Adsorption of Carbon Dioxide and Nitrogen in Co3(ndc)3(dabco) Metal–Organic Framework
Abstract
:1. Introduction
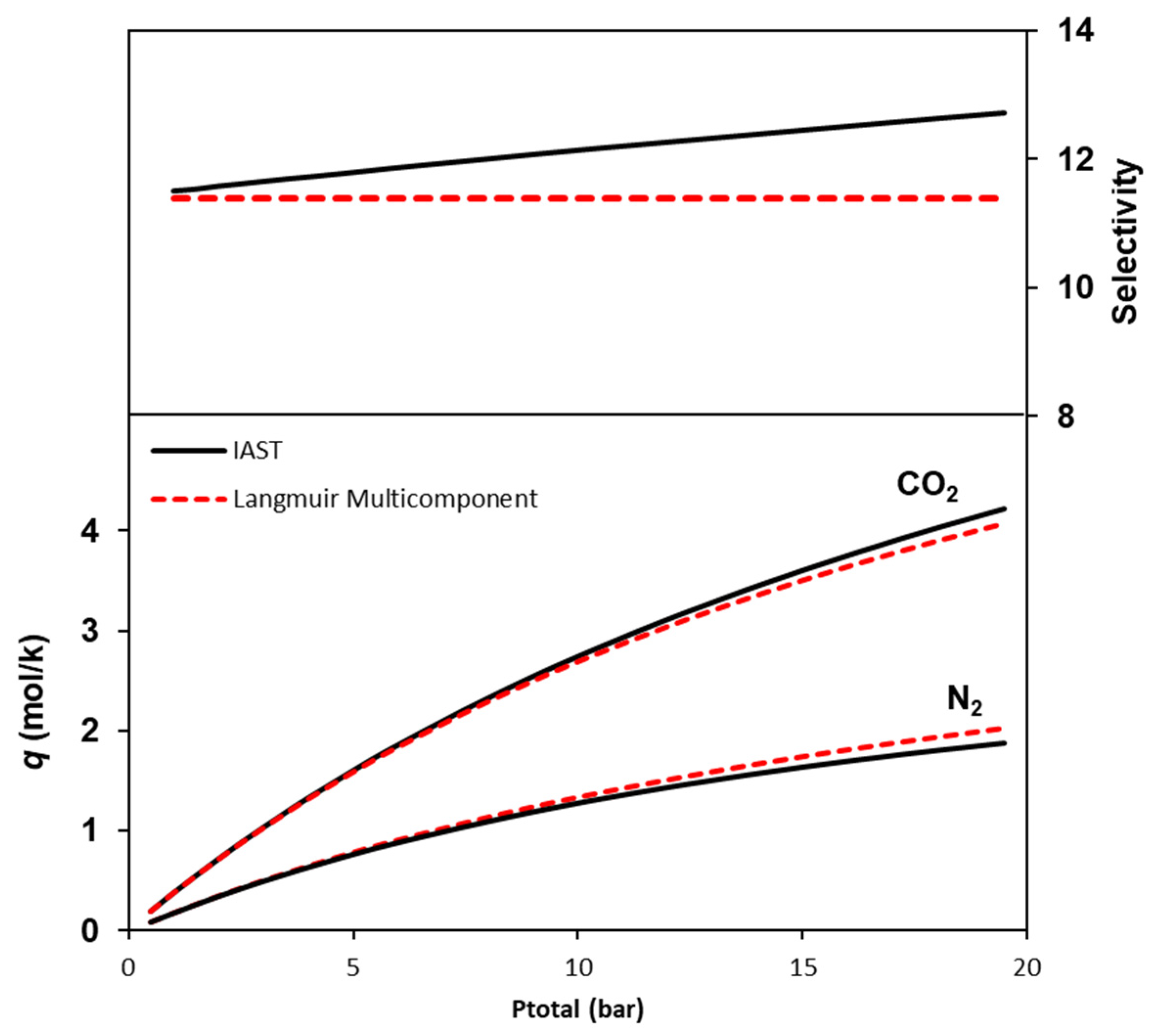
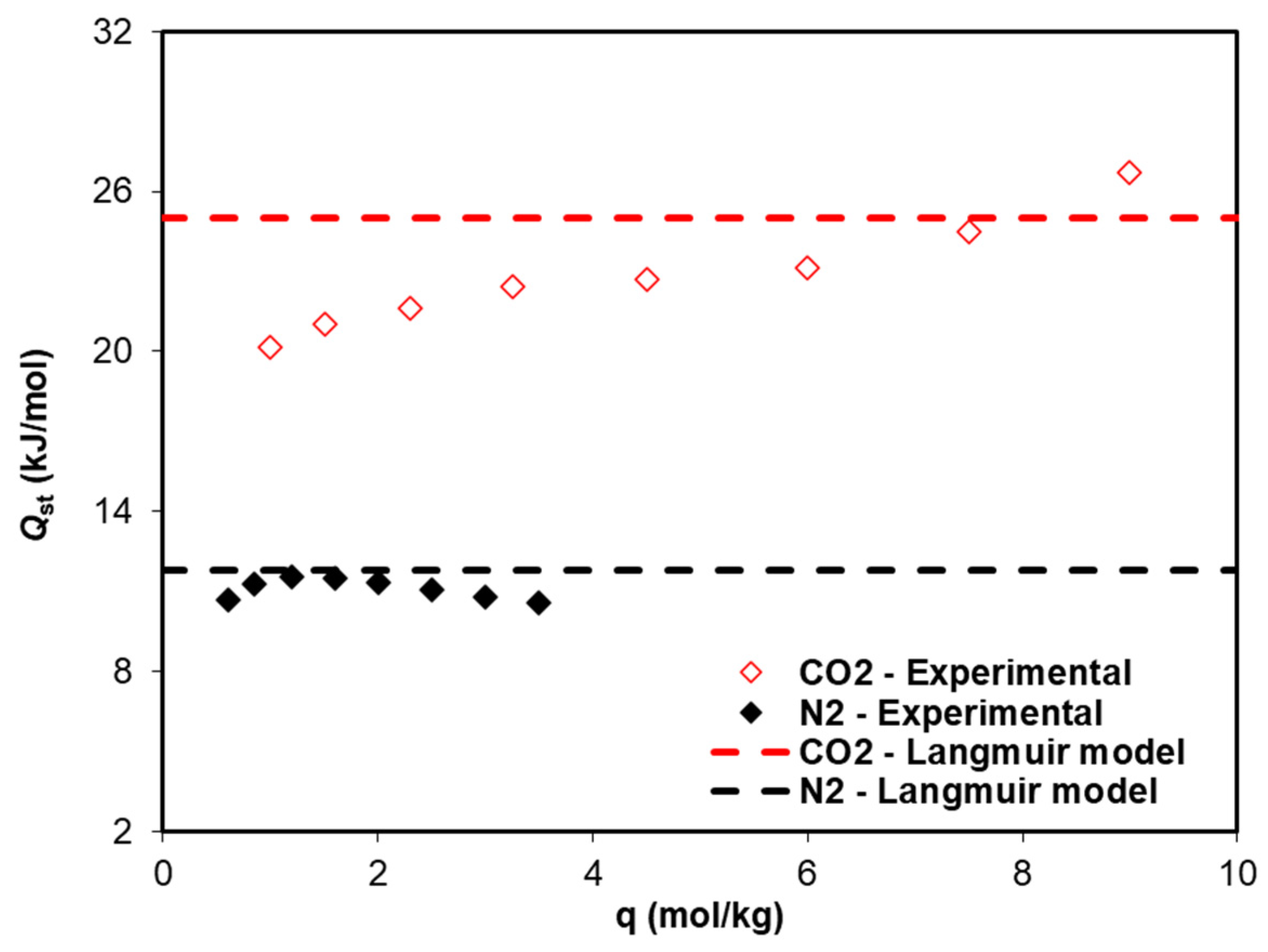
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commision. Stepping Up Europe’s 2030 Climate Ambition. Investing in a Climate-Neutral Future for the Benefit of Our People; European Commision: Brussels, Belgium, 2020. [Google Scholar]
- Ebner, A.D.; Ritter, J.A. State-of-the-art Adsorption and Membrane Separation Processes for Carbon Dioxide Production from Carbon Dioxide Emitting Industries. Sep. Sci. Technol. 2009, 44, 1273–1421. [Google Scholar] [CrossRef]
- Joss, L.; Gazzani, M.; Mazzotti, M. Rational design of temperature swing adsorption cycles for post-combustion CO2 capture. Chem. Eng. Sci. 2017, 158, 381–394. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Grande, C.A.; Rodrigues, A.E. Activated Carbon Honeycomb Monolith—Zeolite 13X Hybrid System to Capture CO2 from Flue Gases employing Electric Swing Adsorption. Chem. Eng. Sci. 2013, 104, 304–318. [Google Scholar] [CrossRef]
- Santos, M.P.S.; Grande, C.A.; Rodrigues, A.E. Pressure Swing Adsorption for Biogas Upgrading. Effect of Recycling Streams in Pressure Swing Adsorption Design. Ind. Eng. Chem. Res. 2011, 50, 974–985. [Google Scholar] [CrossRef]
