Sirtuin 1 Inhibits Fatty Acid Synthesis through Forkhead Box Protein O1-Mediated Adipose Triglyceride Lipase Expression in Goat Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
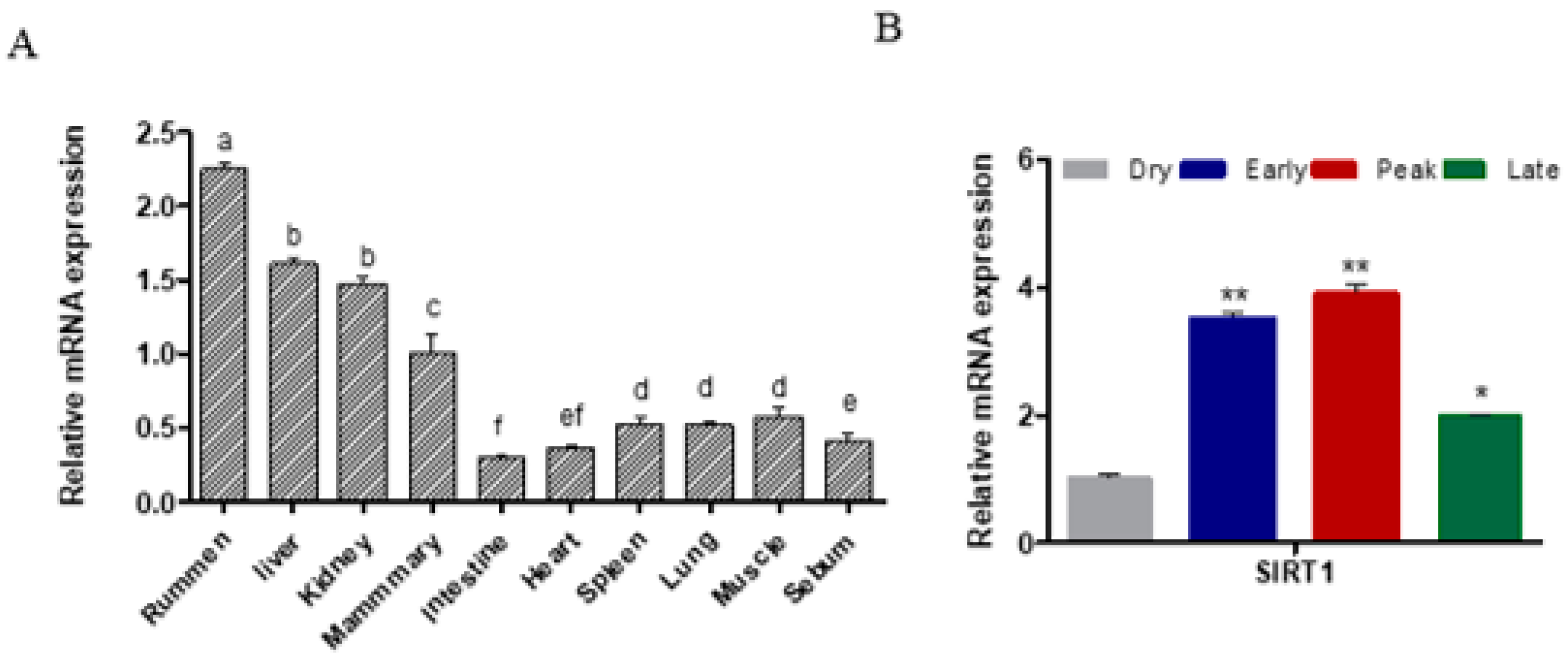
2.1. SIRT1 Expression across Different Tissues and Lactation Stages
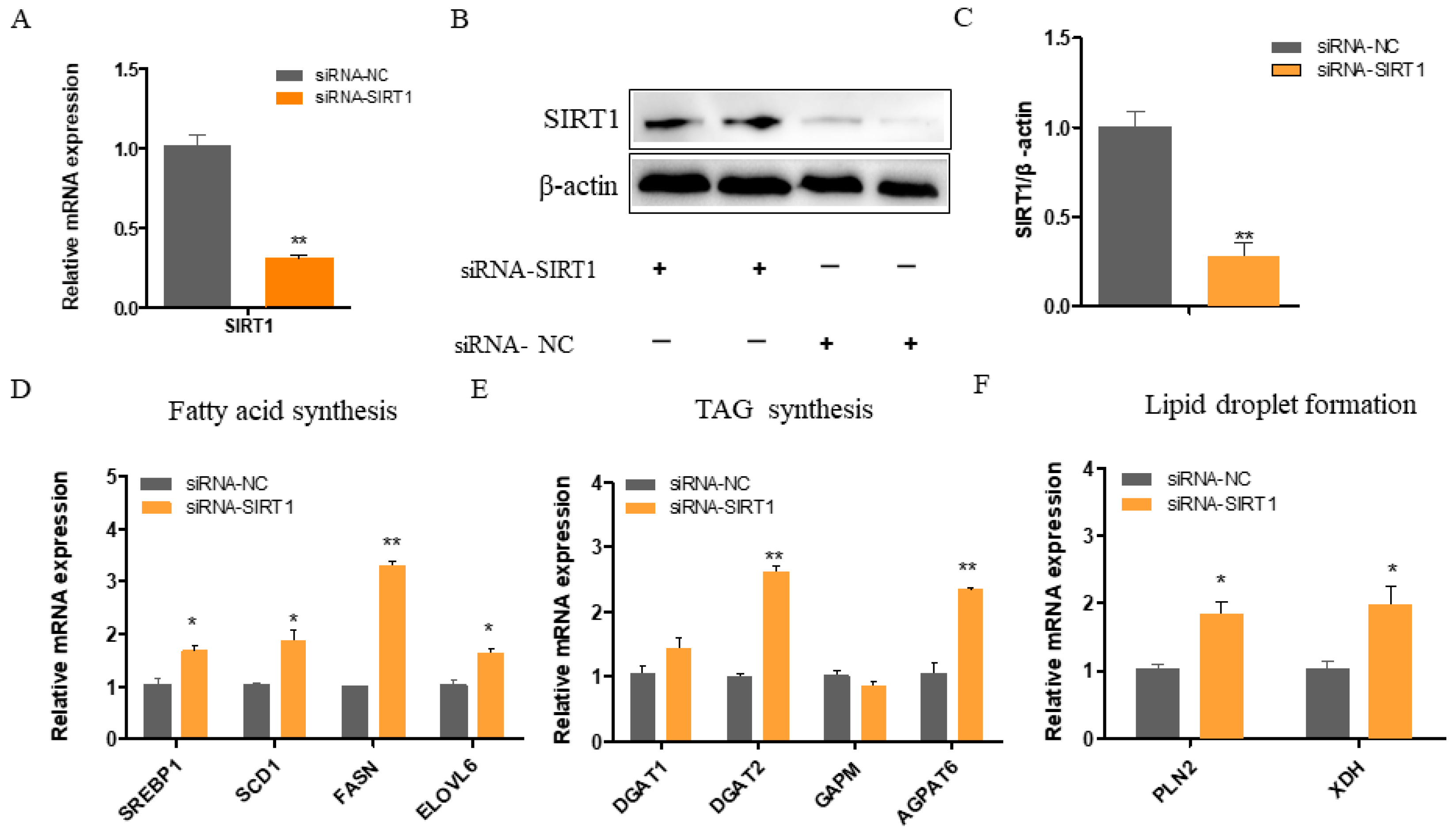
2.2. SIRT1 Deficiency Increases the Expressions of Genes Involved in Fatty Acid Synthesis, TAG Synthesis, and Lipid Droplet Formation
2.3. SIRT1 Overexpression Inhibits Genes Related to Fatty Acid Metabolism and Lipid Accumulation in GMECs
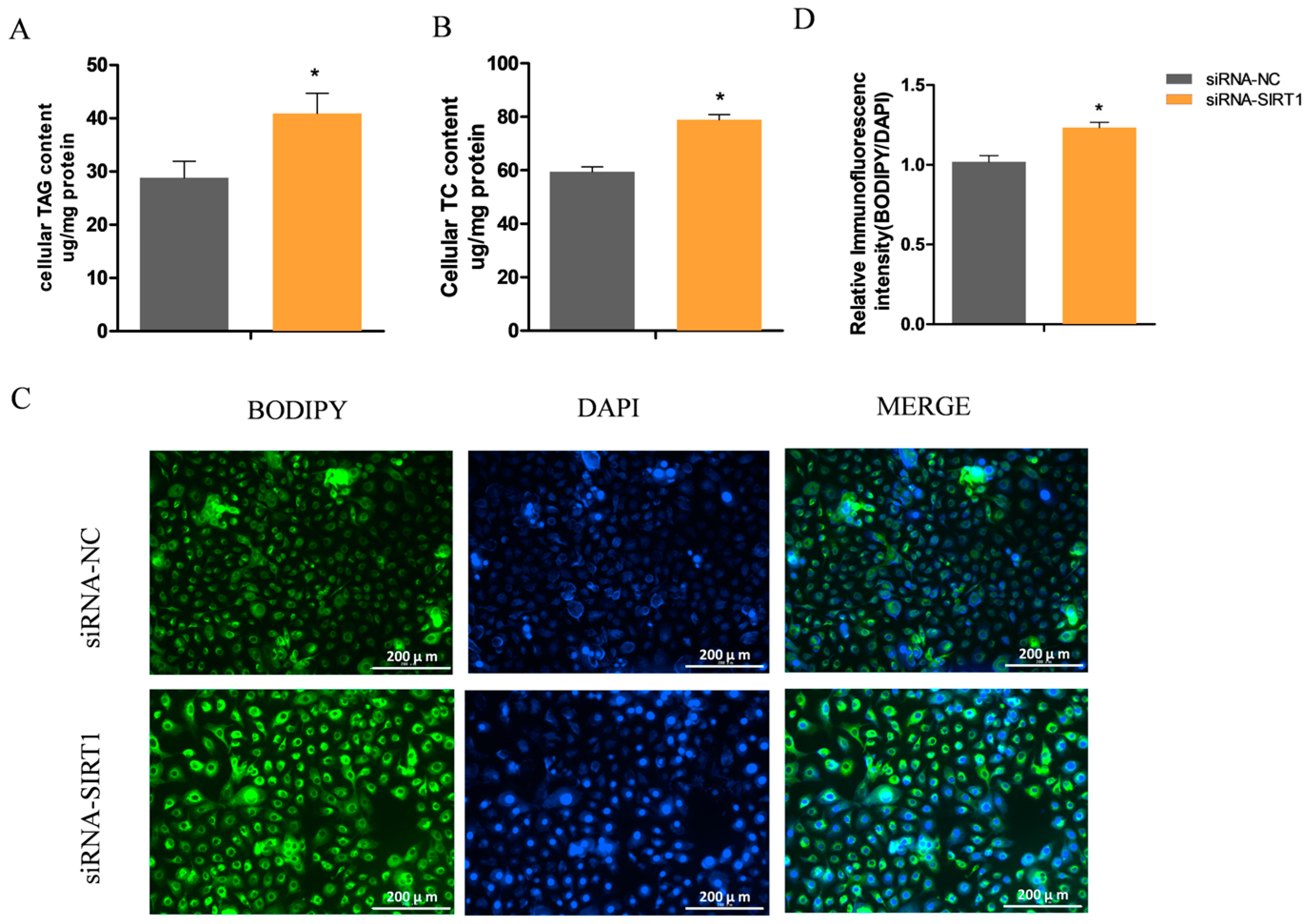
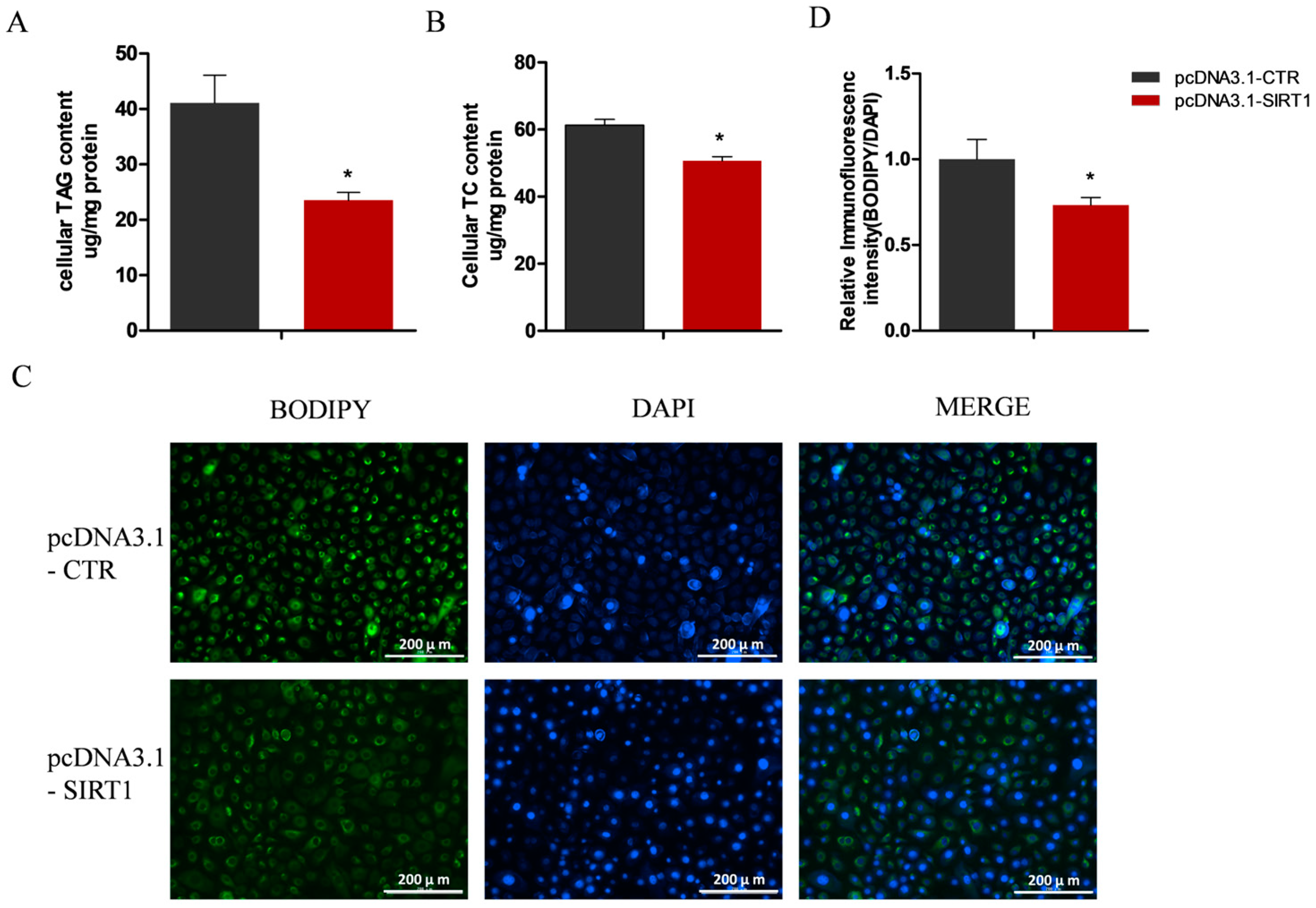
2.4. Manipulating SIRT1 Alters Intracellular TAG and Lipid Droplet Levels
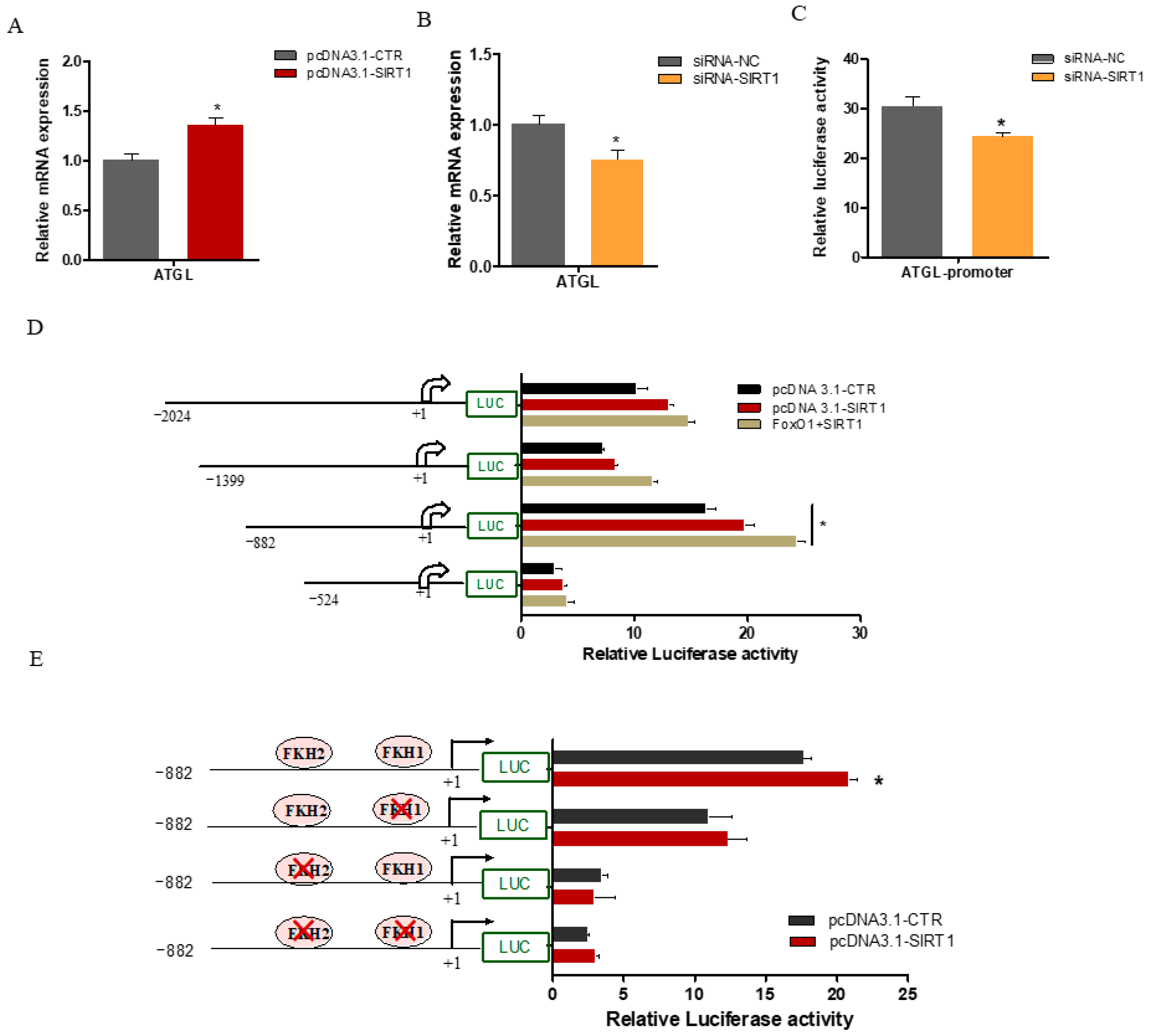
2.5. SIRT1 Enhanced ATGL Promoter Activity via FOXO1 Binding Sites
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Collection
4.2. Cell Culture
4.3. Overexpression Vector Construction
4.4. RNA Interference Experiment
4.5. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.6. Western Blot
4.7. Cellular TAG and Cholesterol Analysis
4.8. BODIPY Staining
4.9. Cell Transfection and Luciferase Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Niu, H.; Tian, X.; Tian, H.; Yao, W.; He, H.; Shi, H.; Li, C.; Luo, J. Goat Milk Improves Glucose Metabolism in Type 2 Diabetic Mice and Protects Pancreatic Beta-Cell Functions. Mol. Nutr. Food Res. 2024, 68, e2200842. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jiang, X.; Ma, H.; Wang, Y.; Liu, Y. SIRT1 and insulin resistance. J. Diabetes Complicat. 2016, 30, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, T.; Peng, J.; Zhou, Z.; Wei, H.; Zhou, R.; Jiang, S.; Peng, J. SIRT1 suppresses adipogenesis by activating Wnt/β-catenin signaling in vivo and in vitro. Oncotarget 2016, 7, 77707–77720. [Google Scholar] [CrossRef]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef]
