Transcriptomic and Biochemical Analysis of the Antimicrobial Mechanism of Lipopeptide Iturin W against Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
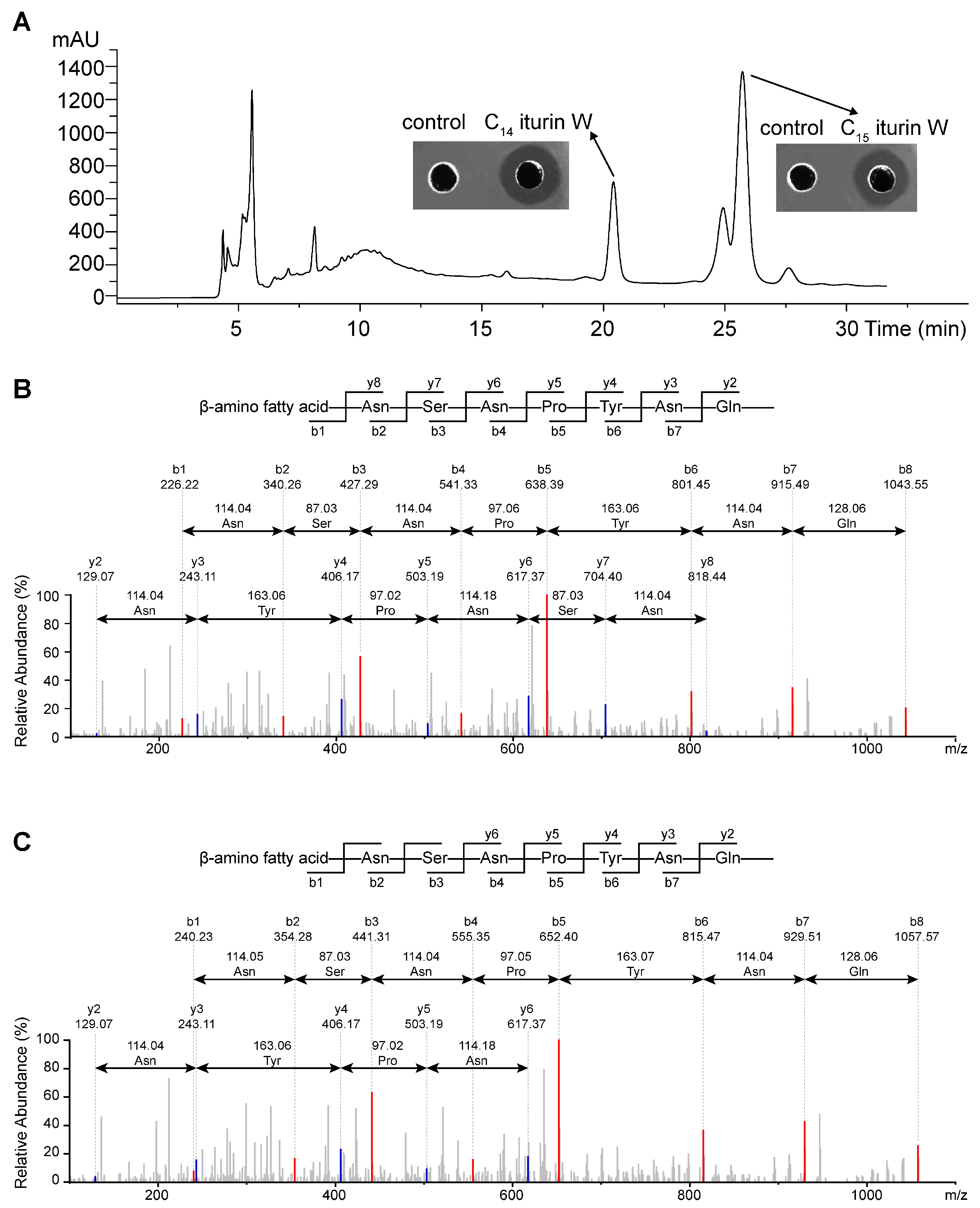
2.1. Screening, Purification and Identification of the Antimicrobial Agents
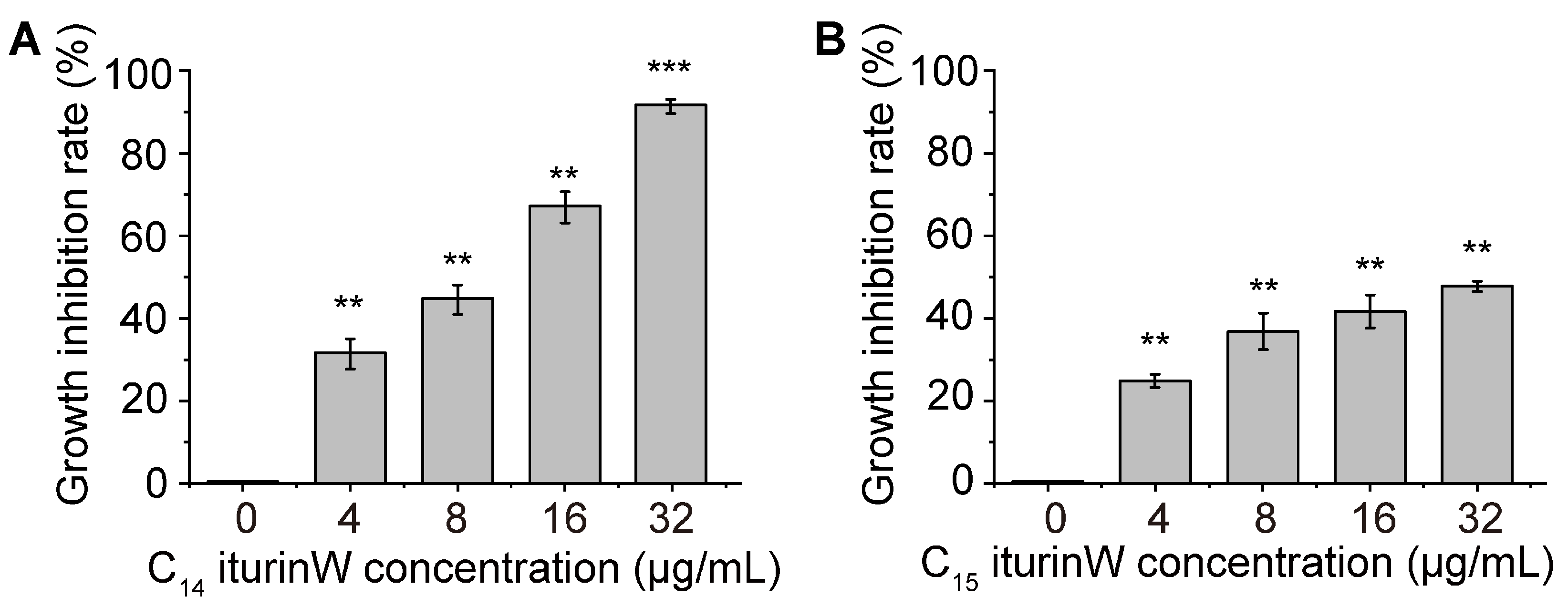
2.2. Antimicrobial Activity Assay of C14 Iturin W and C15 Iturin W
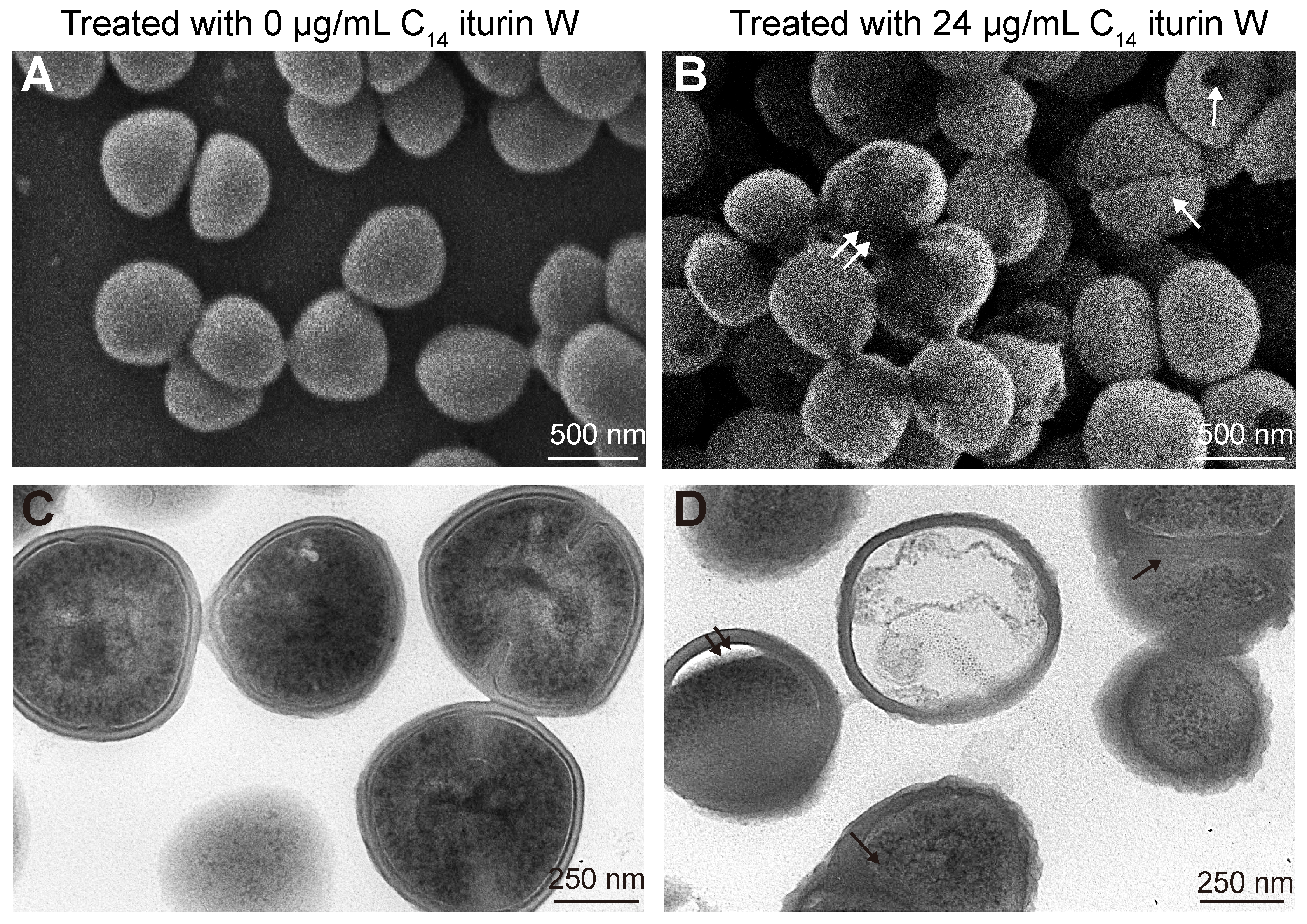
2.3. Morphological and Ultrastructural Changes of MRSA CCARM 3090 Caused by C14 Iturin W
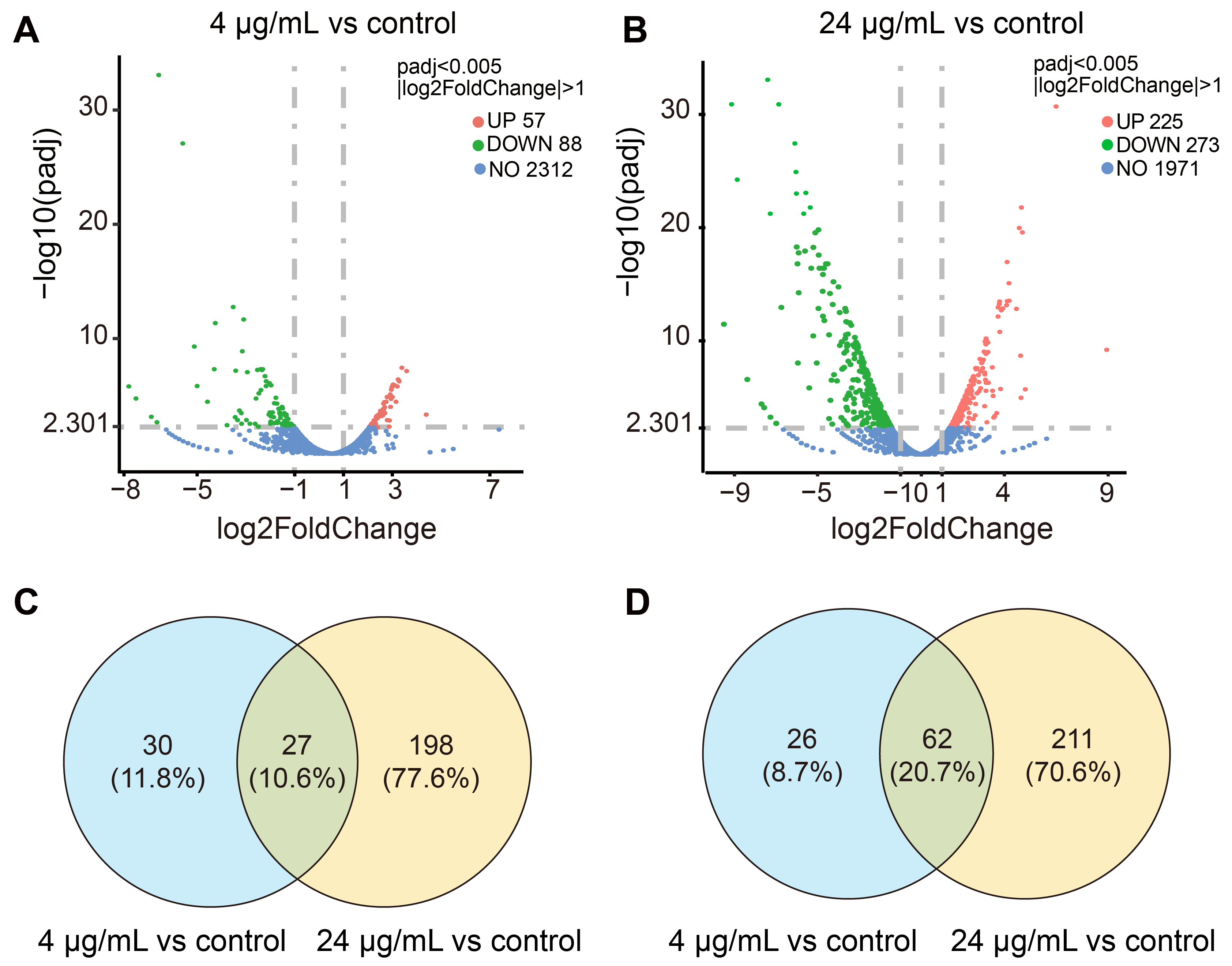
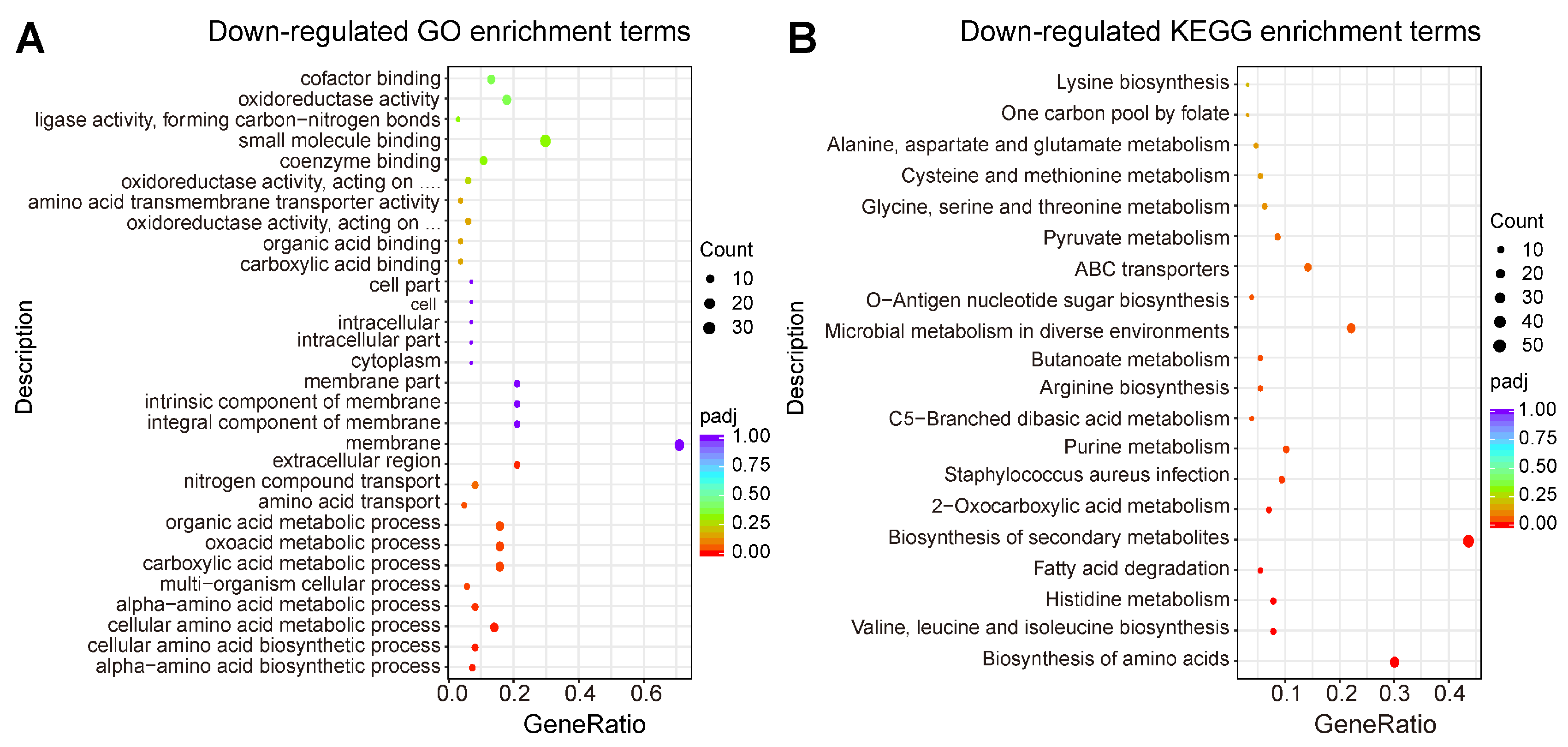
2.4. Transcriptomic Profiling of MRSA CCARM 3090 after Treated with C14 Iturin W
2.5. Functional Analysis of DEGs
2.5.1. DEGs Involved in ROS Accumulation and Proton Motive Force
2.5.2. DEGs Involved in Virulence of MRSA CCARM 3090
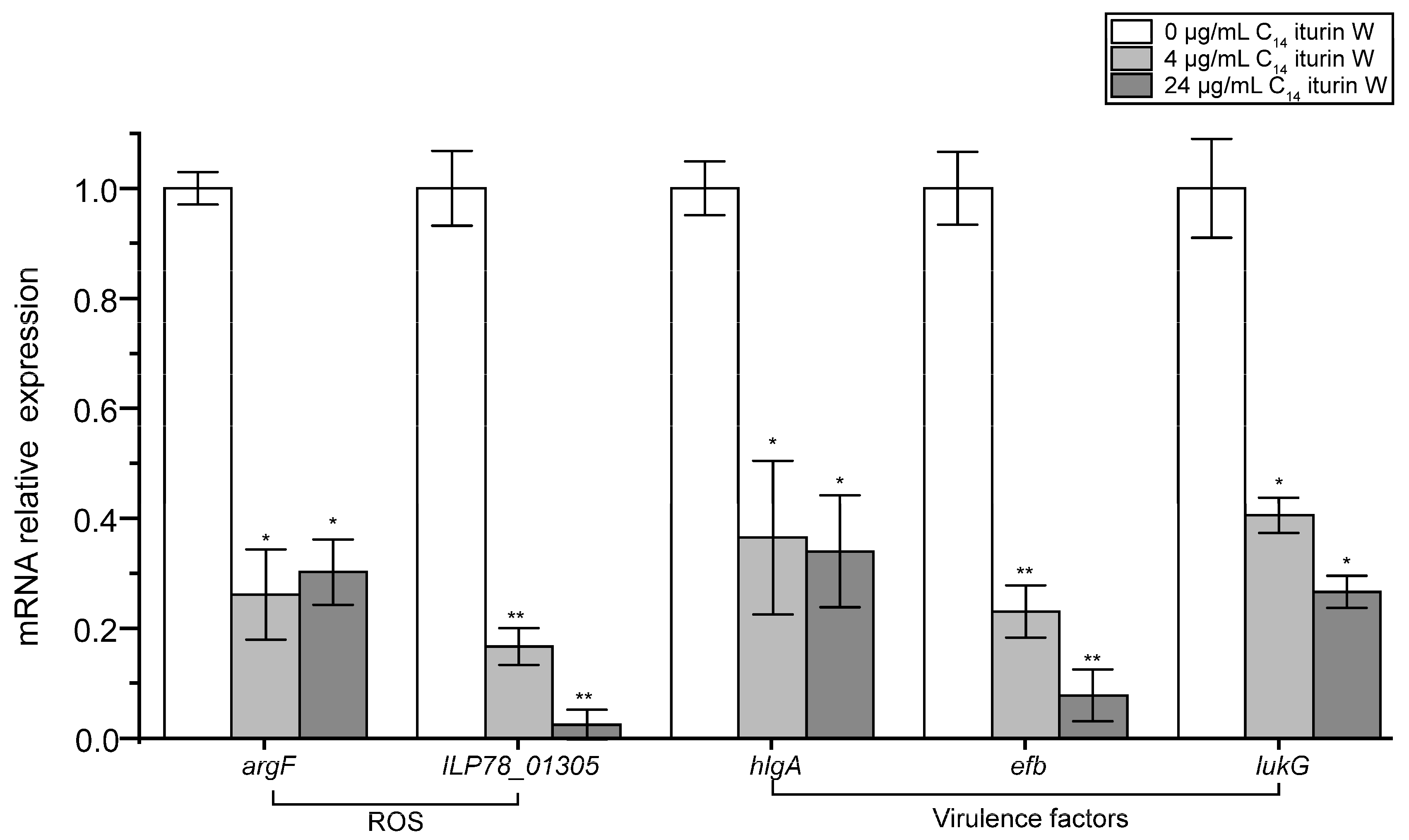
2.6. qRT-PCR Verification of the Related DGEs
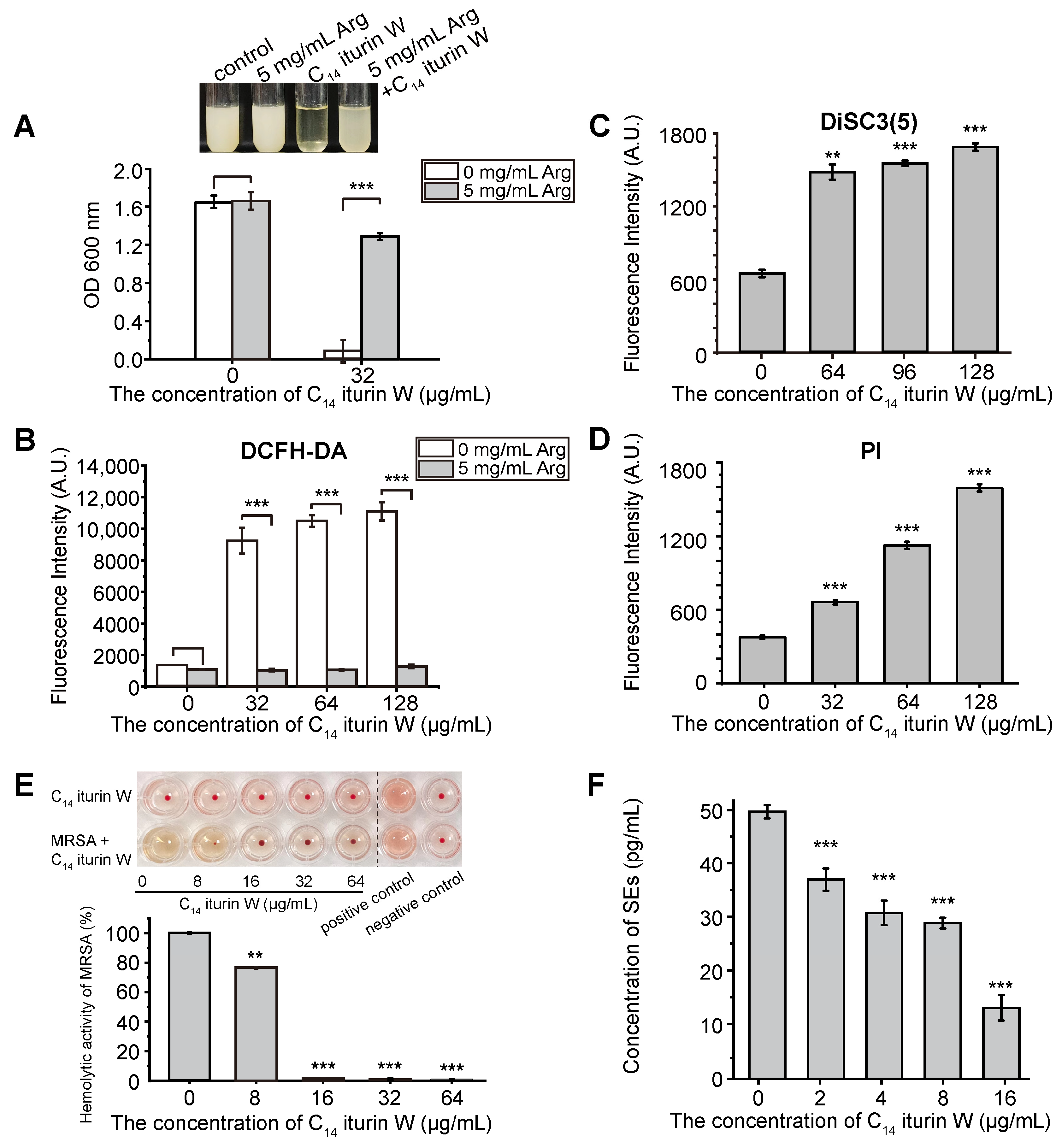
2.7. Biochemical Functional Validation of DEGs
2.8. Inhibition of C14 Iturin W on Virulence of MRSA CCARM 3090
3. Materials and Methods
3.1. Screening of Strains with Inhibitory Activity against S. aureus
3.2. Purification of the Antimicrobial Agents S. aureus
3.3. Identification of the Purified Antimicrobial Agents
3.4. Antimicrobial Activity Assay
3.5. SEM and TEM Observation
3.6. Transcriptomic Analysis
3.7. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Analysis
3.8. Detection of the Correlation between Arginine and the C14 Iturin W
3.9. Determination of ROS
3.10. Membrane Potential Assay
3.11. Membrane Permeability Assay
3.12. Effect of C14 Iturin W on the Hemolytic Activity of S. aureus
3.13. Effect of C14 Iturin W on the Production of SEs