- Grande, C.A. Biogas Upgrading by Pressure Swing Adsorption. In Biofuel’s Engineering Process Technology; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Ribeiro, R.P.; Sauer, T.P.; Lopes, F.V.; Moreira, R.F.; Grande, C.A.; Rodrigues, A.E. Adsorption of CO2, CH4, and N2 in Activated Carbon Honeycomb Monolith. J. Chem. Eng. Data 2008, 53, 2311–2317. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pevida, C.; Rubiera, F. Green Coffee Based CO2 Adsorbent with High Performance in Postcombustion Conditions. Fuel 2015, 140, 633–648. [Google Scholar] [CrossRef]
- Surra, E.; Ribeiro, R.P.P.L.; Santos, T.; Bernardo, M.; Mota, J.P.B.; Lapa, N.; Esteves, I.A.A.C. Evaluation of activated carbons produced from Maize Cob Waste for adsorption-based CO2 separation and biogas upgrading. J. Environ. Chem. Eng. 2022, 10, 107065. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Webley, P.; Li, G.; Singh, R.; Todd, R. Capture of CO2 from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption 2008, 14, 575–582. [Google Scholar] [CrossRef]
- Merel, J.; Clausse, M.; Meunier, F. Experimental Investigation on CO2 Post-Combustion Capture by Indirect Thermal Swing Adsorption using 13X and 5A Zeolites. Ind. Eng. Chem. Res. 2008, 47, 209–215. [Google Scholar] [CrossRef]
- Zelenak, V.; Halamova, D.; Gaberova, L.; Bloch, E.; Llewellyn, P. Amine-Modified SBA-12 Mesoporous Silica for Carbon Dioxide Capture: Effect of Amine Basicity on Sorption Properties. Microporous Mesoporous Mater. 2008, 116, 358–364. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Belmabkhout, Y.; Sayari, A. Polyethylenimine-Impregnated Mesoporous Silica: Effect of Amine Loading and Surface Alkyl Chains on CO2 Adsorption. Langmuir 2011, 27, 12411–12416. [Google Scholar] [CrossRef] [PubMed]
