Rhododendron luteum Sweet Flower Supercritical CO2 Extracts: Terpenes Composition, Pro-Inflammatory Enzymes Inhibition and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
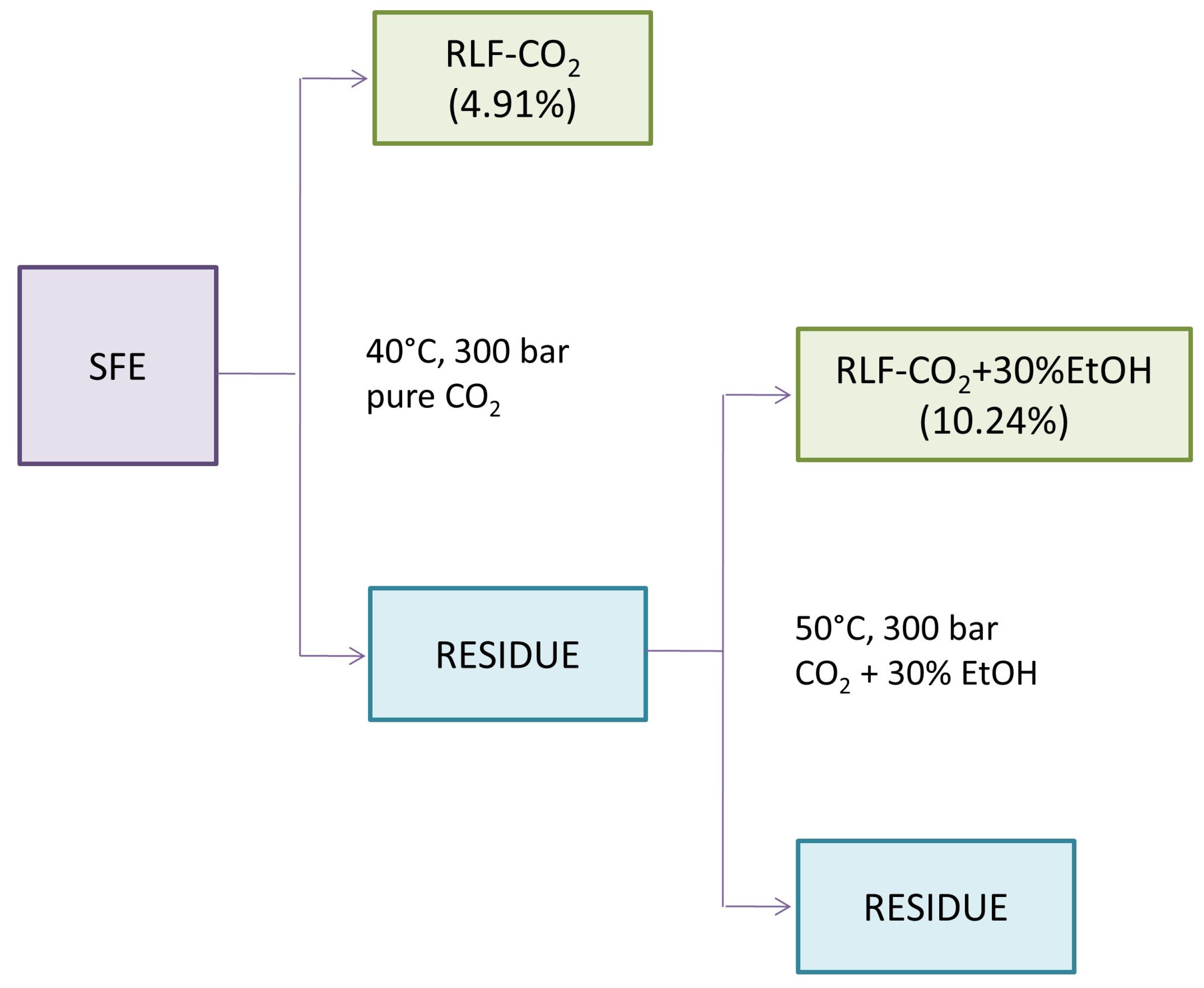
2.1. Extraction Yield and Recovery of Triterpenes
2.2. Content of Volatile Compounds in RL Samples
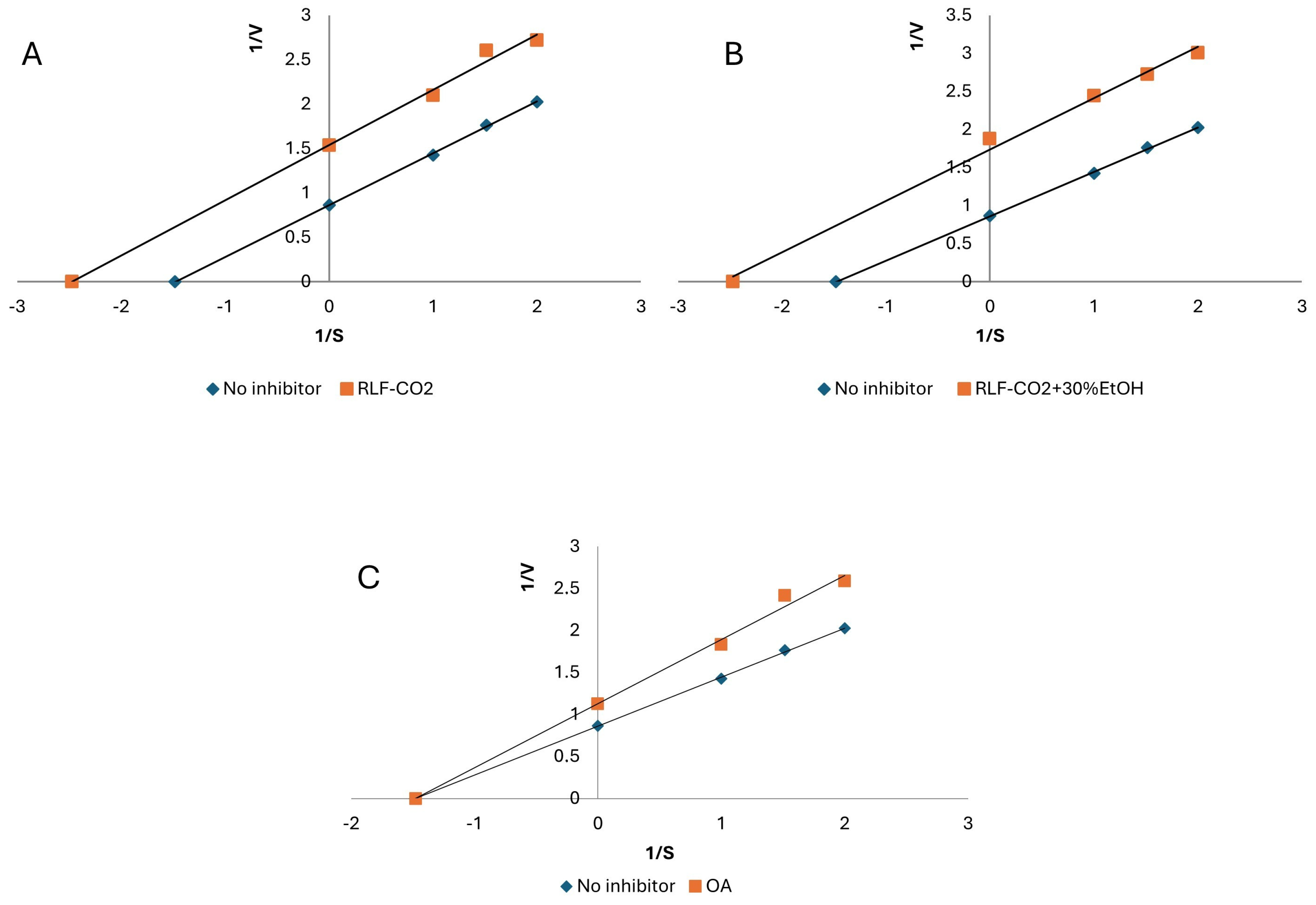
2.3. Biological Activity of RLF Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Apparatus
3.3. Supercritical Fluid Extraction (SFE)
3.4. LC-APCI-MS/MS Analysis of Triterpenes
3.5. Analysis of Volatile Compounds
3.6. Hyaluronidase Inhibition Assay
3.7. Lipoxygenase Inhibition Assay (LOX)
3.8. Xanthine Oxidase Inhibition Assay (XO)
3.9. Oxygen Radical Absorbance Capacity (ORAC) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmid-Schönbein, G.W. Analysis of Inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef] [PubMed]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Coussens, L.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- de Santana Souza, M.T.; Almeida, J.R.G.d.S.; de Souza Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.F.; dos Santos, M.R.V.; Quintans-Júnior, L.J. Structure-Activity Relationship of Terpenes with Anti-Inflammatory Profile-A Systematic Review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Zhou, B.; Gao, K. Chemical Constituents of Plants from the Genus Rhododendron. Chem. Biodivers. 2011, 8, 1958–1967. [Google Scholar] [CrossRef]
- Fu, C.N.; Mo, Z.Q.; Yang, J.B.; Cai, J.; Ye, L.J.; Zou, J.Y.; Qin, H.T.; Zheng, W.; Hollingsworth, P.M.; Li, D.Z.; et al. Testing Genome Skimming for Species Discrimination in the Large and Taxonomically Difficult Genus Rhododendron. Mol. Ecol. Resour. 2022, 22, 404–414. [Google Scholar] [CrossRef]
- Choi, Y.H.; Zhou, W.; Oh, J.; Choe, S.; Kim, D.W.; Lee, S.H.; Na, M. Rhododendric Acid A, a New Ursane-Type PTP1B Inhibitor from the Endangered Plant Rhododendron Brachycarpum G. Don. Bioorg. Med. Chem. Lett. 2012, 22, 6116–6119. [Google Scholar] [CrossRef]
