Effects of Bifidobacterium-Fermented Milk on Obesity: Improved Lipid Metabolism through Suppression of Lipogenesis and Enhanced Muscle Metabolism
Abstract
1. Introduction
2. Results
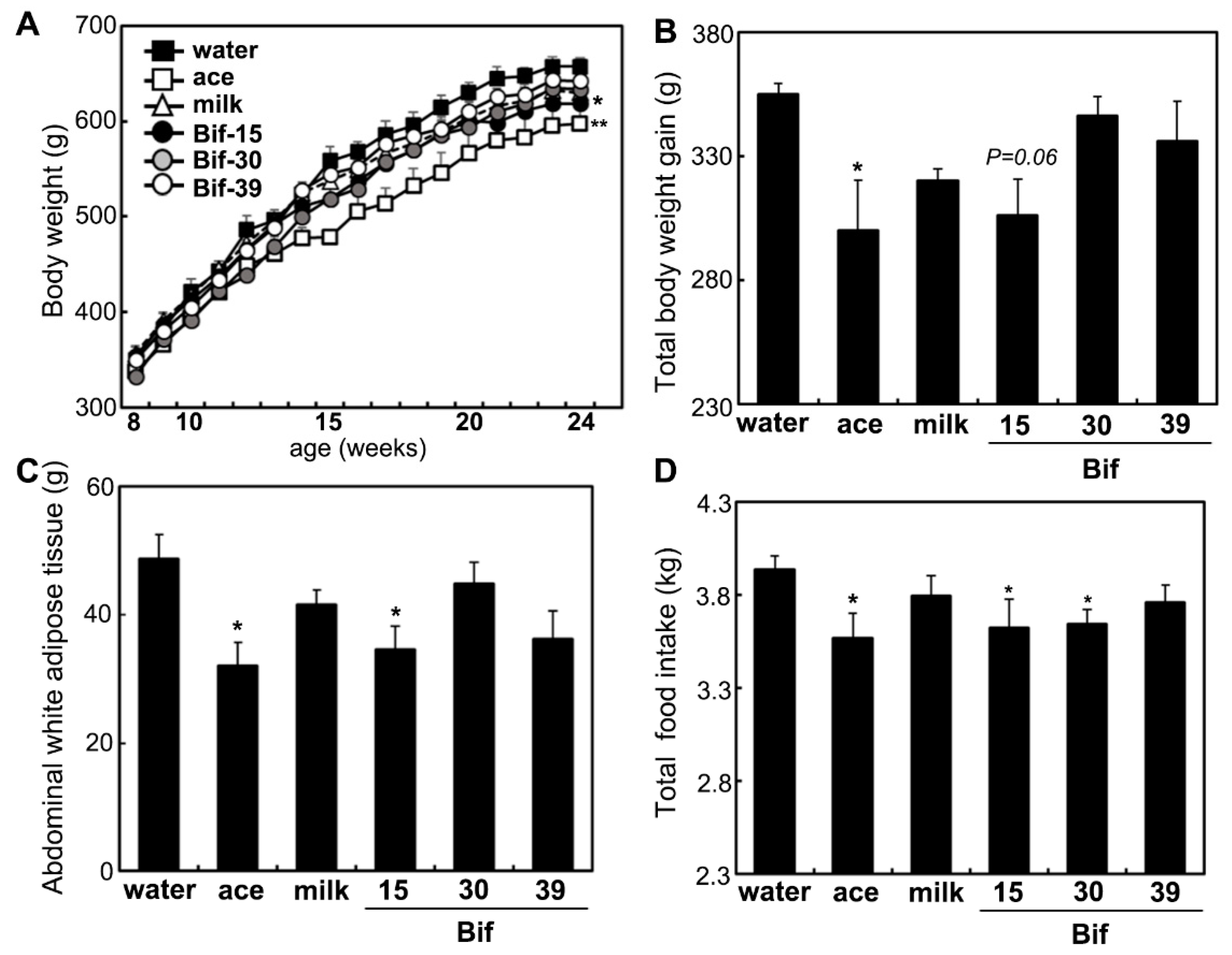
2.1. Milk Fermented with the Bifidobacterium Bif-15 Strain Prevents Obesity
2.2. Long-Term Supplementation with Bif-15-Fermented Milk Increases Plasma Acetic Acid and HDL-C Levels
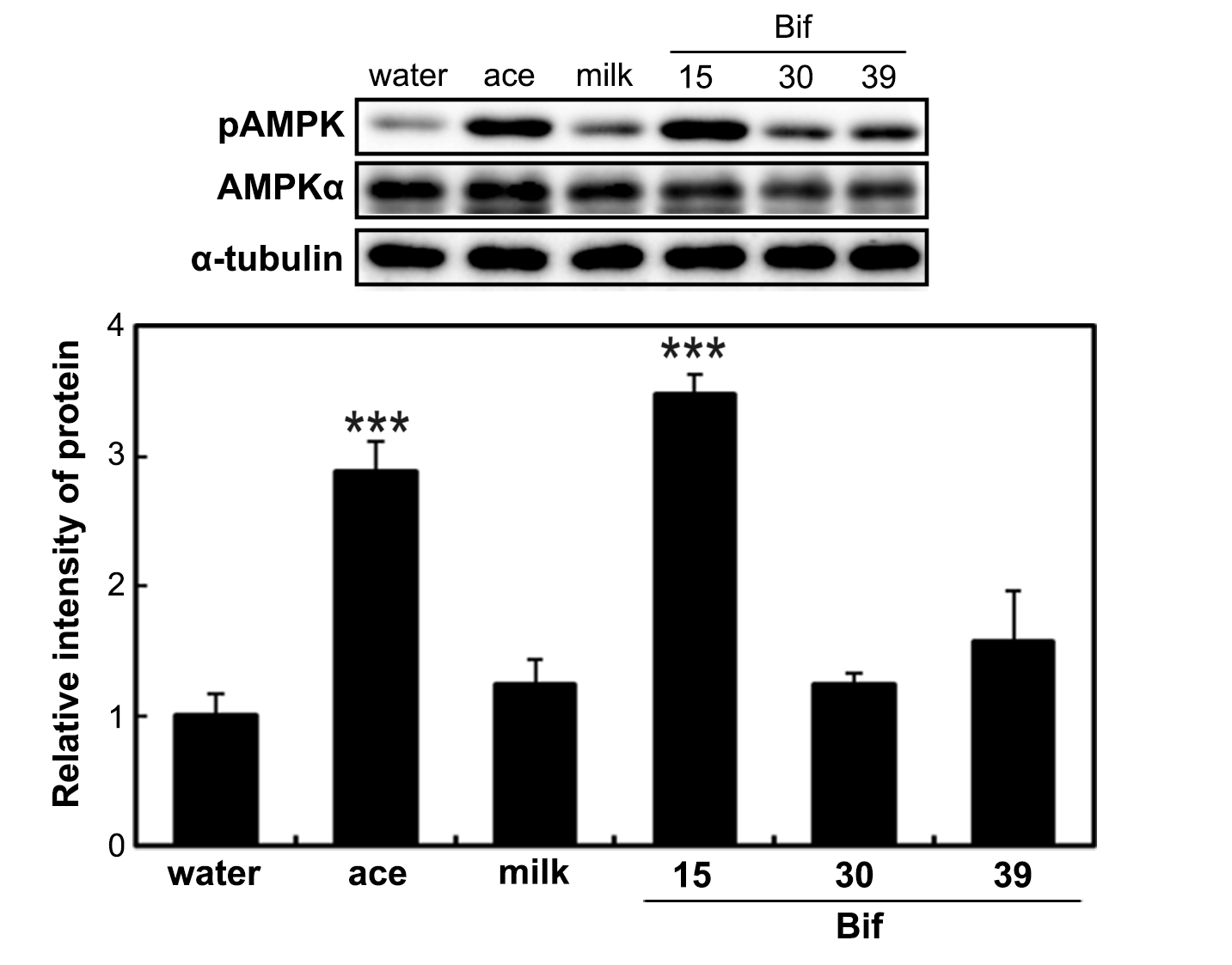
2.3. Effects of Acetic Acid and Bifidobacterium-Fermented Milk on AMPK Phosphorylation in the Liver
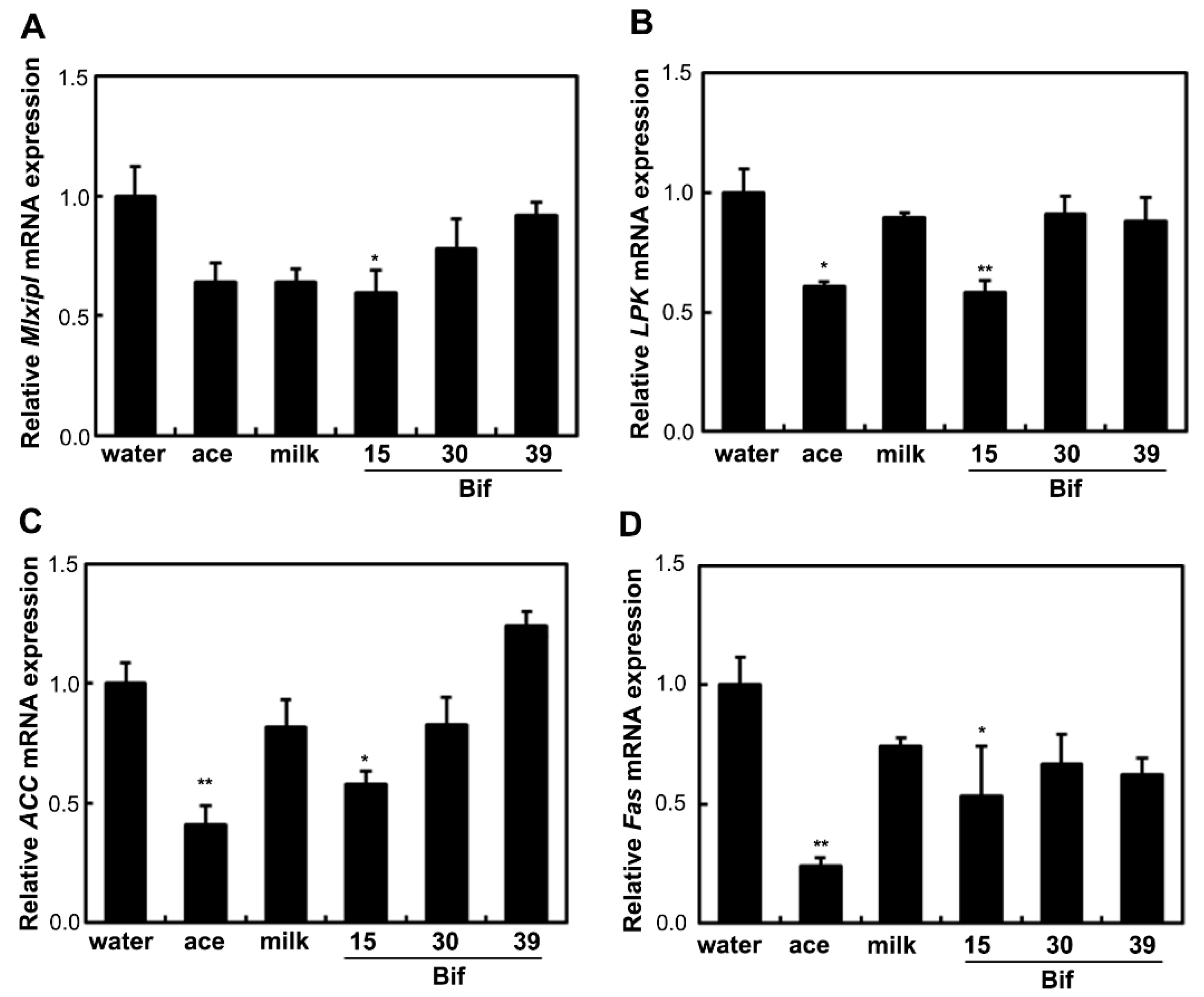
2.4. Bif-15-Fermented Milk Suppresses the Expression of Lipogenic Genes in the Liver
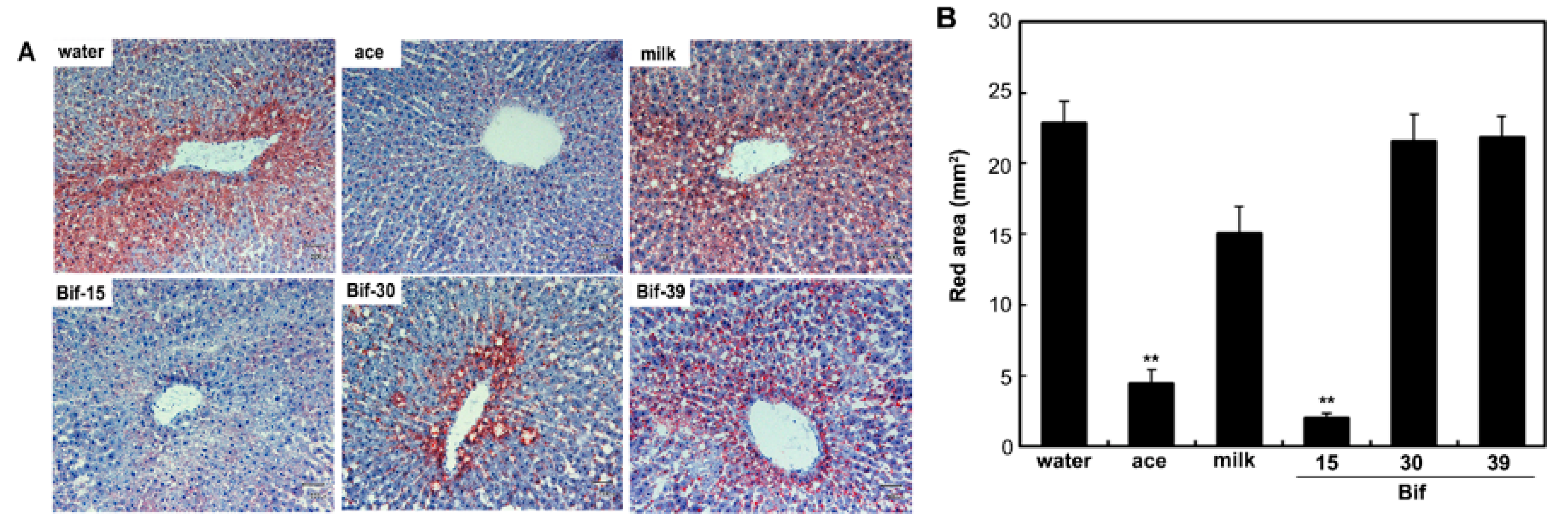
2.5. Effect of Bifidobacterium Bif-15-Fermented Milk on Fatty Liver Modulation
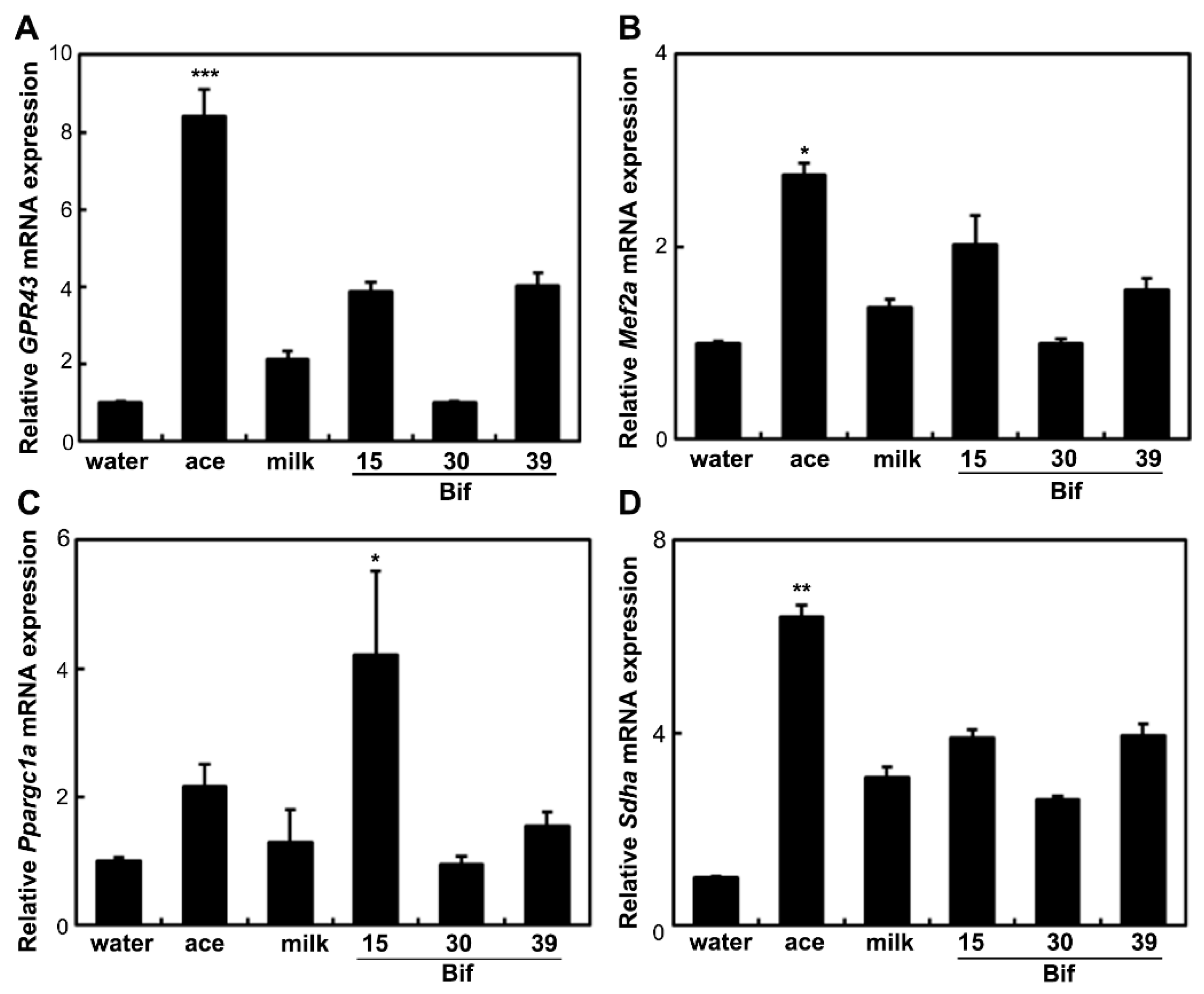
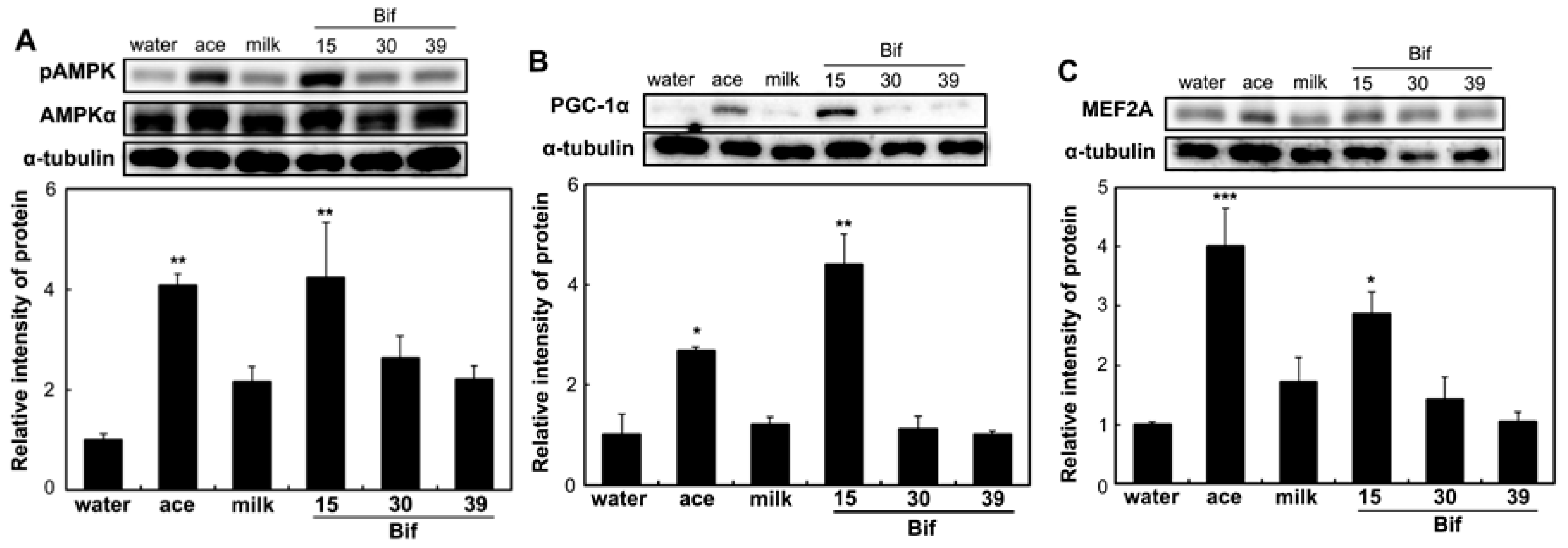
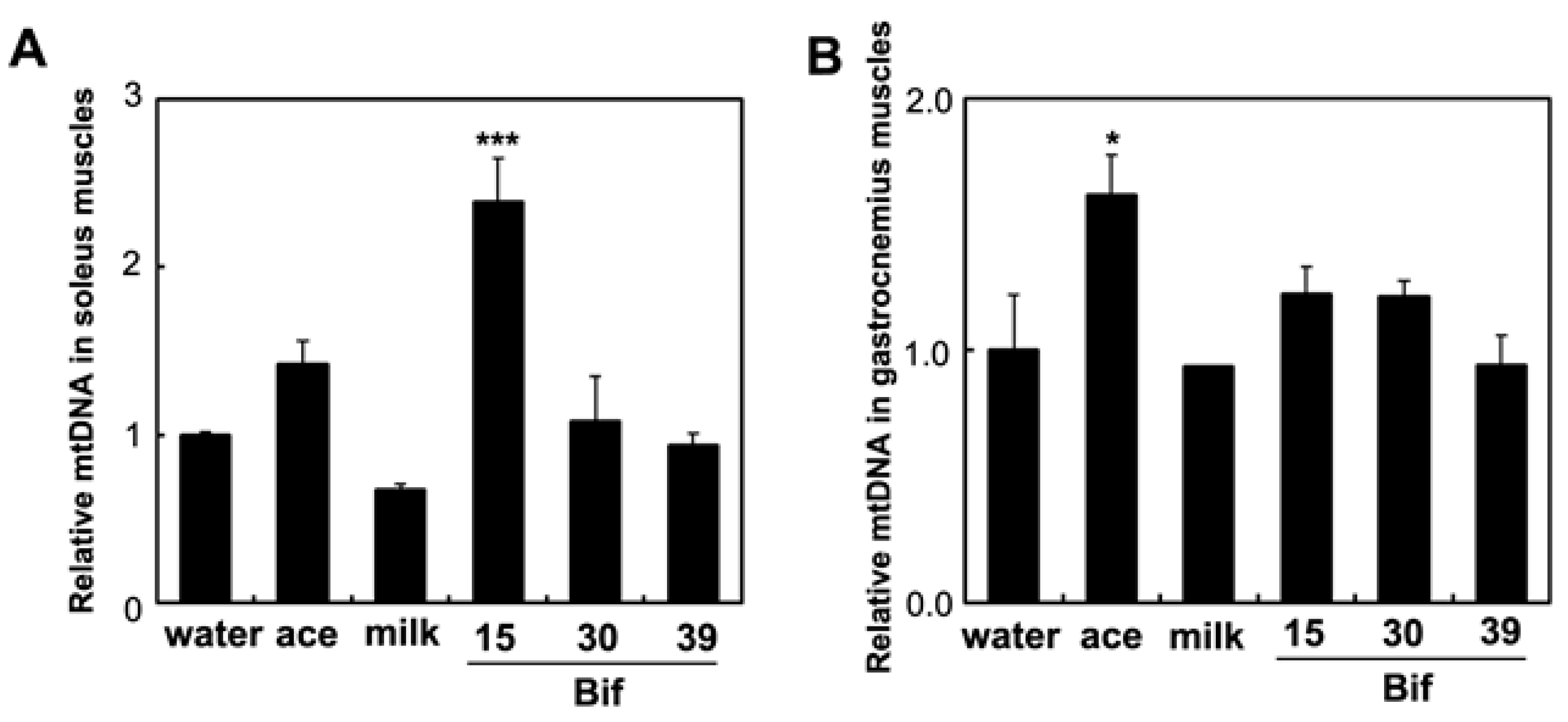
2.6. Effects of Bif-15 Supplementation on Gene and Protein Expression in Skeletal Muscles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Bifidobacterium Strains
4.3. Animal Experiments
4.4. Blood Biochemical Analysis
4.5. Histological Analysis
4.6. Quantitative RT-PCR Analysis
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Kotula, L.; Adams, M.C. The in vivo assessment of safety and gastrointestinal survival of an orally administered novel probiotic, Propionibacterium jensenii 702, in a male Wistar rat model. Food Chem. Toxicol. 2003, 41, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.; Le Lay, C.; Jean, J.; Fliss, I. Growth, acid production and bacteriocin production by probiotic candidates under simulated colonic conditions. J. Appl. Microbiol. 