- Camacho, B.C.R.; Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Mota, J.P.B. Adsorption Equilibrium of Carbon Dioxide and Nitrogen on the MIL-53(Al) Metal Organic Framework. Sep. Purif. Technol. 2015, 141, 150–159. [Google Scholar] [CrossRef]
- Joss, L.; Hefti, M.; Bjelobrk, Z.; Mazzotti, M. On the Potential of Phase-change Adsorbents for CO2 Capture by Temperature Swing Adsorption. Energy Procedia 2017, 114, 2271–2278. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal–Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Mota, J.P.B. Surface Area and Porosity of Co3(ndc)3(dabco) Metal–Organic Framework and Its Methane Storage Capacity: A Combined Experimental and Simulation Study. J. Phys. Chem. C 2021, 125, 2411–2423. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Mota, J.P.B. Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn(dcpa) Metal-Organic Framework. Energies 2021, 14, 5598. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid Porous Solids: Past, Present, Future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Muller, U. Industrial Applications of Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Antunes, C.L.; Garate, A.U.; Portela, A.F.; Plaza, M.G.; Mota, J.P.B.; Esteves, I.A.A.C. Binderless shaped metal-organic framework particles: Impact on carbon dioxide adsorption. Microporous Mesoporous Mater. 2019, 275, 111–121. [Google Scholar] [CrossRef]
