: The Role of the Obstetrician-Gynecologist in the Early Detection of Epithelial Ovarian Cancer in Women at Average Risk. Obstet. Gynecol. 2017, 103, 225–230. [Google Scholar]
- Goff, B.A.; Mandel, L.S.; Drescher, C.W.; Urban, N.; Gough, S.; Schurman, K.M.; Patras, J.; Mahony, B.S.; Andersen, M.R. Development of an ovarian cancer symptom index: Possibilities for earlier detection. Cancer 2007, 62, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Tian, C.; Kesterson, J.P.; Monk, B.J.; Kapp, D.S.; Davidson, B.; Robertson, S.; Copeland, L.J.; Walker, J.L.; Wenham, R.M.; et al. Symptoms of Women With High-Risk Early-Stage Ovarian Cancer. Obstet. Gynecol. 2022, 139, 157–162. [Google Scholar] [CrossRef]
- Petousis, S.; Chatzakis, C.; Westerway, S.C.; Abramowicz, J.S.; Dinas, K.; Dong, Y.; Dietrich, C.F.; Sotiriadis, A. World Federation for Ultrasound in Medicine Review Paper: Incidental Findings during Obstetrical Ultrasound. Ultrasound Med. Biol. 2022, 48, 10–19. [Google Scholar] [CrossRef]
- Sánchez-Lorenzo, L.; Salas-Benito, D.; Villamayor, J.; Patiño-García, A.; González-Martín, A. The BRCA Gene in Epithelial Ovarian Cancer. Cancers 2022, 14, 1235. [Google Scholar] [CrossRef]
- Hinchcliff, E.; Westin, S.N.; Herzog, T.J. State of the science: Contemporary front-line treatment of advanced ovarian cancer. Gynecol. Oncol. 2022, 166, 18–24. [Google Scholar] [CrossRef]
- Lee, A. Niraparib: A Review in First-Line Maintenance Therapy in Advanced Ovarian Cancer. Target. Oncol. 2021, 16, 839–845. [Google Scholar] [CrossRef]
- Fabbro, M.; Colombo, P.-E.; Leaha, C.M.; Rouanet, P.; Carrère, S.; Quenet, F.; Gutowski, M.; Mourregot, A.; D’Hondt, V.; Coupier, I.; et al. Conditional Probability of Survival and Prognostic Factors in Long-Term Survivors of High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Ryan, A.; Singh, N.; Manchanda, R.; Kalsi, J.K.; Woolas, R.; Arora, R.; Casey, L. Tumour stage, treatment, and survival of women with high-grade serous tubo-ovarian cancer in UKCTOCS: An exploratory analysis of a randomised controlled trial. Lancet. Oncol. 2023, 24, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Balihodzic, A.; Prinz, F.; Dengler, M.A.; Calin, G.A.; Jost, P.J.; Pichler, M. Non-coding RNAs and ferroptosis: Potential implications for cancer therapy. Cell Death Differ. 2022, 29, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.; Parveen, R.; Fouad, H.; Yahia, M.E.; Hassanein, A.S. Role of different non-coding RNAs as ovarian cancer biomarkers. J. Ovarian Res. 2022, 15, 72. [Google Scholar] [CrossRef]
- Kim, J.; Piao, H.-L.; Kim, B.-J.; Yao, F.; Han, Z.; Wang, Y.; Xiao, Z.; Siverly, A.N.; Lawhon, S.E.; Ton, B.N.; et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018, 50, 1705–1715. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Grammatikakis, I.; Lal, A. Significance of lncRNA abundance to function. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2022, 33, 271–280. [Google Scholar] [CrossRef]
- Ni, J.; Lu, J.; Lu, D. Abnormal expression and clinical value analysis of long noncoding RNA cancer susceptibility candidate 2 in children with severe pneumonia complicated with respiratory failure. Clin. Respir. J. 2022, 16, 460–466. [Google Scholar] [CrossRef]