- Motta, M.C.; Divecha, N.; Lemieux, M.; Kamel, C.; Chen, D.; Gu, W.; Bultsma, Y.; Mcburney, M.; Guarente, L. Mammalian SIRT1 Represses Forkhead Transcription Factors. Cell Press. 2004, 116, 551–563. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.J.; Topark-Ngarm, A.; Senawong, T.; Oliveira, D. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Q.; Zhang, R.; Du, Q.; Hu, J.; Gao, T.; Gao, S.; Fan, K.; Cheng, X.; Yan, S.; et al. Nobiletin alleviates atherosclerosis by inhibiting lipid uptake via the PPARG/CD36 pathway. Lipids Health Dis. 2024, 23, 76. [Google Scholar] [CrossRef]
- Pham, T.H.; Lee, G.H.; Jin, S.W.; Lee, S.Y.; Han, E.H.; Kim, N.D.; Jeong, H.G. Puerarin attenuates hepatic steatosis via G-protein-coupled estrogen receptor-mediated calcium and SIRT1 signaling pathways. Phytother. Res. PTR 2022, 36, 3601–3618. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Z.; Wang, J. Tetrahydropalmatine ameliorates hepatic steatosis in nonalcoholic fatty liver disease by switching lipid metabolism via AMPK-SREBP-1c-Sirt1 signaling axis. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 119, 155005. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xie, X.; Wang, N.; Wang, Y.; Zhao, J.; Chen, F.; Qu, F.; Wen, W.; Miao, J.; Cui, H. Formononetin promotes fatty acid β-oxidation to treat non-alcoholic steatohepatitis through SIRT1/PGC-1α/PPARα pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 124, 155285. [Google Scholar] [CrossRef] [PubMed]