- Pons Picart, J.; Sanchez, F.J.; Casabó, J.; Rius, J.; Alvarez-Larena, A.; Ros, J. Synthesis and structural characterisation of a new cobalt(II) pentanuclear complex with a tetranucleating pyrazole-derived ligand. Inorg. Chem. Commun. 2002, 5, 130–133. [Google Scholar] [CrossRef]
- Chadghan, A.; Pons, J.; Caubet, A.; Casabó, J.; Ros, J.; Alvarez-Larena, A.; Francesc Piniella, J. Cobalt(II) complexes with pyrazole-derived ligands: Crystal structure of {bis[3-phenyl-5-(2-pyridyl) pyrazole]aquachlorocobalt(II)}chloride monohydrate. Polyhedron 2000, 19, 855–862. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Calvet, T.; Font-Bardia, M.; Choquesillo-Lazarte, D.; Pons, J. Variable behaviour of a flexible bispyrazole ligand: A Co(II) polymer and a unique Cu(II) penta-coordinated dimer. J. Mol. Struct. 2023, 1284, 135419. [Google Scholar] [CrossRef]
- Chun, H.; Jung, H.; Koo, G.; Jeong, H.; Kim, D.K. Efficient hydrogen sorption in 8-connected MOFs based on trinuclear pinwheel motifs. Inorg. Chem. 2008, 47, 5355–5359. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Barreto, J.; Grosso Xavier, M.D.; Martins, D.; Esteves, I.A.A.C.; Branco, M.; Tirolien, T.; Mota, J.P.B.; Bonfait, G. Cryogenic neon adsorption on Co3(ndc)3(dabco) metal-organic framework. Microporous Mesoporous Mater. 2020, 298, 110055. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark Alexander, V.; Olivier James, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing Kenneth, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef]