2012, 114, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Endres, J.; Clewell, A.; Jade, K.; Farber, T.; Hauswirth, J.; Schauss, A. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef]
- Sugahara, H.; Odamaki, T.; Fukuda, S.; Kato, T.; Xiao, J.-Z.; Abe, F.; Kikuchi, J.; Ohno, H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci. Rep. 2015, 5, 13548. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Yoshida, Y.; Makino, K.; Kounoshi, Y.; Kunihiro, S.; Takahashi, R.; Matsuzaki, T.; Miyazaki, K.; Ishikawa, F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J. Appl. Microbiol. 2011, 110, 650–657. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Lu, T.-Y.; Tseng, Y.-Y.; Pan, T.-M. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2006, 71, 238–245. [Google Scholar] [CrossRef]
- Daniali, M.; Nikfar, S.; Abdollahi, M. A brief overview on the use of probiotics to treat overweight and obese patients. Expert Rev. Endocrinol. Metab. 2020, 15, 1–4. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-Z.; Kondo, S.; Yanagisawa, N.; Miyaji, K.; Enomoto, K.; Sakoda, T.; Iwatsuki, K.; Enomoto, T. Clinical Efficacy of Probiotic Bifidobacterium longum for the Treatment of Symptoms of Japanese Cedar Pollen Allergy in Subjects Evaluated in an Environmental Exposure Unit. Allergol. Int. 2007, 56, 67–75. [Google Scholar] [CrossRef]