- Ali, S.; Nisar, M.; Qaisar, M.; Khan, A.; Khan, A.A. Evaluation of the Cytotoxic Potential of a New Pentacyclic Triterpene from Rhododendron Arboreum Stem Bark. Pharm. Biol. 2017, 55, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Turan, I.; Aliyazicioglu, Y. Cytotoxic Effect of Rhododendron Luteum Leaf Extract on Human Cancer Cell Lines Selim. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Doğa Derg 2018, 21, 950–956. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Sieniawska, E.; Sinan, K.I.; Nancy Picot-Allain, M.C.; Yerlikaya, S.; Cengiz Baloglu, M.; Altunoglu, Y.C.; Senkardes, I.; Rengasamy, K.R.; Zengin, G.; et al. Utilisation of Rhododendron Luteum Sweet Bioactive Compounds as Valuable Source of Enzymes Inhibitors, Antioxidant, and Anticancer Agents. Food Chem. Toxicol. 2020, 135, 111052. [Google Scholar] [CrossRef]
- Usta, A.; Yayli, B.; Kahrinman, N.; Karaoglu, S.A.; Yayli, N. Composition and Antimicrobial Activity of Essential Oil from the Flower of Rhododendron Luteum Sweet. Asian J. Chem. 2012, 24, 1927–1930. [Google Scholar]
- Olech, M.; Łyko, L.; Nowak, R. Influence of Accelerated Solvent Extraction Conditions on the LC-ESI-MS/MS Polyphenolic Profile, Triterpenoid Content, and Antioxidant and Anti-Lipoxygenase Activity of Rhododendron Luteum Sweet Leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Łyko, L.; Olech, M.; Nowak, R. LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron Luteum and Their Anti-Inflammatory and Antioxidant Activities. Molecules 2022, 27, 827. [Google Scholar] [CrossRef]
- Shi, Q.; Li, T.T.; Wu, Y.M.; Sun, X.Y.; Lei, C.; Li, J.Y.; Hou, A.J. Meroterpenoids with Diverse Structures and Anti-Inflammatory Activities from Rhododendron anthopogonoides. Phytochemistry 2020, 180, 112524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, M.; Jin, C.; Ye, C.; Zhou, Y.; Wang, R.; Cui, H.; Zhou, W.; Li, G. A New Pentacyclic Triterpenoid from the Leaves of Rhododendron dauricum L. with Inhibition of NO Production in LPS-Induced RAW 264.7 Cells. Nat. Prod. Res. 2020, 34, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.G.; Hong, K.; Tang, M.; Tang, J.; Liu, L.X.; Gao, G.F.; Shen, Z.J.; Zhang, X.M.; Yi, Y. Untargeted Metabolite Profiling of Petal Blight in Field-Grown Rhododendron Agastum Using GC-TOF-MS and UHPLC-QTOF-MS/MS. Phytochemistry 2021, 184, 112655. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Sanchez-Martinez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibanez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice By-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef]
- Safayhi, H.; Sailer, E.R. Anti-Inflammatory Actions of Pentacyclic Triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef]
- Wang, J.; Shi, D.; Zheng, M.; Ma, B. Screening, Separation, and Evaluation of Xanthine Oxidase Inhibitors from Paeonia lactiflora Using Chromatography Combined with a Multi-Mode Microplate Reader. J. Sep. Sci. 2017, 40, 4160–4167. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Verónico Sánchez, F.J.; Solis, O.E.; Zamilpa, A.; Morales, R.G.; Dolores Pérez García, M.; Jiménez Ferrer, J.E.; Tortoriello, J. Extraction of Galphimines from Galphimia glauca with Supercritical Carbon Dioxide. Molecules 2020, 25, 477. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Wai, C.M. Supercritical Fluid Extraction in Herbal and Natural Product Studies-A Practical Review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- De la Peña Armada, R.; Bronze, M.R.; Matias, A.; Mateos-Aparicio, I. Triterpene-Rich Supercritical CO2 Extracts from Apple By-Product Protect Human Keratinocytes Against ROS. Food Bioprocess Technol. 2021, 14, 909–919. [Google Scholar] [CrossRef]
- Fokina, G.A. Triterpene Acids of Rhododendron Plant of the Flora of the USSR. Khimiya Prir. Soedin. 1980, 11, 734–735. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Ercisli, S. Comparative Analysis of Far East Sikhotinsky Rhododendron (Rh. Sichotense) and East Siberian Rhododendron (Rh. Adamsii) Using Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Molecules 2020, 25, 3774. [Google Scholar] [CrossRef]
- Sajfrtová, M.; Ličková, I.; Wimmerová, M.; Sovová, H.; Wimmer, Z. β-Sitosterol: Supercritical Carbon Dioxide Extraction from Sea Buckthorn (Hippophae rhamnoides L.) Seeds. Int. J. Mol. Sci. 2010, 11, 1842–1850. [Google Scholar] [CrossRef]