- Nabais, A.R.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Alves, V.D.; Esteves, I.A.A.C.; Neves, L.A. CO2/N2 gas separation using Fe(BTC)-based mixed matrix membranes: A view on the adsorptive and filler properties of metal-organic frameworks. Sep. Purif. Technol. 2018, 202, 174–184. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Ribeiro, R.P.P.L.; Camacho, B.C.R.; Lyubchyk, A.; Esteves, I.A.A.C.; Cruz, F.J.A.L.; Mota, J.P.B. Experimental and Computational Study of Ethane and Ethylene Adsorption in the MIL-53(Al) Metal Organic Framework. Microporous Mesoporous Mater. 2016, 230, 154–165. [Google Scholar] [CrossRef]
- Gumma, S.; Talu, O. Net Adsorption: A Thermodynamic Framework for Supercritical Gas Adsorption and Storage in Porous Solids. Langmuir 2010, 26, 17013–17023. [Google Scholar] [CrossRef]
- Brandani, S.; Mangano, E.; Sarkisov, L. Net, Excess and Absolute Adsorption and Adsorption of Helium. Adsorption 2016, 22, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L. Adsorption of Gas Mixtures—A Thermodynamic Approach. Ind. Eng. Chem. 1968, 60, 45–49. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of Mixed-Gas Adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]

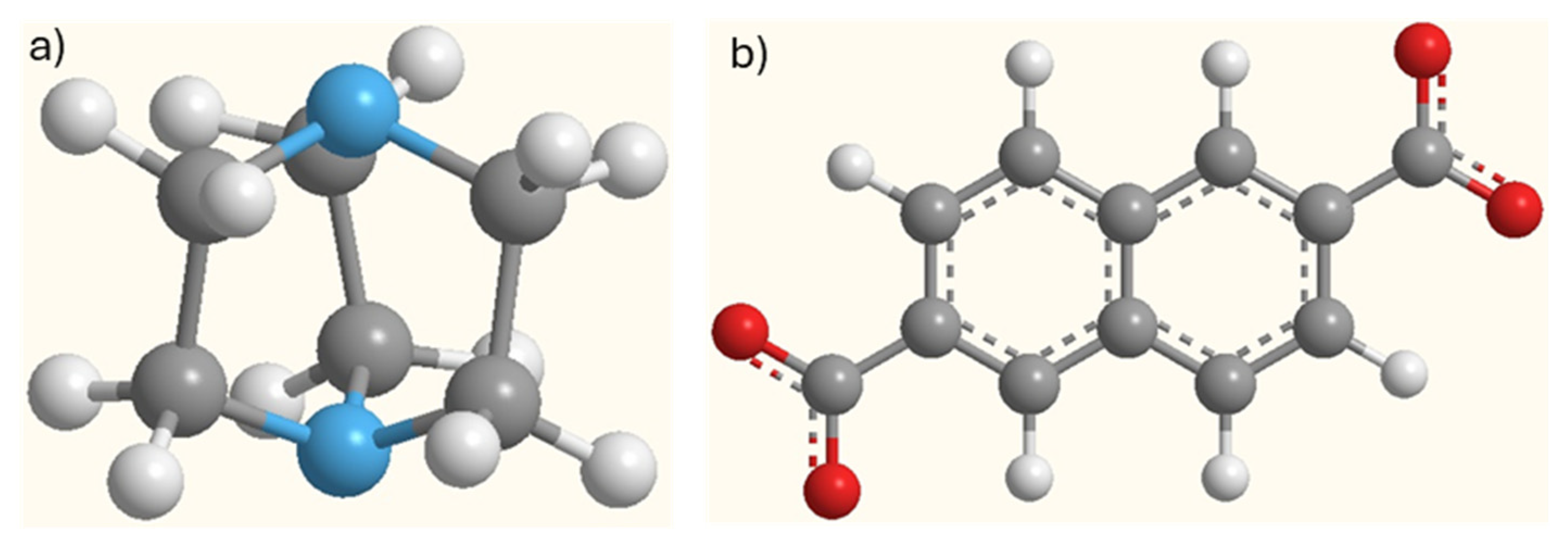

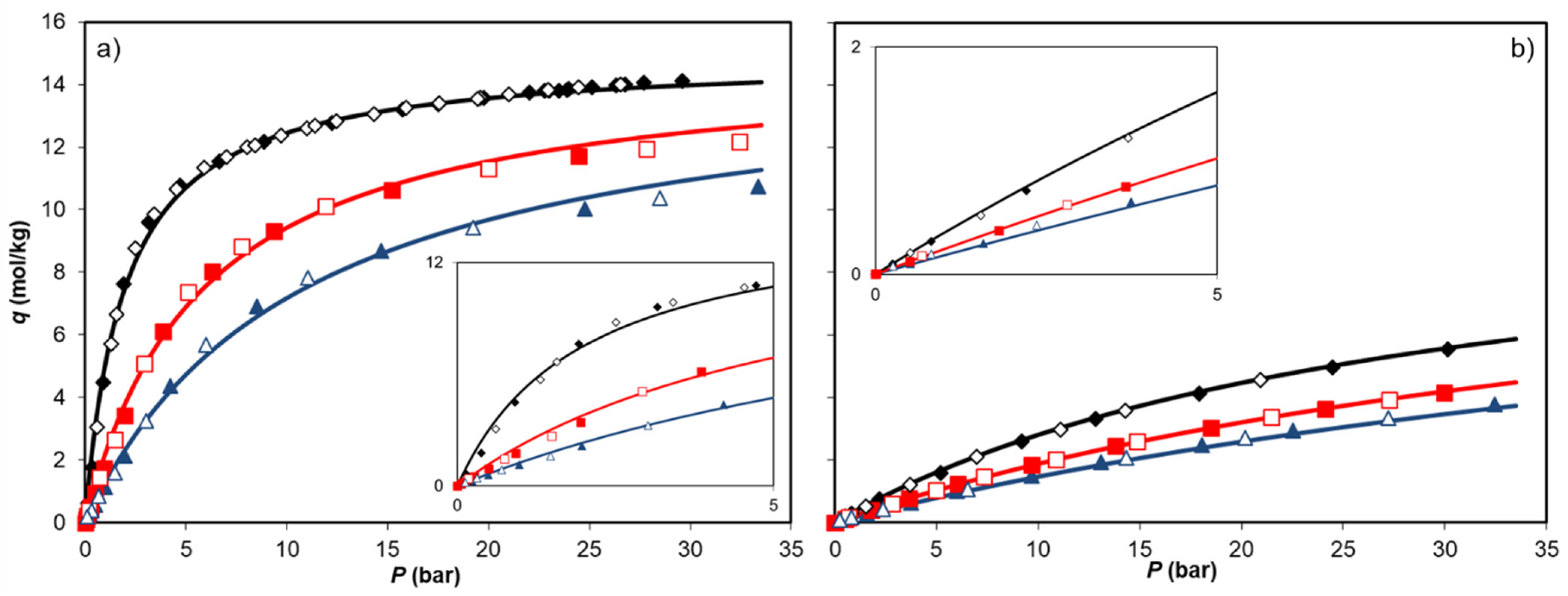
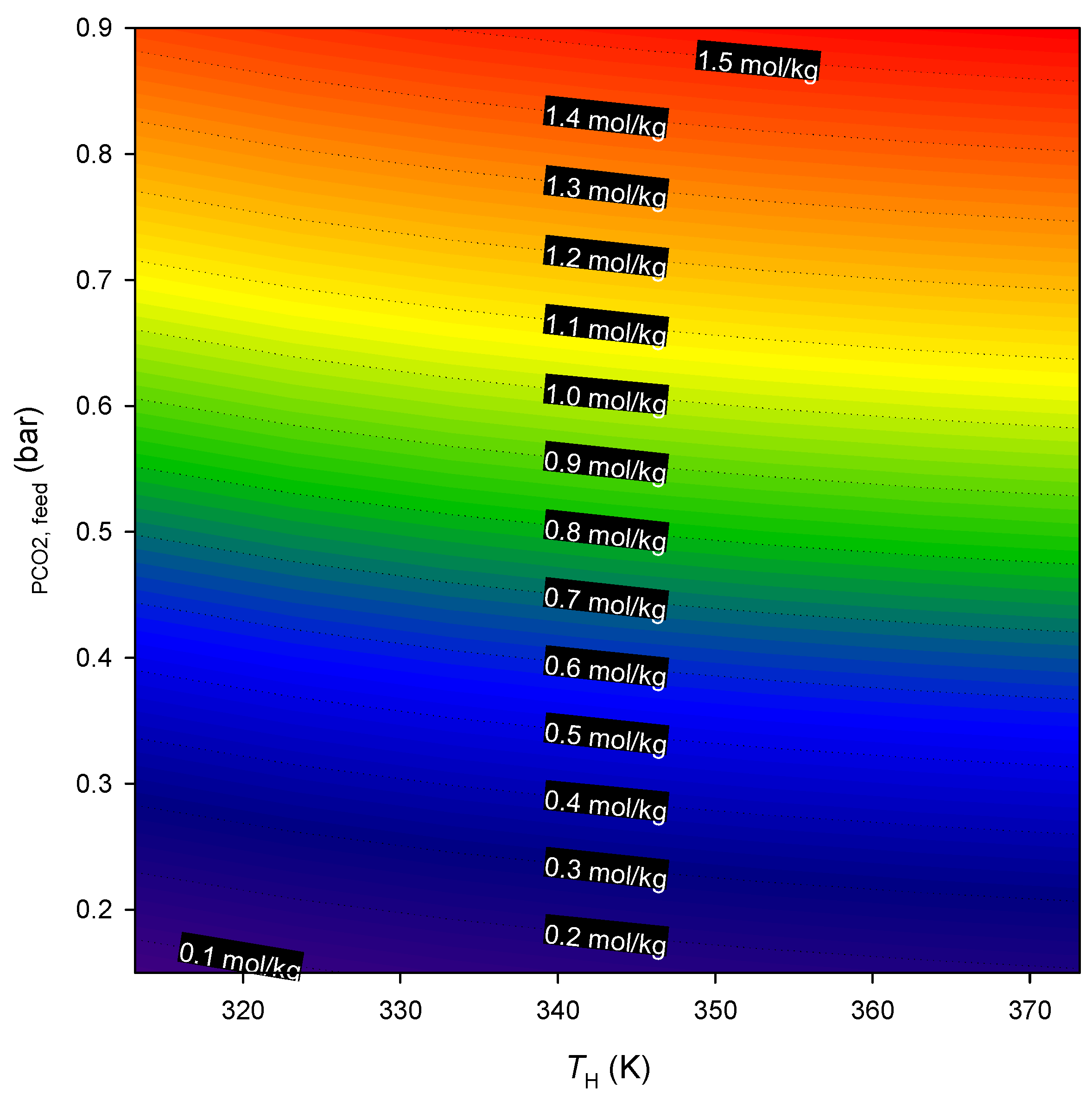
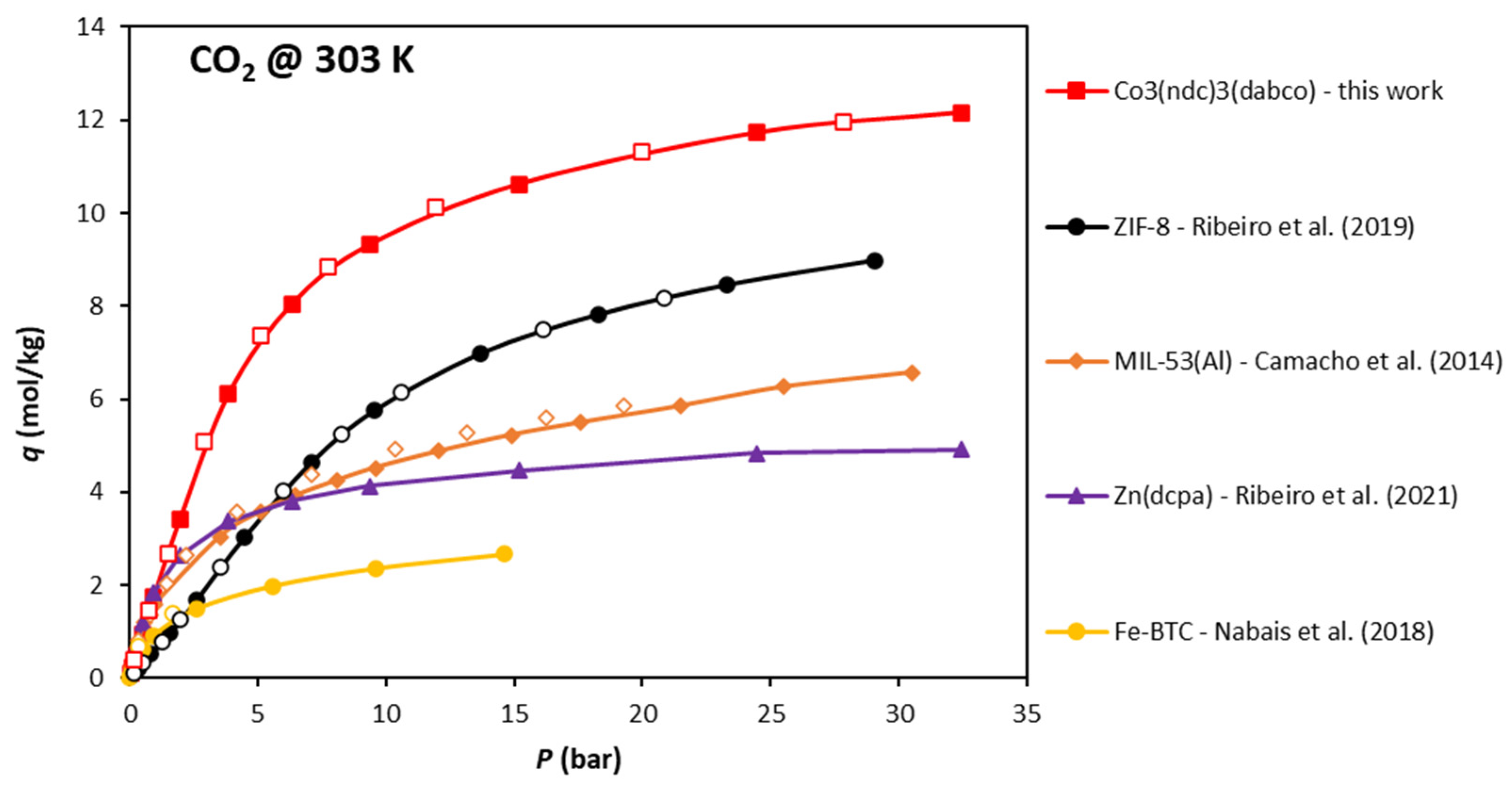
| T = 273 K | T = 303 K | T = 323 K | ||||||
|---|---|---|---|---|---|---|---|---|
(bar) | (mol/kg) | (mol/kg) | (bar) | (mol/kg) | (mol/kg) | (bar) | (mol/kg) | (mol/kg) |
| 0.04 | 0.13 | 0.13 | 0.06 | 0.11 | 0.11 | 0.07 | 0.08 | 0.09 |
| 0.15 | 0.61 | 0.62 | 0.13 | 0.23 | 0.24 | 0.23 | 0.26 | 0.27 |