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Canning, M.; Birhane, M.G.; Dewey-Mattia, D.; Lawinger, H.; Cote, A.; Gieraltowski, L.; Schwensohn, C.; Tagg, K.A.; Francois Watkins, L.K.; Park Robyn, M.; et al. Salmonella Outbreaks Linked to Beef, United States, 2012–2019. J. Food Prot. 2023, 86, 100071. [Google Scholar] [CrossRef] [PubMed]
- Manchal, N.; Young, M.K.; Castellanos, M.E.; Leggat, P.; Adegboye, O. A systematic review and meta-analysis of ambient temperature and precipitation with infections from five food-borne bacterial pathogens. Epidemiol. Infect. 2024, 152, e98. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, L.; Zhang, Y.; Luo, L.; Yang, H.; Wen, K.; Yu, X.; Shen, J.; Wang, Z. A comprehensive review on the detection of Staphylococcus aureus enterotoxins in food samples. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, Y.; Ma, L.; Huang, K.; Liang, Z. Co-occurrence of Staphylococcus aureus and Ochratoxin A in psteurized milk. Toxins 2022, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhang, H.; Shi, X.; Wang, J.; Li, K.; Liang, J.; Xu, X.; Zhao, W.; Zhao, C. Genomic analysis, antibiotic resistance, and virulence of Staphylococcus aureus from food and food outbreaks: A potential public concern. Int. J. Food Microbiol. 2022, 377, 109825. [Google Scholar] [CrossRef]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Kwak, Y.K.; Vikstrom, E.; Magnusson, K.E.; Vecsey-Semjen, B.; Colque-Navarro, P.; Mollby, R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect. Immun. 2012, 80, 1670–1680. [Google Scholar] [CrossRef]
- Ashker, M.E.; Gwida, M.; Monecke, S.; El-Gohary, F.; Ehricht, R.; Elsayed, M.; Akinduti, P.; El-Fateh, M.; Maurischat, S. Antimicrobial resistance pattern and virulence profile of S. aureus isolated from household cattle and buffalo with mastitis in Egypt. Vet. Microbiol. 2020, 240, 108535. [Google Scholar]