- Mus, A.A.; Goh, L.P.W.; Marbawi, H.; Gansau, J.A. The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines 2022, 10, 807. [Google Scholar] [CrossRef]
- Belova, N.V.; Fokina, G.A. The Triterpenoids of Some Species of Rhododendron. Khimiya Prir. Soedin. 1970, 6, 137–138. [Google Scholar] [CrossRef]
- Korpinen, R.I.; Välimaa, A.L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare)—Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef] [PubMed]
- Komissarenko, N.F.; Levashova, I.G. Biologically Active Substances of Rhododendron Luteum Sweet. Leaves. Rastit. Resur. 1980, 16, 406–411. [Google Scholar]
- Rhourri-Frih, B.; Chaimbault, P.; Claude, B.; Lamy, C.; André, P.; Lafosse, M. Analysis of Pentacyclic Triterpenes by LC-MS. A Comparative Study between APCI and APPI. J. Mass Spectrom. 2009, 44, 71–80. [Google Scholar] [CrossRef]
- Falev, D.I.; Ul’yanovskii, N.V.; Ovchinnikov, D.V.; Faleva, A.V.; Kosyakov, D.S. Screening and Semi-Quantitative Determination of Pentacyclic Triterpenoids in Plants by Liquid Chromatography–Tandem Mass Spectrometry in Precursor Ion Scan Mode. Phytochem. Anal. 2021, 32, 252–261. [Google Scholar] [CrossRef]
- García-Risco, M.R.; Vázquez, E.; Sheldon, J.; Steinmann, E.; Riebesehl, N.; Fornari, T.; Reglero, G. Supercritical Fluid Extraction of Heather (Calluna vulgaris) and Evaluation of Anti-Hepatitis C Virus Activity of the Extracts. Virus Res. 2015, 198, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.; Ramos-Romero, S.; Perona, J. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Michel, P.; Owczarek, A.; Matczak, M.; Kosno, M.; Szymański, P.; Mikiciuk-Olasik, E.; Kilanowicz, A.; Wesołowski, W.; Olszewska, M.A. Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules 2017, 22, 412. [Google Scholar] [CrossRef]
- Ullah, F.; Hussain, H.; Hussain, J.; Bukhari, I.A.; Khan, M.T.H.; Choudhary, M.I.; Gilani, A.H.; Ahmad, V.U. Tyrosinase Inhibitory Pentacyclic Triterpenes and Analgesic and Spasmolytic Activities of Methanol Extracts of Rhododendron collettianum. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 1213, 1076–1081. [Google Scholar] [CrossRef]