- Zhai, W.; Zhu, R.; Ma, J.; Gong, D.; Zhang, H.; Zhang, J.; Chen, Y.; Huang, Y.; Zheng, J.; Xue, W. A positive feed-forward loop between LncRNA-URRCC and EGFL7/P-AKT/FOXO3 signaling promotes proliferation and metastasis of clear cell renal cell carcinoma. Mol. Cancer. 2019, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Yuan, K.; Yan, X.; Xu, L.; Liao, H.; Hao, X.; Wang, J.; Liu, H.; Chen, X.; Xie, K.; et al. LncRNA SNHG10 Facilitates Hepatocarcinogenesis and Metastasis by Modulating Its Homolog SCARNA13 via a Positive Feedback Loop. Cancer Res. 2019, 79, 3220–3234. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, P.; Zhang, J.; Wu, H.; Sui, S.; Zhang, J.; Wang, Q.; Qiao, K.; Yang, W.; Xu, H.; et al. Ai-lncRNA EGOT enhancing autophagy sensitizes paclitaxel cytotoxicity via upregulation of ITPR1 expression by RNA-RNA and RNA-protein interactions in human cancer. Mol. Cancer 2019, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Guo, C.; Xia, T.; Zhang, R.; Zen, K.; Pan, Y.; Jin, L. LncCCAT1 Promotes Breast Cancer Stem Cell Function through Activating WNT/β-catenin Signaling. Theranostics 2019, 9, 7384–7402. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Lu, B.; Gu, Y.; Chen, Q.; Lei, T.; Nie, F.; Gu, J.; Huang, J.; Wei, C.; et al. Up-regulated LINC01234 promotes non-small-cell lung cancer cell metastasis by activating VAV3 and repressing BTG2 expression. J. Hematol. Oncol. 2020, 13, 7. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, J.; Li, M.; Li, L.; Li, A. Long noncoding RNA NEAT1 promotes the growth of human retinoblastoma cells via regulation of miR-204/CXCR4 axis. J. Cell. Physiol. 2019, 234, 11567–11576. [Google Scholar] [CrossRef]
- Snyder, M.; Iraola-Guzmán, S.; Saus, E.; Gabaldón, T. Discovery and Validation of Clinically Relevant Long Non-Coding RNAs in Colorectal Cancer. Cancers 2022, 14, 3866. [Google Scholar] [CrossRef]
- Knutsen, E.; Harris, A.L.; Perander, M. Expression and functions of long non-coding RNA NEAT1 and isoforms in breast cancer. Br. J. Cancer 2022, 126, 551–561. [Google Scholar] [CrossRef]
- Li, K.; Yao, T.; Zhang, Y.; Li, W.; Wang, Z. NEAT1 as a competing endogenous RNA in tumorigenesis of various cancers: Role, mechanism and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 3428. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, L.; Yang, H.; Luo, K.; Qing, C. Long non-coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR-1321 and regulating tight junction protein 3 expression. Mol. Med. Rep. 2020, 22, 3429–3439. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Zhang, Z.; Xie, B.-B.; Zhang, H.-Y. Clinical significance of up-regulated lncRNA NEAT1 in prognosis of ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3373–3377. [Google Scholar]
- Chai, Y.; Liu, J.; Zhang, Z.; Liu, L. HuR-regulated lncRNA NEAT1 stability in tumorigenesis and progression of ovarian cancer. Cancer Med. 2016, 5, 1588–1598. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef]
- Ding, N.; Wu, H.; Tao, T.; Peng, E. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther. 2017, 10, 4905–4915. [Google Scholar] [CrossRef]
- Yong, W.; Yu, D.; Jun, Z.; Yachen, D.; Weiwei, W.; Midie, X.; Xingzhu, J.; Xiaohua, W. Long noncoding RNA NEAT1, regulated by LIN28B, promotes cell proliferation and migration through sponging miR-506 in high-grade serous ovarian cancer. Cell Death Dis. 2018, 9, 861. [Google Scholar] [CrossRef]
- Xu, H.; Sun, X.; Huang, Y.; Si, Q.; Li, M. Long non-coding RNA NEAT1 modifies cell proliferation, colony formation, apoptosis, migration and invasion via the miR-4500/BZW1 axis in ovarian cancer. Mol. Med. Rep. 2020, 22, 3347–3357. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, K.; Yang, L. LncRNA NEAT1 promotes proliferation of ovarian cancer cells and angiogenesis of co-incubated human umbilical vein endothelial cells by regulating FGF9 through sponging miR-365: An experimental study. Medicine 2021, 100, e23423. [Google Scholar] [CrossRef]