- Takahashi, N.; Kitazawa, H.; Iwabuchi, N.; Xiao, J.-Z.; Miyaji, K.; Iwatsuki, K.; Saito, T. Oral Administration of an Immunostimulatory DNA Sequence from Bifidobacterium longum Improves Th1/Th2 Balance in a Murine Model. Biosci. Biotechnol. Biochem. 2006, 70, 2013–2017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, N.; Kitazawa, H.; Shimosato, T.; Iwabuchi, N.; Xiao, J.-Z.; Iwatsuki, K.; Kokubo, S.; Saito, T. An immunostimulatory DNA sequence from a probiotic strain of Bifidobacterium longum inhibits IgE production in vitro. FEMS Immunol. Med Microbiol. 2006, 46, 461–469. [Google Scholar] [CrossRef]
- Odamaki, T.; Sugahara, H.; Yonezawa, S.; Yaeshima, T.; Iwatsuki, K.; Tanabe, S.; Tominaga, T.; Togashi, H.; Benno, Y.; Xiao, J.-Z. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe 2012, 18, 14–18. [Google Scholar] [CrossRef]
- Namba, K.; Yaeshima, T.; Ishibashi, N.; Hayasawa, H.; Yamazaki, S. Inhibitory Effects ofBifidobacterium longumon EnterohemorrhagicEscherichia coliO157: H7. Biosci. Microflora 2003, 22, 85–91. [Google Scholar] [CrossRef]
- Ogata, T.; Nakamura, T.; Anjitsu, K.; Yaeshima, T.; Takahashi, S.; Fukuwatari, Y.; Ishibashi, N.; Hayasawa, H.; Fujisawa, T.; Iino, H. Effect of Bifidobacterium longum BB536 Administration on the Intestinal Environment, Defecation Frequency and Fecal Characteristics of Human Volunteers. Biosci. Microflora 1997, 16, 53–58. [Google Scholar] [CrossRef]
- Yaeshima, T.; Takahashi, S.; Matsumoto, N.; Ishibashi, N.; Hayasawa, H.; Iino, H. Effect of Yogurt Containing Bifidobacterium longum BB536 on the Intestinal Environment, Fecal Characteristics and Defecation Frequenc. Biosci. Microflora 1997, 16, 73–77. [Google Scholar] [CrossRef][Green Version]