| 0.38 | 1.74 | 1.76 | 0.27 | 0.49 | 0.50 | 0.49 | 0.55 | 0.58 |
| 0.91 | 4.43 | 4.48 | 0.50 | 0.91 | 0.93 | 0.98 | 1.09 | 1.14 |
| 1.92 | 7.51 | 7.63 | 0.93 | 1.69 | 1.74 | 1.97 | 2.04 | 2.14 |
| 3.16 | 9.41 | 9.60 | 1.95 | 3.31 | 3.42 | 4.21 | 4.15 | 4.36 |
| 4.73 | 10.47 | 10.76 | 3.86 | 5.91 | 6.12 | 8.52 | 6.47 | 6.91 |
| 6.66 | 11.14 | 11.55 | 6.31 | 7.69 | 8.04 | 14.66 | 7.90 | 8.68 |
| 8.87 | 11.61 | 12.18 | 9.36 | 8.79 | 9.32 | 24.79 | 8.65 | 10.04 |
| 12.26 | 11.98 | 12.78 | 15.20 | 9.74 | 10.62 | 33.37 | 8.79 | 10.74 |
| 15.73 | 12.14 | 13.20 | 24.46 | 10.23 | 11.73 | 28.48 | 8.76 | 10.37 |
| 17.50 | 12.19 | 13.38 | 32.46 | 10.06 | 12.16 | 19.22 | 8.38 | 9.43 |
| 19.75 | 12.19 | 13.57 | 27.81 | 10.21 | 11.95 | 11.04 | 7.24 | 7.82 |
| 22.03 | 12.17 | 13.75 | 19.97 | 10.13 | 11.32 | 6.02 | 5.37 | 5.68 |
| 22.80 | 12.15 | 13.79 | 11.94 | 9.44 | 10.12 | 3.02 | 3.10 | 3.25 |
| 22.99 | 12.14 | 13.80 | 7.75 | 8.41 | 8.84 | 1.48 | 1.53 | 1.60 |
| 23.49 | 12.10 | 13.80 | 5.13 | 7.08 | 7.36 | 0.70 | 0.83 | 0.86 |
| 23.89 | 12.08 | 13.83 | 2.93 | 4.91 | 5.07 | 0.31 | 0.39 | 0.41 |
| 23.99 | 12.11 | 13.86 | 1.49 | 2.58 | 2.66 | 0.12 | 0.18 | 0.19 |
| 25.11 | 12.06 | 13.92 | 0.74 | 1.42 | 1.46 | |||
| 26.34 | 12.00 | 13.98 | 0.18 | 0.38 | 0.39 | |||
| 26.78 | 11.99 | 14.01 | ||||||
| 27.71 | 11.92 | 14.05 | ||||||
| 29.62 | 11.79 | 14.12 | ||||||
| 26.54 | 12.00 | 14.00 | ||||||
| 24.46 | 12.11 | 13.90 | ||||||
| 22.97 | 12.16 | 13.82 | ||||||
| 21.02 | 12.20 | 13.69 | ||||||