- Trites, M.J.; Clugston, R.D. The role of adipose triglyceride lipase in lipid and glucose homeostasis: Lessons from transgenic mice. Lipids Health Dis. 2019, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yang, P.; Han, H.; Zhang, G.; Xu, H.; Quan, K. Overexpression of ATGL impairs lipid droplet accumulation by accelerating lipolysis in goat mammary epithelial cells. Anim. Biotechnol. 2022, 34, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gao, L.; Zhang, F.; Yao, W.; Wu, J.; Song, N.; Luo, J.; Zhang, Y. The FoxO1-ATGL axis alters milk lipolysis homeostasis through PI3K/AKT signaling pathway in dairy goat mammary epithelial cells. J. Anim. Sci. 2023, 101, skad286. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; English, T.; Karki, S.; Qiang, L.; Tao, R.; Kim, J.; Luo, Z.; Farmer, S.R.; Kandror, K.V. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 2011, 52, 1693–1701. [Google Scholar] [CrossRef]
- Bernard, L.; Rouel, J.; Leroux, C.; Ferlay, A.; Faulconnier, Y.; Legrand, P.; Chilliard, Y. Mammary lipid metabolism and milk fatty acid secretion in alpine goats fed vegetable lipids. J. Dairy. Sci. 2005, 88, 1478–1489. [Google Scholar] [CrossRef]
- Clark, S.; Mora García, M.B. A 100-Year Review: Advances in goat milk research. J. Dairy. Sci. 2017, 100, 10026–10044. [Google Scholar] [CrossRef]
- Lajnaf, R.; Feki, S.; Ben, A.S.; Attia, H.; Kammoun, T.; Ayadi, M.A.; Masmoudi, H. Recent advances in selective allergies to mammalian milk proteins not associated with Cow’s Milk Proteins Allergy. Food Chem. Toxico 2023, 178, 113929. [Google Scholar] [CrossRef]
- Sakkas, L.; Evageliou, V.; Igoumenidis, P.E.; Moatsou, G. Properties of Sweet Buttermilk Released from the Churning of Cream Separated from Sheep or Cow Milk or Sheep Cheese Whey: Effect of Heat Treatment and Storage of Cream. Foods 2022, 11, 465. [Google Scholar] [CrossRef]