- Al-Sheraji, S.; Amin, I.; Azlan, A.; Manap, M.; Hassan, F. Effects of Bifidobacterium longum BB536 on lipid profile and histopathological changes in hypercholesterolaemic rats. Benef. Microbes 2015, 6, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Hypocholesterolaemic effect of yoghurt containing Bifidobacterium pseudocatenulatum G4 or Bifidobacterium longum BB536. Food Chem. 2012, 135, 356–361. [Google Scholar] [CrossRef]
- Karimi, G.; Jamaluddin, R.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Parvaneh, M. Single-species versus dual-species probiotic supplementation as an emerging therapeutic strategy for obesity. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 910–918. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Ugajin, S.; Kaga, T. Vinegar Intake Reduces Body Weight, Body Fat Mass, and Serum Triglyceride Levels in Obese Japanese Subjects. Biosci. Biotechnol. Biochem. 2009, 73, 1837–1843. [Google Scholar] [CrossRef]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the Addition of Probiotic Cultures to Foods. Crit. Rev. Food Sci. Nutr. 2005, 45, 61–84. [Google Scholar] [CrossRef]
- Abe, F.; Tomita, S.; Yaeshima, T.; Iwatsuki, K. Effect of production conditions on the stability of a human bifidobacterial species Bifidobacterium longum in yogurt. Lett. Appl. Microbiol. 2009, 49, 715–720. [Google Scholar] [CrossRef]
- Kondo, S.; Xiao, J.-Z.; Satoh, T.; Odamaki, T.; Takahashi, S.; Sugahara, H.; Yaeshima, T.; Iwatsuki, K.; Kamei, A.; Abe, K. Antiobesity Effects of Bifidobacterium breve Strain B-3 Supplementation in a Mouse Model with High-Fat Diet-Induced Obesity. Biosci. Biotechnol. Biochem. 2010, 74, 1656–1661. [Google Scholar] [CrossRef]