- An, J.; Lv, W.; Zhang, Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017, 10, 5377–5390. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Y. Long non-coding RNA NEAT1 facilitates the growth, migration, and invasion of ovarian cancer cells via the let-7 g/MEST/ATGL axis. Cancer Cell Int. 2021, 21, 437. [Google Scholar] [CrossRef]
- Wei, C.; Wang, H.; Xu, F.; Liu, Z.; Jiang, R. LncRNA SOX21-AS1 is associated with progression of hepatocellular carcinoma and predicts prognosis through epigenetically silencing p21. Biomed. Pharmacother. 2018, 104, 137–144. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, J.; Cao, B.; Jin, C. Long non-coding RNA SOX21-AS1: A potential tumor oncogene in human cancers. Pathol. Res. Pract. 2023, 249, 154774. [Google Scholar] [CrossRef]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 1990, 10, 28–36. [Google Scholar]
- Wang, L.; Cai, Y.; Zhao, X.; Jia, X.; Zhang, J.; Liu, J.; Zhen, H.; Wang, T.; Tang, X.; Liu, Y.; et al. Down-regulated long non-coding RNA H19 inhibits carcinogenesis of renal cell carcinoma. Neoplasma 2015, 62, 412–418. [Google Scholar] [CrossRef]
- Matouk, I.J.; DeGroot, N.; Mezan, S.; Ayesh, S.; Abu-lail, R.; Hochberg, A.; Galun, E. The H19 non-coding RNA is essential for human tumor growth. PLoS ONE 2007, 2, e845. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Wang, J.; Li, X.; Fan, Z.; Zhao, J.; Liu, L.; Zhang, M.; Goscinski, M.A.; Wang, J.; et al. High level of lncRNA H19 expression is associated with shorter survival in esophageal squamous cell cancer patients. Pathol. Res. Pract. 2019, 215, 152638. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, W.; Zou, H.; Lv, Q.; Shao, P. Circulating exosomal long non-coding RNA H19 as a potential novel diagnostic and prognostic biomarker for gastric cancer. J. Int. Med. Res. 2021, 48, 0300060520934297. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Yang, F. The role of long non-coding RNA H19 in breast cancer. Oncol. Lett. 2020, 19, 7–16. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Xu, H.; Suo, S.-S.; Xu, X.-L.; Ni, M.-W.; Gu, L.-H.; Chen, W.; Wang, L.-Y.; Zhao, Y.; Tian, B.; et al. The Essential Role of H19 Contributing to Cisplatin Resistance by Regulating Glutathione Metabolism in High-Grade Serous Ovarian Cancer. Sci. Rep. 2016, 6, 26093. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Deng, X.; Luo, M.; Wang, X.; Hu, H.; Liu, C.; Zhong, M. Long noncoding RNA H19 promotes transforming growth factor-β-induced epithelial-mesenchymal transition by acting as a competing endogenous RNA of miR-370-3p in ovarian cancer cells. Onco Targets Ther. 2018, 11, 427–440. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, Y.; Chen, W.; Chen, L.; Lu, J.; He, F.; Li, X.; Zhao, L. Ginsenoside 20(S)-Rg3 Prevents PKM2-Targeting miR-324-5p from H19 Sponging to Antagonize the Warburg Effect in Ovarian Cancer Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 1340–1353. [Google Scholar] [CrossRef]
- Sajadpoor, Z.; Amini-Farsani, Z.; Teimori, H.; Shamsara, M.; Sangtarash, M.H.; Ghasemi-Dehkordi, P.; Yadollahi, F. Valproic Acid Promotes Apoptosis and Cisplatin Sensitivity Through Downregulation of H19 Noncoding RNA in Ovarian A2780 Cells. Appl. Biochem. Biotechnol. 2018, 185, 1132–1144. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; He, J.; Sun, H.; Jin, Z. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int. J. Clin. Exp. Pathol. 2019, 12, 2506. [Google Scholar]
- Zhao, L.; Sun, W.; Zheng, A.; Zhang, Y.; Fang, C.; Zhang, P. Ginsenoside Rg3 suppresses ovarian cancer cell proliferation and invasion by inhibiting the expression of lncRNA H19. Acta Biochim. Pol. 2021, 68, 575–582. [Google Scholar] [CrossRef]