- Geerlofs, L.; He, Z.; Xiao, S.; Xiao, Z.C. Repeated Dose (90 Days) Oral Toxicity Study of Ursolic Acid in Han-Wistar Rats. Toxicol. Rep. 2020, 7, 610–623. [Google Scholar] [CrossRef]
- Ray, S.; Krmic, M.; Hussain, A.; Marvilli, C.; Fabian, R. Toxicity of Natural Products. In Encyclopedia of Toxicology; Academic Press: Cambridge, MA, USA, 2024; pp. 257–282. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar] [CrossRef]
- Lashkari, A.; Najafi, F.; Kavoosi, G.; Niazi, S. Evaluating the In Vitro Anti-Cancer Potential of Estragole from the Essential Oil of Agastache foeniculum [Pursh.] Kuntze. Biocatal. Agric. Biotechnol. 2020, 27, 101727. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Oliveira Brito Pereira Bezerra Martins, A.; Cesário, F.R.A.S.; Ferreira e Castro, F.; de Albuquerque, T.R.; Martins Fernandes, M.N.; Fernandes da Silva, B.A.; Quintans Júnior, L.J.; da Costa, J.G.M.; Melo Coutinho, H.D.; et al. Anti-Inflammatory and Antiedematogenic Activity of the Ocimum basilicum Essential Oil and Its Main Compound Estragole: In Vivo Mouse Models. Chem. Biol. Interact. 2016, 257, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.; Ennaji, H.; Maaghloud, F.E.; El Azhary, K.; Badou, A.; Elmakssoudi, A.; Aboulmouhajir, A.; Ibenmoussa, S.; JamalEddine, J.; Dakir, M. In Silico and in Vivo Anti-Inflammatory Effect of Eugenol and Acetyleugenol. Sci. Afr. 2024, 24, e02205. [Google Scholar] [CrossRef]
- Alexandre Carvalho, F.; Valadares de Moraes, N.; Eduardo Miller Crotti, A.; José Crevelin, E.; Gonzaga dos Santos, A. Casearia Essential Oil: An Updated Review on the Chemistry and Pharmacological Activities. Chem. Biodivers. 2023, 20, e202300492. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-Inflammation Activities of Essential Oil and Its Constituents from Indigenous Cinnamon (Cinnamomum osmophloeum) Twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8182 (accessed on 6 September 2024).