- Ruiz, P.; Barragán, I.; Seseña, S.; Palop, M.L. Is staphylococci population from milk of healthy goats safe? Int. J. Food Microbiol. 2016, 238, 146–152. [Google Scholar] [CrossRef]
- Ghabbour, R.; Awad, A.; Younis, G. Genetic characterization and antimicrobial-resistant profiles of Staphylococcus aureus isolated from different food sources. Biocontrol Sci. 2022, 27, 87–97. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-Inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial peptides: Opportunities and challenges in overcoming resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef]
- Vaca, J.; Ortiz, A.; Sansinenea, E. Bacillus sp. bacteriocins: Natural weapons against bacterial enemies. Curr. Med. Chem. 2022, 29, 2093–2108. [Google Scholar] [CrossRef]
- Puan, S.L.; Erriah, P.; Baharudin, M.M.A.; Yahaya, N.M.; Kamil, W.; Ali, M.S.M.; Ahmad, S.A.; Oslan, S.N.; Lim, S.; Sabri, S. Antimicrobial peptides from Bacillus spp. and strategies to enhance their yield. Appl. Microbiol. Biotechnol. 2023, 107, 5569–5593. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Shi, Y.; Huang, M.; Song, Z.; Simal-Gandara, J.; Li, N.; Shi, J. Classification, application, multifarious activities and production improvement of lipopeptides produced by Bacillus. Crit. Rev. Food Sci. Nutr. 2024, 64, 7451–7464. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, G.; Zheng, R.; Sun, C.; Wu, S. Structural and functional insights into iturin W, a novel lipopeptide produced by the Deep-sea bacterium Bacillus sp. strain wsm-1. Appl. Environ. Microbiol. 2020, 86, e01597-20. [Google Scholar] [CrossRef]
- Su, Y.; Gao, L.; Li, C.; Wang, L.; Zhou, H.; Zhang, C.; Xia, X. Regulation mechanism and bioactivity characteristic of surfactin homologues with C14 and C15 fatty acid chains. Microb. Cell Fact. 2024, 23, 94. [Google Scholar] [CrossRef]