- A McKinsey, T.; Zhang, C.L.; Olson, E.N. MEF2: A calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 2002, 27, 40–47. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Mangum, J.E.; Hardee, J.P.; Fix, D.K.; Puppa, M.J.; Elkes, J.; Altomare, D.; Bykhovskaya, Y.; Campagna, D.R.; Schmidt, P.J.; Sendamarai, A.K.; et al. Pseudouridine synthase 1 deficient mice, a model for Mitochondrial Myopathy with Sideroblastic Anemia, exhibit muscle morphology and physiology alterations. Sci. Rep. 2016, 6, 26202. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaard, S.; de Ruiter, C.J.; Noordhof, D.A.; Sterrenburg, R.; Bloemers, F.W.; de Koning, J.J.; Jaspers, R.T.; van der Laarse, W.J. Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J. Appl. Physiol. 2016, 121, 636–645. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Baltgalvis, K.A.; Puppa, M.J.; Sato, S.; Baynes, J.W.; Carson, J.A. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R201–R211. [Google Scholar] [CrossRef]
- Hu, J.; Kyrou, I.; Tan, B.K.; Dimitriadis, G.K.; Ramanjaneya, M.; Tripathi, G.; Patel, V.; James, S.; Kawan, M.; Chen, J.; et al. Short-Chain Fatty Acid Acetate Stimulates Adipogenesis and Mitochondrial Biogenesis via GPR43 in Brown Adipocytes. Endocrinology 2016, 157, 1881–1894. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA Receptor GPR43 and Energy Metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Stoddart, L.A.; Smith, N.J.; Jenkins, L.; Brown, A.J.; Milligan, G. Conserved Polar Residues in Transmembrane Domains V, VI, and VII of Free Fatty Acid Receptor 2 and Free Fatty Acid Receptor 3 Are Required for the Binding and Function of Short Chain Fatty Acids. J. Biol. Chem. 2008, 283, 32913–32924. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Yamashita, H. Acetic acid stimulates G-protein-coupled receptor GPR43 and induces intracellular calcium influx in L6 myotube cells. PLOS ONE 2020, 15, e0239428. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Abe, R.; Yamashita, H. Effect of Long-Term Supplementation with Acetic Acid on the Skeletal Muscle of Aging Sprague Dawley Rats. Int. J. Mol. Sci. 2022, 23, 4691. [Google Scholar] [CrossRef]
- Li, Y.; Arai, S.; Kato, K.; Iwabuchi, S.; Iwabuchi, N.; Muto, N.; Motobayashi, H.; Ebihara, S.; Tanaka, M.; Hashimoto, S. The Potential Immunomodulatory Effect of Bifidobacterium longum subsp. longum BB536 on Healthy Adults through Plasmacytoid Dendritic Cell Activation in the Peripheral Blood. Nutrients 2023, 16, 42. [Google Scholar] [CrossRef]
- Toda, K.; Yamauchi, Y.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Yoshimoto, S.; Xiao, J.-Z. Heat-Killed Bifidobacterium breve B-3 Enhances Muscle Functions: Possible Involvement of Increases in Muscle Mass and Mitochondrial Biogenesis. Nutrients 2020, 12, 219. [Google Scholar] [CrossRef]
- Beutler, H.-O. Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany; Deerfield Berach, FL, USA, 1964; Volume VI. [Google Scholar]


| Glucose (mg/dL) | TG (mg/dL) | TC (mig/dL) | HDL-C (mg/dL) | Insulin (ng/mL) | Acetic Acid (μM) | |
|---|---|---|---|---|---|---|
| Water | 258 ± 33 | 350 ± 10 | 210 ± 3.1 | 69 ± 5.3 | 7.0 ± 2.2 | 94 ± 11 |