- Liu, J.; Zhai, C.; Liu, D.; Liu, J. The Long Noncoding RNA LOXL1-AS1 Promotes the Proliferation, Migration, and Invasion in Hepatocellular Carcinoma. Anal. Cell. Pathol. 2020, 2020, 4182092. [Google Scholar] [CrossRef]
- Tang, M.; Rong, Y.; Liu, S.; Wu, Z.; Ma, G.; Li, X.; Cai, H. Potential role of lncRNA LOXL1-AS1 in human cancer development: A narrative review. Transl. Cancer Res. 2024, 13, 1997–2011. [Google Scholar] [CrossRef]
- Liu, C.-N.; Zhang, H.-Y. Serum lncRNA LOXL1-AS1 is a diagnostic and prognostic marker for epithelial ovarian cancer. J. Gene Med. 2020, 22, e3233. [Google Scholar] [CrossRef]
- Su, D.; Deng, T.; Xie, M. LncRNALOXL1-AS1 Regulates the proliferation and apoptosis of ovarian cancer cells by targeting miR-761. Pak. J. Zool. 2022, 55, 1831–1837. [Google Scholar] [CrossRef]
- Xue, F.; Xu, Y.H.; Shen, C.C.; Qin, Z.L.; Zhou, H.B. Non-coding RNA LOXL1-AS1 exhibits oncogenic activity in ovarian cancer via regulation of miR-18b-5p/VMA21 axis. Biomed. Pharmacother. 2020, 125, 109568. [Google Scholar] [CrossRef]
- Ahmadi, M.; Morshedzadeh, F.; Ghaderian, S.M.H.; Mousavi, P.; Habibipour, L.; Peymani, M.; Abbaszadegan, M.R.; Ghafouri-Fard, S. Carcinogenic roles of MAFG-AS1 in human cancers. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2024, 26, 52–68. [Google Scholar] [CrossRef]
- Bai, Y.; Ren, C.; Wang, B.; Xue, J.; Li, F.; Liu, J.; Yang, L. LncRNA MAFG-AS1 promotes the malignant phenotype of ovarian cancer by upregulating NFKB1-dependent IGF1. Cancer Gene Ther. 2021, 29, 277–291. [Google Scholar] [CrossRef]
- Lin, G.; Liu, H.; Lin, J.; Liu, X.; Xu, L. Correlation between long non-coding RNA MAFG-AS1 and cancer prognosis: A meta-analysis. Front. Oncol. 2023, 13, 1286610. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5’-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol. Cell. Biol. 1998, 18, 6897–6909. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, Y.; Lyu, H.; Xu, X.; Wu, Q.; Liu, N.; Yin, Q.; Li, J.; Sheng, X. Long Noncoding RNA GAS5, Which Acts as a Tumor Suppressor via microRNA 21, Regulates Cisplatin Resistance Expression in Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, M.; Zou, Y.; Mao, M.; Shen, T.; Zhang, C.; Song, S.; Sun, M.; Zhang, S.; Wang, B.; et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol. Rep. 2015, 34, 3212–3221. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Li, Y.; Li, L.; Hou, W.; You, Z. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol. Rep. 2016, 36, 3241–3250. [Google Scholar] [CrossRef]
- Dong, Q.; Long, X.; Cheng, J.; Wang, W.; Tian, Q.; Di, W. LncRNA GAS5 suppresses ovarian cancer progression by targeting the miR-96-5p/PTEN axis. Ann. Transl. Med. 2021, 9, 1770. [Google Scholar] [CrossRef]
- McComas, K.N.; Torgeson, A.M.; Ager, B.J.; Hellekson, C.; Burt, L.M.; Maurer, K.A.; Werner, T.L.; Gaffney, D.K. The variable impact of positive lymph nodes in cervical cancer: Implications of the new FIGO staging system. Gynecol. Oncol. 2020, 156, 85–92. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Zheng, J.; Ning, N.; Tang, F.; Yang, Y.; Wang, Y. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol. Oncol. 2018, 151, 345–355. [Google Scholar] [CrossRef]
- Ma, N.; Li, S.; Zhang, Q.; Wang, H.; Qin, H.; Wang, S. Long non-coding RNA GAS5 inhibits ovarian cancer cell proliferation via the control of microRNA-21 and SPRY2 expression. Exp. Ther. Med. 2018, 16, 73–82. [Google Scholar] [CrossRef]
- Long, X.; Song, K.; Hu, H.; Tian, Q.; Wang, W.; Dong, Q.; Yin, X.; Di, W. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J. Exp. Clin. Cancer Res. 2019, 38, 345. [Google Scholar] [CrossRef]