- Shabeer Ali, H.; Ajesh, K.; Dileep, K.V.; Prajosh, P.; Sreejith, K. Structural characterization of Kannurin isoforms and evaluation of the role of beta-hydroxy fatty acid tail length in functional specificity. Sci. Rep. 2020, 10, 2839. [Google Scholar] [CrossRef]
- Sobral, R.; Tomasz, A. The Staphylococcal Cell Wall. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef]
- Ganesan, N.; Mishra, B.; Felix, L.; Mylonakis, E. Antimicrobial Peptides and Small Molecules Targeting the Cell Membrane of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2023, 87, e0003722. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plant-pathogenic fungus fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Wu, H.; Wang, S.; Tian, J.; Dai, C.; Liu, T. Zeolitic imidazolate framework-8 triggers the inhibition of arginine biosynthesis to combat methicillin-resistant Staphylococcus aureus. Small 2023, 19, e2205682. [Google Scholar] [CrossRef]
- Van Kessel, J.S.; Russell, J.B. Energetics of arginine and lysine transport by whole cells and membrane vesicles of strain SR, a monensin-sensitive ruminal bacterium. Appl. Environ. Microbiol. 1992, 58, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Verhoogt, H.J.; Smit, H.; Abee, T.; Gamper, M.; Driessen, A.J.; Haas, D.; Konings, W.N. arcD, the first gene of the arc operon for anaerobic arginine catabolism in Pseudomonas aeruginosa, encodes an arginine-ornithine exchanger. J. Bacteriol. 1992, 174, 1568–1573. [Google Scholar] [CrossRef]
- Pinmanee, P.; Sompinit, K.; Jantimaporn, A.; Khongkow, M.; Haltrich, D.; Nimchua, T.; Sukyai, P. Purification and immobilization of superoxide dismutase obtained from Saccharomyces cerevisiae TBRC657 on bacterial cellulose and its protective effect against oxidative damage in fibroblasts. Biomolecules. 2023, 13, 1156. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, H.; Wu, X.; Wang, Q.; Chen, M.; Feng, Z.; Chen, H. The functions of glutathione peroxidase in ROS homeostasis and fruiting body development in Hypsizygus marmoreus. Appl. Microbiol. Biotechnol. 2020, 104, 10555–10570. [Google Scholar] [CrossRef]