- Noh, H.J.; Yoon, J.Y.; Kim, G.S.; Lee, S.E.; Lee, D.Y.; Choi, J.H.; Kim, S.Y.; Kang, K.S.; Cho, J.Y.; Kim, K.H. Benzyl Alcohol Derivatives from the Mushroom Hericium erinaceum Attenuate LPS-Stimulated Inflammatory Response through the Regulation of NF-ΚB and AP-1 Activity. Immunopharmacol. Immunotoxicol. 2014, 36, 349–354. [Google Scholar] [CrossRef] [PubMed]
- De Micalizzi, Y.C.; Pappano, N.B.; Debattista, N.B. First and Second Order Derivative Spectrophotometric Determination of Benzyl Alcohol and Diclofenac in Pharmaceutical Forms. Talanta 1998, 47, 525–530. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the Anti-Inflammatory, Anti-Catabolic and pro-Anabolic Effects of E-Caryophyllene, Myrcene and Limonene in a Cell Model of Osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Zou, L.; Li, C.; Chen, X.; Yu, F.; Huang, Q.; Chen, L.; Wu, W.; Liu, Q. The Anti-Inflammatory Effects of Cinnamyl Alcohol on Sepsis-Induced Mice via the NLRP3 Inflammasome Pathway. Ann. Transl. Med. 2022, 10, 48. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Esters of Some Non-Steroidal Anti-Inflammatory Drugs with Cinnamyl Alcohol Are Potent Lipoxygenase Inhibitors with Enhanced Anti-Inflammatory Activity. Bioorg. Med. Chem. Lett. 2015, 25, 5028–5031. [Google Scholar] [CrossRef]
- Chuang, L.T.; Shih, Y.H.; Huang, W.C.; Lin, L.C.; Hsu, C.; Chyuan, J.H.; Tsai, T.H.; Tsai, P.J. In Vitro and in Vivo Screening of Wild Bitter Melon Leaf for Anti-Inflammatory Activity against Cutibacterium acnes. Molecules 2020, 25, 4277. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of Cosmeceutical Potentials of Selected Mushroom Fruitbody Extracts through Evaluation of Antioxidant, Anti-Hyaluronidase and Anti-Tyrosinase Activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Yoshimura, R.; Matsuyama, M.; Mitsuhashi, M.; Takemoto, Y.; Tsuchida, K.; Kawahito, Y.; Sano, H.; Nakatani, T. Relationship between Lipoxygenase and Human Testicular Cancer. Int. J. Mol. Med. 2004, 13, 389–393. [Google Scholar] [CrossRef]
- Kostic, D.A.; Dimitrijevic, D.S.; Stojanovic, G.S.; Palic, I.R. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]
- Tekulu, G.H.; Hiluf, T.; Brhanu, H.; Araya, E.M.; Bitew, H.; Haile, T. Anti-Inflammatory and Anti-Nociceptive Property of Capparis Tomentosa Lam. Root Extracts. J. Ethnopharmacol. 2020, 253, 112654. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Rój, E.; Nowak, R. Effects of Supercritical Carbon Dioxide Extraction (SC-CO2) on the Content of Tiliroside in the Extracts from Tilia L. Flowers. Open Chem. 2019, 17, 302–312. [Google Scholar] [CrossRef]
- Desmiaty, Y.; Hanafi, M.; Saputri, F.C.; Elya, B.; Rifai, E.A.; Syahdi, R.R. Two Triterpenoids from Rubus fraxinifolius Leaves and Their Tyrosinase and Elastase Inhibitory Activities. Sci. Rep. 2021, 11, 20452. [Google Scholar] [CrossRef]