| 19.57 | 12.21 | 13.57 | ||||||
| 19.48 | 12.19 | 13.55 | ||||||
| 17.56 | 12.21 | 13.41 | ||||||
| 15.93 | 12.19 | 13.26 | ||||||
| 14.34 | 12.11 | 13.06 | ||||||
| 12.46 | 12.02 | 12.83 | ||||||
| 11.39 | 11.94 | 12.68 | ||||||
| 11.01 | 11.90 | 12.61 | ||||||
| 9.73 | 11.76 | 12.38 | ||||||
| 8.41 | 11.54 | 12.06 | ||||||
| 8.04 | 11.49 | 12.00 | ||||||
| 7.03 | 11.26 | 11.70 | ||||||
| 5.92 | 10.97 | 11.34 | ||||||
| 4.54 | 10.38 | 10.66 | ||||||
| 3.41 | 9.64 | 9.85 | ||||||
| 2.51 | 8.63 | 8.78 | ||||||
| 1.58 | 6.56 | 6.66 | ||||||
| 1.31 | 5.63 | 5.71 | ||||||
| 0.61 | 3.01 | 3.04 | ||||||
| T = 273 K | T = 303 K | T = 323 K | ||||||
|---|---|---|---|---|---|---|---|---|
(bar) | (mol/kg) | (mol/kg) | (bar) | (mol/kg) | (mol/kg) | (bar) | (mol/kg) | (mol/kg) |
| 0.25 | 0.08 | 0.09 | 0.01 | 0.003 | 0.003 | 0.01 | 0.004 | 0.004 |
| 0.81 | 0.24 | 0.29 | 0.50 | 0.09 | 0.110 | 0.51 | 0.070 | 0.090 |
| 2.21 | 0.60 | 0.74 | 1.81 | 0.29 | 0.390 | 1.58 | 0.190 | 0.270 |
| 5.21 | 1.26 | 1.57 | 3.66 | 0.57 | 0.770 | 3.74 | 0.450 | 0.640 |
| 9.18 | 2.03 | 2.58 | 6.05 | 0.90 | 1.220 | 6.01 | 0.690 | 0.990 |
| 12.81 | 2.54 | 3.30 | 9.68 | 1.31 | 1.830 | 9.67 | 1.020 | 1.500 |
| 17.93 | 3.06 | 4.14 | 13.80 | 1.70 | 2.440 | 13.09 | 1.280 | 1.940 |
| 24.45 | 3.49 | 4.96 | 18.49 | 2.03 | 3.030 | 18.03 | 1.580 | 2.480 |
| 30.13 | 3.74 | 5.55 | 24.10 | 2.33 | 3.620 | 22.52 | 1.800 | 2.930 |
| 20.95 | 3.29 | 4.55 | 29.96 | 2.54 | 4.150 | 32.46 | 2.140 | 3.770 |
| 14.28 | 2.71 | 3.57 | 27.28 | 2.46 | 3.920 | 27.22 | 1.980 | 3.340 |