- Bayer, A.S.; McNamara, P.; Yeaman, M.R.; Lucindo, N.; Jones, T.; Cheung, A.L.; Sahl, H.G.; Proctor, R.A. Transposon disruption of the complex I NADH oxidoreductase gene (snoD) in Staphylococcus aureus is associated with reduced susceptibility to the microbicidal activity of thrombin-induced platelet microbicidal protein 1. J. Bacteriol. 2006, 188, 211–222. [Google Scholar] [CrossRef]
- Vaish, M.; Whelan, A.P.; Robles, T.R.; Liu, J.; Jereen, A.; Christie, S.; Alonzo, F., 3rd; Benson, M.A.; Torres, V.J.; Krulwich, T.A. Roles of Staphylococcus aureus Mnh1 and Mnh2 antiporters in salt tolerance, alkali tolerance, and pathogenesis. J. Bacteriol. 2018, 200, e00611-17. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H. Halotolerance, stress mechanisms, and circadian clock of salt-tolerant cyanobacteria. Appl. Microbiol. Biotechnol. 2023, 107, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Yang, B.; Tong, Z.; Shi, J.; Wang, Z.; Liu, Y. Bacterial proton motive force as an unprecedented target to control antimicrobial resistance. Med. Res. Rev. 2023, 43, 1068–1090. [Google Scholar] [CrossRef]
- Feng, J.; Sun, D.; Wang, L.; Li, X.; Guan, J.; Wei, L.; Yue, D.; Wang, X.; Zhao, Y.; Yang, H.; et al. Biochanin A as an alpha-hemolysin inhibitor for combating methicillin-resistant Staphylococcus aureus infection. World J. Microbiol. Biotechnol. 2021, 38, 6. [Google Scholar]
- Zarrineh, M.; Mashhadi, I.S.; Farhadpour, M.; Ghassempour, A. Mechanism of antibodies purification by protein A. Anal. Biochem. 2020, 609, 113909. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, T.; Gao, L.; Wang, Y.; Liu, L.; Tian, T.; Shi, Y.; Zhang, J.; Zhao, Z.; Lu, D.; et al. Extracellular fibrinogen-binding protein released by intracellular Staphylococcus aureus suppresses host immunity by targeting TRAF3. Nat. Commun. 2022, 13, 5493. [Google Scholar] [CrossRef] [PubMed]