- Tian, H.; Luo, J.; Zhang, Z.; Wu, J.; Zhang, T.; Busato, S.; Huang, L.; Song, N.; Bionaz, M. CRISPR/Cas9-mediated Stearoyl-CoA Desaturase 1 (SCD1) Deficiency Affects Fatty Acid Metabolism in Goat Mammary Epithelial Cells. J. Agric. Food Chem. 2018, 66, 10041–10052. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Tsukamoto, K.; Shimizu, T.; Hara, M.; Yatomi, Y. Apolipoprotein M/sphingosine 1-phosphate protects against diabetic nephropathy. Transl. Res. 2023, 258, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Shen, M.; Kuang, L.; Yang, K.; Wu, S.; Liu, X.; Wang, Y.; Wang, Y. SIRT1/SREBPs-mediated regulation of lipid metabolism. Pharmacol. Res. 2024, 199, 107037. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating miR-34a In Vitro. Int. J. Mol. Sci. 2021, 22, 13163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Wang, J.; Yang, L.; Yu, X.; Huang, P.; Song, H.; Zheng, P. Salvia-Nelumbinis naturalis improves lipid metabolism of NAFLD by regulating the SIRT1/AMPK signaling pathway. BMC Complement. Med. Ther. 2022, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Castro-Navarro, I.; McGuire, M.A.; Williams, J.E.; Holdsworth, E.A.; Meehan, C.L.; McGuire, M.K. Maternal Cannabis Use during Lactation and Potential Effects on Human Milk Composition and Production: A Narrative Review. Adv. Nutr. 2024, 15, 100196. [Google Scholar] [CrossRef]
- Yasar, C.; Oguzhan, O.; Ebubekir, S.; Ilyas, T.; Elif, Y.; Gupse, A.; Levent, D.; Yilmaz, E.F. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med. Sci. Monit. 2011, 17, HY5–HY9. [Google Scholar]
- Meng, D.; Zhang, F.; Yu, W.; Zhang, X.; Yin, G.; Liang, P.; Feng, Y.; Chen, S.; Liu, H. Biological Role and Related Natural Products of SIRT1 in Nonalcoholic Fatty Liver. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 4043–4064. [Google Scholar] [CrossRef]