| 11.08 | 2.31 | 2.98 | 21.46 | 2.21 | 3.360 | 20.18 | 1.690 | 2.700 |
| 6.99 | 1.67 | 2.09 | 14.87 | 1.78 | 2.580 | 14.32 | 1.350 | 2.070 |
| 3.70 | 0.98 | 1.20 | 10.89 | 1.44 | 2.020 | 6.54 | 0.740 | 1.070 |
| 1.54 | 0.42 | 0.52 | 7.32 | 1.07 | 1.460 | 2.36 | 0.310 | 0.430 |
| 0.51 | 0.16 | 0.19 | 5.00 | 0.77 | 1.040 | 0.81 | 0.140 | 0.180 |
| 2.80 | 0.46 | 0.610 | 0.26 | 0.060 | 0.070 | |||
| 0.68 | 0.13 | 0.160 | ||||||
| CO2 | N2 | |
|---|---|---|
| (mol/kg) | 14.9 | 11.0 |
| (bar−1) | 8.47 × 10−6 | 1.09 × 10−4 |
| Q (kJ/mol) | 25.00 | 11.78 |
| ARE (%) | 7.3 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.P.P.L.; Mota, J.P.B. Adsorption of Carbon Dioxide and Nitrogen in Co3(ndc)3(dabco) Metal–Organic Framework. Int. J. Mol. Sci. 2024, 25, 9951. https://doi.org/10.3390/ijms25189951
Ribeiro RPPL, Mota JPB. Adsorption of Carbon Dioxide and Nitrogen in Co3(ndc)3(dabco) Metal–Organic Framework. International Journal of Molecular Sciences. 2024; 25(18):9951. https://doi.org/10.3390/ijms25189951
Chicago/Turabian StyleRibeiro, Rui Pedro Pinto Lopes, and José Paulo Barbosa Mota. 2024. "Adsorption of Carbon Dioxide and Nitrogen in Co3(ndc)3(dabco) Metal–Organic Framework" International Journal of Molecular Sciences 25, no. 18: 9951. https://doi.org/10.3390/ijms25189951
APA StyleRibeiro, R. P. P. L., & Mota, J. P. B. (2024). Adsorption of Carbon Dioxide and Nitrogen in Co3(ndc)3(dabco) Metal–Organic Framework. International Journal of Molecular Sciences, 25(18), 9951. https://doi.org/10.3390/ijms25189951