- Jura, G.; Masiuk, H.; Pruss, A.; Kurzawski, M.; Sienkiewicz, M.; Wojciechowska-Koszko, I.; Kwiatkowski, P. Prevalence of selected immune evasion genes and clonal diversity in methicillin-susceptible Staphylococcus aureus isolated from nasal carriers and outpatients with cut wound infections. Antibiotics 2024, 13, 730. [Google Scholar] [CrossRef]
- Trstenjak, N.; Milic, D.; Graewert, M.A.; Rouha, H.; Svergun, D.; Djinovic-Carugo, K.; Nagy, E.; Badarau, A. Molecular mechanism of leukocidin GH-integrin CD11b/CD18 recognition and species specificity. Proc. Natl. Acad. Sci. USA 2020, 117, 317–327. [Google Scholar] [CrossRef]
- Winstel, V.; Schneewind, O.; Missiakas, D. Staphylococcus aureus exploits the host apoptotic pathway to persist during infection. mBio. 2019, 10, e02270-19. [Google Scholar] [CrossRef]
- Abril, A.G.; Villa, T.G.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Guo, N.; Liu, Z.; Yan, Z.; Liu, Z.; Hao, K.; Liu, C.; Wang, J. Subinhibitory concentrations of Honokiol reduce alpha-Hemolysin (Hla) secretion by Staphylococcus aureus and the Hla-induced inflammatory response by inactivating the NLRP3 inflammasome. Emerg Microbes Infect 2019, 8, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shen, Y.; Qin, Y.; Pang, Y.; Fan, H.; Peng, J.; Pei, X.; Liu, X. Isolation and purification of antibacterial lipopeptides from Bacillus velezensis YA215 isolated from sea mangroves. Front. Nutr. 2022, 9, 1064764. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation and structural elucidation of brevibacillin, an antimicrobial lipopeptide from Brevibacillus laterosporus that combats drug-resistant Gram-positive bacteria. Appl. Environ. Microbiol. 