- Brovkina, O.I.; Pronina, I.V.; Uroshlev, L.A.; Fridman, M.V.; Loginov, V.I.; Kazubskaya, T.P.; Utkin, D.O.; Kushlinskii, N.E.; Braga, E.A. Identification of Novel Differentially Expressing Long Non- Coding RNAs with Oncogenic Potential. Mol. Biol. 2021, 55, 548–554. [Google Scholar] [CrossRef]
- Wang, B.; Yang, S.; Zhao, W. Long Non-Coding RNA NRAD1 and LINC00152 are Highly Expressed and Associated with Prognosis in Patients with Hepatocellular Carcinoma. Onco Targets Ther. 2020, 13, 10409–10416. [Google Scholar] [CrossRef]
- Endo, I.; Amatya, V.J.; Kushitani, K.; Nakagiri, T.; Aoe, K.; Takeshima, Y. Long Non-coding RNA LINC00152 Requires EZH2 to Promote Mesothelioma Cell Proliferation, Migration, and Invasion. Anticancer Res. 2023, 43, 5367–5376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Su, H. Long non-coding RNA LINC00152/miR-613/CD164 axis regulates cell proliferation, apoptosis, migration and invasion in glioma via PI3K/AKT pathway. Neoplasma 2020, 67, 762. [Google Scholar] [CrossRef]
- Ni, H.; Niu, L.-L.; Tian, S.-C.; Jing, L.-K.; Zhang, L.-T.; Lin, Q.-Q.; Cai, Y.-H.; Liang, H.-M.; Du, Q.; Li, H. Long non-coding RNA LINC00152 is up-regulated in ovarian cancer tissues and regulates proliferation and cell cycle of SKOV3 cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9803–9813. [Google Scholar]
- Chen, P.; Fang, X.; Xia, B.; Zhao, Y.; Li, Q.; Wu, X. Long noncoding rna Linc00152 promotes cell proliferation through competitively binding endogenous mir-125b with mcl-1 by regulating mitochondrial apoptosis pathways in ovarian cancer. Cancer Med. 2018, 7, 4530–4541. [Google Scholar] [CrossRef]
- Zou, H.; Li, H. Knockdown of long non-coding rna Linc00152 increases cisplatin sensitivity in ovarian cancer cells. Exp. Ther. Med. 2019, 18, 4510–4516. [Google Scholar] [CrossRef]
- Wang, S.; Weng, W.; Chen, T.; Xu, M.; Wei, P.; Li, J.; Lu, L.; Wang, Y. LINC00152 Promotes Tumor Progression and Predicts Poor Prognosis by Stabilizing BCL6 From Degradation in the Epithelial Ovarian Cancer. Front. Oncol. 2020, 10, 555132. [Google Scholar] [CrossRef]
- Pink, R.C.; Wicks, K.; Caley, D.P.; Punch, E.K.; Jacobs, L.; Carter, D.R.F. Pseudogenes: Pseudo-functional or key regulators in health and disease? RNA 2011, 17, 792–798. [Google Scholar] [CrossRef]
- Poliseno, L. Pseudogenes: Newly discovered players in human cancer. Sci. Signal. 2012, 5, re5. [Google Scholar] [CrossRef]
- Hu, X.; Yang, L.; Mo, Y.-Y. Role of Pseudogenes in Tumorigenesis. Cancers 2018, 10, 256. [Google Scholar] [CrossRef]
- Esposito, F.; De Martino, M.; Petti, M.G.; Forzati, F.; Tornincasa, M.; Federico, A.; Arra, C.; Pierantoni, G.M.; Fusco, A. HMGA1 pseudogenes as candidate proto-oncogenic competitive endogenous RNAs. Oncotarget 2014, 5, 8341–8354. [Google Scholar] [CrossRef]
- Tian, X.; Song, J.; Zhang, X.; Yan, M.; Wang, S.; Wang, Y.; Xu, L.; Zhao, L.; Wei, J.-J.; Shao, C. MYC-regulated pseudogene HMGA1P6 promotes ovarian cancer malignancy via augmenting the oncogenic HMGA1/2. Cell Death Dis. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, M.; Agostini, A.; Staurseth, J.; Davidson, B.; Heim, S.; Micci, F. Molecular characterization of carcinosarcomas arising in the uterus and ovaries. Oncotarget 2019, 10, 3614–3624. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.-S.; Ai, Y.; Wilusz, J.E. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020, 30, 226–240. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Du, W.W.; Zhang, C.; Yang, W.; Yong, T.; Awan, F.M.; Yang, B.B. Identifying and Characterizing circRNA-Protein Interaction. Theranostics 2017, 7, 4183. [Google Scholar] [CrossRef]