- Yao, D.; Luo, J.; He, Q.; Shi, H.; Li, J.; Wang, H.; Xu, H.; Chen, Z.; Yi, Y.; Loor, J.J. SCD1 Alters Long-Chain Fatty Acid (LCFA) Composition and Its Expression Is Directly Regulated by SREBP-1 and PPARγ 1 in Dairy Goat Mammary Cells. J. Cell Physiol. 2016, 232, 635–649. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, W.; Ming, R.; Deng, Q.; Zuo, A.; Zhang, S.; Ying, Y.; Zhao, Y.; Ma, J. Noninvasive 40-Hz Light Flicker Rescues Circadian Behavior and Abnormal Lipid Metabolism Induced by Acute Ethanol Exposure. Front. Pharmacol. 2020, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, S.; Noh, R.M.; Daga, S.; Langrish, J.P.; Newby, D.E. Cardiovascular Effects of a Novel SIRT1 Activator, SRT2104, in Otherwise Healthy Cigarette Smokers. Randomized Control. Trial 2013, 2, e000042. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, C.; Niu, Y.; Chi, D.; Xu, M.; Si, L.; Qu, X.; Li, J. The influence of delipidation on triglyceride and LIPIN1 of porcine embryos derived from parthenogenetic activation. Reprod. Domest. Anim. Zuchthyg. 2017, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Hu, M.; Liang, X.; Ajmo, J.M.; Li, X.; Bataller, R.; Odena, G.; Stevens, S.M., Jr.; You, M. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology 2014, 146, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Fan, X.; Miao, Y. LPIN1 promotes triglycerides synthesis and is transcriptionally regulated by PPARG in buffalo mammary epithelial cells. Sci. Rep. 2022, 12, 2390. [Google Scholar] [CrossRef] [PubMed]
- Bhatt-Wessel, B.; Jordan, T.W.; Miller, J.H.; Peng, L. Role of DGAT enzymes in triacylglycerol metabolism. Arch. Biochem. Biophys. 2018, 655, 1–11. [Google Scholar] [CrossRef]