2016, 82, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Q.; Huang, Y.; Qi, M.; Yan, H.; Li, W.; Zhuang, H. Antibacterial efficacy and mechanisms of curcumin-based photodynamic treatment against Staphylococcus aureus and its application in juices. Molecules 2022, 27, 7136. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Bai, T.; Zheng, X.; Yang, L.; Li, Q.; Wang, X. Lysine inhibits hemolytic activity of Staphylococcus aureus and its application in food model contaminated with Staphylococcus aureus. Toxins 2022, 14, 867. [Google Scholar] [CrossRef]
| Bacterial Strain 1 | MIC (μg/mL) |
|---|---|
| MRSA CCARM 3090 | 64 |
| MRSA QD-1 | 64 |
| S. aureus QD-2 | 32 |
| S. aureus ATCC 25923 | 32 |
| Gene | 4 μg/mL log2FoldChange | 24 μg/mL log2FoldChange | Gene Description |
|---|---|---|---|
| ROS-related genes | |||
| ILP78_10355 | −6.115 | −7.143 | argininosuccinate synthase |
| argH | −7.100 | −6.613 | argininosuccinate lyase |
| argF | −1.965 | −2.346 | ornithine carbamoyltransferase |
| ILP78_00430 | −2.230 | −2.689 | superoxide dismutase |
| ILP78_01305 | −1.248 | −1.693 | glutathione peroxidase |
| proton motive force | |||
| mnhA2 | −0.776 | −1.207 | Na+/H+ antiporter Mnh2 subunit A |
| mnhB2 | −0.618 | −1.087 | Na+/H+ antiporter Mnh2 subunit B |
| virulence factor-related genes | |||
| hlgA | −1.408 | −4.341 | bi-component gamma-hemolysin HlgAB subunit A |
| hlgB | −1.872 | −4.502 | bi-component gamma-hemolysin HlgAB/HlgCB subunit B |
| hlgC | −1.795 | −5.407 | bi-component gamma-hemolysin HlgCB subunit C |
| hyl | −2.700 | −4.238 | alpha-hemolysin |
| spa | −2.869 | −4.204 | staphylococcal protein A |
| efb | −3.676 | −5.711 | complement convertase inhibitor Efb |
| ecb | −2.695 | −4.651 | complement convertase inhibitor Ecb |