| Ace | 263 ± 6.5 | 210 ± 41 | 124 ± 17 ** | 74 ± 6.7 | 6.4 ± 1.8 | 180 ± 0.1 |
| Milk | 271 ± 18 | 371 ± 46 | 164 ± 3.5 | 52 ± 4.0 | 4.8 ± 1.6 | 67 ± 10 |
| Bif-15 | 269 ± 23 | 314 ± 69 | 145 ± 17 * | 85 ± 2.6 * | 6.2 ± 2.7 | 250 ± 47 * |
| Bif-30 | 414 ± 47 * | 469 ± 85 | 225 ± 5.6 | 70 ± 5.8 | 7.4 ± 0.7 | 128 ± 28 |
| Bif-39 | 329 ± 49 | 257 ± 33 | 158 ± 19 | 63 ± 3.4 | 5.9 ± 0.4 | 102 ± 31 |
| Culture (hr) | Concentrations of Organic Acids (%) | (cfu/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Citric Acid | Malic Acid | Succinic Acid | Lactic Acid | Formic Acid | Acetic Acid | |||
| Milk | - | 0.219 | 0.004 | 0.002 | 0.002 | 0.001 | 0.002 | - |
| Control B. longum | 24 | 0.211 | 0.007 | 0.019 | 0.525 | 0.004 | 0.562 | - |
| Bif-15 | 48 | 0.191 | 0.006 | 0.008 | 0.482 | 0.004 | 0.555 | 1.2 × 107 |
| Bif-30 | 22 | 0.207 | 0.005 | 0.007 | 0.553 | 0.002 | 0.623 | 7.6 × 106 |
| Bif-39 | 21 | 0.213 | 0.004 | 0.007 | 0.541 | 0.003 | 0.602 | 1.4 × 109 |
| Gene | Forward | Reverse |
|---|---|---|
| β-actin (actb) | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
| ChREBP (Mlxipl) | GAAGACCCAAAGACCAAGATGC | TCTGACAACAAAGCAGGAGGTG |
| SREBP (Srebp-1c) | AGCACAGCAACCAGAAACTC | AGGTTTCATGCCCTCCATAG |
| L-type pyruvate kinase (LPK) | AACCTCCCCACTCAGCTACA | CCCTTCACAATTTCCACCTC |
| Acetyl-CoA carboxylase (ACC) | TACAACGCAGGCATCAGAAG | TGTGCTGCAGGAAGATTGAC |
| Fatty acid synthase (Fas) | CAGGAACAACTCATCCGTTCTCT | GGACCGAGTAATGCCGTTCA |
| GPR43 (ffar2, GPR43) | CAGAGGAGAACCAGGTGGAAG | GGCAGGGACCCCAGTAAGAA |