- Littlejohn, M.D.; Tiplady, K.; Lopdell, T.; Law, T.A.; Scott, A.; Harland, C.; Sherlock, R.; Henty, K.; Obolonkin, V.; Lehnert, K.; et al. Expression variants of the lipogenic AGPAT6 gene affect diverse milk composition phenotypes in Bos taurus. PLoS ONE 2014, 9, e85757. [Google Scholar] [CrossRef]
- Viale, E.; Tiezzi, F.; Maretto, F.; De Marchi, M.; Penasa, M.; Cassandro, M. Association of candidate gene polymorphisms with milk technological traits, yield, composition, and somatic cell score in Italian Holstein-Friesian sires. J. Dairy. Sci. 2017, 100, 7271–7281. [Google Scholar] [CrossRef]
- Chong, B.M.; Reigan, P.; Mayle-Combs, K.D.; Orlicky, D.J.; McManaman, J.L. Determinants of adipophilin function in milk lipid formation and secretion. Trends Endocrinol. Metab. 2011, 22, 211–217. [Google Scholar] [CrossRef][Green Version]
- Sathyanarayan, A.; Mashek, M.T.; Mashek, D.G. ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism. Cell Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Zhou, R.; Yi, L.; Ye, X.; Zeng, X.; Liu, K.; Qin, Y.; Zhang, Q.; Mi, M. Resveratrol Ameliorates Lipid Droplet Accumulation in Liver Through a SIRT1/ ATF6-Dependent Mechanism. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 2397–2420. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Deol, K.K.; Mathiowetz, A.J.; Lange, M.; Leto, D.E.; Stevenson, J.; Hashemi, S.H.; Morgens, D.W.; Easter, E.; Heydari, K.; et al. Parallel CRISPR-Cas9 screens identify mechanisms of PLIN2 and lipid droplet regulation. Dev. Cell 2023, 58, 1782–1800.e1710. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, A.I.; Li, Y.; Khanal, P.; Hjorth, M.; Kolset, S.O.; Norheim, F.A.; Kimmel, A.R.; Dalen, K.T. Altered hepatic lipid droplet morphology and lipid metabolism in fasted Plin2-null mice. J. Lipid Res. 2023, 64, 100461. [Google Scholar] [CrossRef] [PubMed]