| lukG | −1.933 | −3.232 | bi-component leukocidin LukGH subunit G |
| lukH | −0.947 | −1.595 | bi-component leukocidin LukGH subunit H |
| adsA | −1.439 | −1.701 | LPXTG-anchored adenosine synthase AdsA |
| Primers | Sequence (5′ → 3′) |
|---|---|
| 16S-F | CGCGTGAGTGATGAAGGTCT |
| 16S-R | ATTCCGGATAACGCTTGCCA |
| icaA-F | GTGAAACGATTGAAGATACG |
| icaA-R | GCTTTACCTCTGTTTTCTTG |
| icaB-F | CTTATGGCTTGATGAATGAC |
| icaB-R | GACTGCTTTTTCCTCTAATG |
| fnbA-F | AGATGAACTACCTGAAGAAC |
| fnbA-R | CCATTTTCAGTTCCTAAACC |
| fnbB-F | AGTGGATGAATTACCTGAAG |
| fnbB-R | CCATTTTCAGTTCCTAAACC |
| ILP78_12790-F | GATGGATCAAAGAACTTGAG |
| ILP78_12790-R | CTTTTAGCATGGAAAGGAAG |
| ILP78_01305-F | ATATAAGGATCGTGGGTTTG |
| ILP78_01305-R | CCGTTCACAGATATTTTAGC |
| argF-F | CCTGATGAAGTATGGAAAGA |
| argF-R | CGTATCAGCATTATGGAAAG |
| hlgA-F | GACTATTTCGTCCCAGATAA |
| hlgA-R | CTCGCTTTTATCACCTTTAC |
| efb-F | TAACAATAGCGGCAATAGG |
| efb-R | CTCACTGGTTTCTTTTCTCT |
| lukG-F | AAAGGAACAATAGGTAGTGG |
| lukG-R | ACTTCTCTTGATTCATCCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Sun, C.; Wu, S. Transcriptomic and Biochemical Analysis of the Antimicrobial Mechanism of Lipopeptide Iturin W against Staphylococcus aureus. Int. J. Mol. Sci. 2024, 25, 9949. https://doi.org/10.3390/ijms25189949
Ji Y, Sun C, Wu S. Transcriptomic and Biochemical Analysis of the Antimicrobial Mechanism of Lipopeptide Iturin W against Staphylococcus aureus. International Journal of Molecular Sciences. 2024; 25(18):9949. https://doi.org/10.3390/ijms25189949
Chicago/Turabian StyleJi, Yingyu, Chaomin Sun, and Shimei Wu. 2024. "Transcriptomic and Biochemical Analysis of the Antimicrobial Mechanism of Lipopeptide Iturin W against Staphylococcus aureus" International Journal of Molecular Sciences 25, no. 18: 9949. https://doi.org/10.3390/ijms25189949