| PGC-1α (ppargc1a) | GACCCCAGAGTCACCAAATGA | GGCCTGCAGTTCCAGAGAGT |
| MEF2A (mef2a) | ATGAGAGGAACCGACAGGTG | TATCCGAGTTCGTCCTGCTT |
| Succinate dehydrogenase (sdha) | TGGGGCGACTCGTGGCTTTC | CCCCGCCTGCACCTACAACC |
| NADH dehydrogenase 1, mitochondrial (mt-Nd1) | CTCCCTATTCGGAGCCCTAC | ATTTGTTTCTGCTAGGGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruta, H.; Fujii, Y.; Toyokawa, N.; Nakamura, S.; Yamashita, H. Effects of Bifidobacterium-Fermented Milk on Obesity: Improved Lipid Metabolism through Suppression of Lipogenesis and Enhanced Muscle Metabolism. Int. J. Mol. Sci. 2024, 25, 9934. https://doi.org/10.3390/ijms25189934
Maruta H, Fujii Y, Toyokawa N, Nakamura S, Yamashita H. Effects of Bifidobacterium-Fermented Milk on Obesity: Improved Lipid Metabolism through Suppression of Lipogenesis and Enhanced Muscle Metabolism. International Journal of Molecular Sciences. 2024; 25(18):9934. https://doi.org/10.3390/ijms25189934
Chicago/Turabian StyleMaruta, Hitomi, Yusuke Fujii, Naoki Toyokawa, Shoji Nakamura, and Hiromi Yamashita. 2024. "Effects of Bifidobacterium-Fermented Milk on Obesity: Improved Lipid Metabolism through Suppression of Lipogenesis and Enhanced Muscle Metabolism" International Journal of Molecular Sciences 25, no. 18: 9934. https://doi.org/10.3390/ijms25189934
APA StyleMaruta, H., Fujii, Y., Toyokawa, N., Nakamura, S., & Yamashita, H. (2024). Effects of Bifidobacterium-Fermented Milk on Obesity: Improved Lipid Metabolism through Suppression of Lipogenesis and Enhanced Muscle Metabolism. International Journal of Molecular Sciences, 25(18), 9934. https://doi.org/10.3390/ijms25189934