- Schott, M.B.; Weller, S.G.; Schulze, R.J.; Krueger, E.W.; Mcniven, M.A. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 2019, 218, 3320–3335. [Google Scholar] [CrossRef] [PubMed]
- Jalgaonkar, M.P.; Parmar, U.M.; Kulkarni, Y.A.; Oza, M.J. SIRT1-FOXOs activity regulates diabetic complications. Pharmacol. Res. 2022, 175, 106014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, Y.; Luo, J.; Wu, M.; Li, J.; Cao, Y. Specificity Protein 1 Regulates Gene Expression Related to Fatty Acid Metabolism in Goat Mammary Epithelial Cells. Int. J. Mol. Sci. 2015, 16, 1806–1820. [Google Scholar] [CrossRef]
- Shi, H.; Shi, H.; Luo, J.; Wang, W.; Haile, A.B.; Xu, H.; Li, J. Establishment and characterization of a dairy goat mammary epithelial cell line with human telomerase (hT-MECs). Anim. Sci. J. 2014, 85, 735–743. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, J.; Wang, W.; Zhao, W.; Lin, X. Characterization and culture of isolated primary dairy goat mammary gland epithelial cells. Chin. J. Biotechnol. 2010, 26, 1123–1127. [Google Scholar]

| Gene | Primer Sequence (5′-3′) | Size (bp) | |
|---|---|---|---|
| UXT | F R | CAGCTGGCCAAATACCTTCAA GTGTCTGGGACCACTGTGTCAA | 125 |
| RPS9 | F R | CCTCGACCAAGAGCTGAAG CCTCCAGACCTCACGTTTGTTC | 64 |
| FASN | F R | GGGCTCCACCACCGTGTTCCA GCTCTGCTGGGCCTGCAGCTG | 226 |
| SCD1 | F R | CCATCGCCTGTGGAGTCAC GTCGGATAAATCTAGCGTAGCA | 257 |
| ELOVL6 | F R | GGAAGCCTTTAGTGCTCTGGTC ATTGTATCTCCTAGTTCGGGTGC | 205 |
| XDH | F R | GATCATCCACTTTTCTGCCAATG CCTCGTCTTGGTGCTTCCAA | 100 |
| PLIN2 | F R | GATGAGACCACGGCAGATG GTCAACTATTTCCCGCACAAG | 120 |