- Yang, Q.; Li, F.; He, A.T.; Yang, B.B. Circular RNAs: Expression, localization, and therapeutic potentials. Mol. Ther. 2021, 29, 1683–1702. [Google Scholar] [CrossRef]
- Verduci, L.; Tarcitano, E.; Strano, S.; Yarden, Y.; Blandino, G. CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 2021, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, J.; Zhang, L.-Y.; Wang, L. CircHIPK3 is upregulated and predicts a poor prognosis in epithelial ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3713–3718. [Google Scholar] [PubMed]
- Ge, L.; Sun, Y.; Shi, Y.; Liu, G.; Teng, F.; Geng, Z.; Chen, X.; Xu, H.; Xu, J.; Jia, X. Plasma circRNA microarray profiling identifies novel circRNA biomarkers for the diagnosis of ovarian cancer. J. Ovarian Res. 2022, 15, 58. [Google Scholar] [CrossRef]
- Ning, L.; Lang, J.; Wu, L. Plasma circN4BP2L2 is a promising novel diagnostic biomarker for epithelial ovarian cancer. BMC Cancer 2022, 22, 6. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, L.; Zou, X. Circular RNA circ-BNC2 (hsa_circ_0008732) inhibits the progression of ovarian cancer through microRNA-223-3p/ FBXW7 axis. J. Ovarian Res. 2022, 15, 95. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Qiu, Q.; Hou, L.; Wu, M.; Li, J.; Li, X.; Lu, B.; Cheng, X.; Liu, P.; et al. CircPLEKHM3 acts as a tumor suppressor through regulation of the miR-9/BRCA1/DNAJB6/KLF4/AKT1 axis in ovarian cancer. Mol. Cancer 2019, 18, 144. [Google Scholar] [CrossRef]
- Wu, M.; Qiu, Q.; Zhou, Q.; Li, J.; Yang, J.; Zheng, C.; Luo, A.; Li, X.; Zhang, H.; Cheng, X.; et al. circFBXO7/miR-96-5p/MTSS1 axis is an important regulator in the Wnt signaling pathway in ovarian cancer. Mol. Cancer 2022, 21, 137. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, C.; Liu, Y.; Wang, M. Circular RNA profiling reveals circRNA1656 as a novel biomarker in high grade serous ovarian cancer. Biosci. Trends 2019, 13, 204–211. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, Y.; Shi, X.; Shen, F.; Chen, M.; Chen, Y.; Wang, J. Hsa_circ_0001445 works as a cancer suppressor via miR-576-5p/SFRP1 axis regulation in ovarian cancer. Cancer Med. 2023, 12, 5736–5750. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, H. The molecular mechanism of circRHOBTB3 inhibits the proliferation and invasion of epithelial ovarian cancer by serving as the ceRNA of miR-23a-3p. J. Ovarian Res. 2022, 15, 66. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H. Circ_0078607 inhibits the progression of ovarian cancer via regulating the miR-32-5p/SIK1 network. J. Ovarian Res. 2022, 15, 3. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Shen, Q.; Chen, Q.; Zhu, X.-J.; Jiang, S.-S.; Zhang, Q. CircRNA_MYLK promotes malignant progression of ovarian cancer through regulating microRNA-652. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5281–5291. [Google Scholar]
- Chen, Y.; Ye, X.; Xia, X.; Lin, X. Circular RNA ABCB10 correlates with advanced clinicopathological features and unfavorable survival, and promotes cell proliferation while reduces cell apoptosis in epithelial ovarian cancer. Cancer Biomark. 2019, 26, 151–161. [Google Scholar] [CrossRef]
- Makdissy, N. Precision Therapy for Solid Tumors: Synergistic CDK4/6 Inhibition and Anti-VEGF Targeting LncRNA (PTST_PALBEVA); Lebanese University: Beirut, Lebanon, 2024. [Google Scholar]
- Xu, J.; Wu, J.; Fu, C.; Teng, F.; Liu, S.; Dai, C.; Shen, R.; Jia, X. Multidrug resistant lncRNA profile in chemotherapeutic sensitive and resistant ovarian cancer cells. J. Cell. Physiol. 2017, 233, 5034–5043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).