| DGAT1 | F R | CCACTGGGACCTGAGGTGTC GCATCACCACACACCAATTCA | 111 |
| GPAM | F R | ATTGACCCTTGGCACGATAG AACAGCACCTTCCCACAAAG | 188 |
| AGPAT6 | F R | AAGCAAGTTGCCCATCCTCA AAACTGTGGCTCCAATTTCGA | 101 |
| SREBP1 | F R | ACGCCATCGAGAAACGCTAC GTGCGCAGACTCAGGTTCTC | 181 |
| PPARG | F R | CCTTCACCACCGTTGACTTCT GTATCAGGCTCCACTTTGATTGC | 145 |
| LIPIN1 | F R | GAGGGGAAGAAACACCACAA GTAGCTGACGCTGGACAACA | 202 |
| XBP1 | F R | AGTTAAGACAGCGGTTGGGG CACTCCATTCCCCTTGGTCT | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Yao, W.; Lv, L.; Zhang, X.; Wu, J.; Luo, J. Sirtuin 1 Inhibits Fatty Acid Synthesis through Forkhead Box Protein O1-Mediated Adipose Triglyceride Lipase Expression in Goat Mammary Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 9923. https://doi.org/10.3390/ijms25189923
He Q, Yao W, Lv L, Zhang X, Wu J, Luo J. Sirtuin 1 Inhibits Fatty Acid Synthesis through Forkhead Box Protein O1-Mediated Adipose Triglyceride Lipase Expression in Goat Mammary Epithelial Cells. International Journal of Molecular Sciences. 2024; 25(18):9923. https://doi.org/10.3390/ijms25189923
Chicago/Turabian StyleHe, Qiuya, Weiwei Yao, Li Lv, Xuelin Zhang, Jiao Wu, and Jun Luo. 2024. "Sirtuin 1 Inhibits Fatty Acid Synthesis through Forkhead Box Protein O1-Mediated Adipose Triglyceride Lipase Expression in Goat Mammary Epithelial Cells" International Journal of Molecular Sciences 25, no. 18: 9923. https://doi.org/10.3390/ijms25189923
APA StyleHe, Q., Yao, W., Lv, L., Zhang, X., Wu, J., & Luo, J. (2024). Sirtuin 1 Inhibits Fatty Acid Synthesis through Forkhead Box Protein O1-Mediated Adipose Triglyceride Lipase Expression in Goat Mammary Epithelial Cells. International Journal of Molecular Sciences, 25(18), 9923. https://doi.org/10.3390/ijms25189923