- Grabowska, K.; Wróbel, D.; Żmudzki, P.; Podolak, I. Anti-Inflammatory Activity of Saponins from Roots of Impatiens parviflora DC. Nat. Prod. Res. 2020, 34, 1581–1585. [Google Scholar] [CrossRef]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the Way towards Effective Plant-Based Inhibitors of Hyaluronidase and Tyrosinase: A Critical Review on a Structure–Activity Relationship. J. Enzym. Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Lysaght, J.; Krishnamoorthy, S.; Reynolds, J.V.; O’Byrne, K.; Nie, D.; Honn, K.V. Lipoxygenase Metabolism: Roles in Tumor Progression and Survival. Cancer Metastasis Rev. 2007, 26, 503–524. [Google Scholar] [CrossRef]
- Baylac, S.; Racine, P. Inhibition of 5-Lipoxygenase by Essential Oils and Other Natural Fragment Extracts. Int. J. Aromather. 2003, 13, 138–142. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a Novel Anti-Inflammatory and Anti-Cancer Dietary Triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Habza-Kowalska, E.; Gawlik-Dziki, U.; Dziki, D. Mechanism of Action and Interactions between Thyroid Peroxidase and Lipoxygenase Inhibitors Derived from Plant Sources. Biomolecules 2019, 9, 663. [Google Scholar] [CrossRef]
- Werz, O. Inhibition of 5-Lipoxygenase Product Synthesis by Natural Compounds of Plant Origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef]
- Mawa, S.; Husain, K.; Jantan, I. Triterpenes with 5-Lipoxigenase (5-LOX) and Xanthine Oxidase (XOD) Inhibitory Activity from the Stem of Ficus Aurantiaca Griff. Open Conf. Proccedings J. 2013, 4, 2013. [Google Scholar] [CrossRef]
- Yin, M.-C.; Chan, K.-C. Nonenzymatic Antioxidative and Antiglycative Effects of Oleanolic Acid and Ursolic Acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, Free Radicals and Antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and Antiatherogenic Activities of Pentacyclic Triterpenic Diols and Acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef]
- Ibarra, A.; Cases, J.; Bily, A.; Bai, N.; Roller, M. Importance of Extract Standardization and In Vitro/Ex Vivo Assay Selection for the Evaluation of Antioxidant Activity of Botanicals: A Case Study on Three Rosmarinus officinalis L. Extracts. J. Med. Food 2010, 13, 1167–1175. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Gawlik-Dziki, U.; Nowak, R. Antioxidant, Anti-Inflammatory, and Anti-Diabetic Activity of Phenolic Acids Fractions Obtained from Aerva lanata (L.) Juss. Molecules 2021, 26, 3486. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Angelov, G.; Gawlik-dziki, U. LC-ESI-MS/MS-MRM Profiling of Polyphenols and Antioxidant Activity Evaluation of Junipers of Different Origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]



| RLF-CO2 | RLF-CO2 + 30%EtOH | |
|---|---|---|
| β-Sitosterol | 80.10 ± 0.42 | 26.87 ± 0.61 |
| 3β-Taraxerol | 49.39 ± 0.05 | 7.72 ± 0.08 |
| α-Amyrin | 28.45 ± 0.07 | 4.81 ± 0.01 |
| Lupeol | 17.00 ± 0.14 | 1.95 ± 0.02 |
| Erythrodiol/Uvaol | 3.60 ± 0.03 | 2.98 ± 0.04 |
| Oleanolic/Ursolic acids | 10.43 ± 0.12 | 94.35 ± 0.35 |
| Corosolic acid | BQL | 0.88 ± 0.00 |
| Maslinic acid | 0.83 ± 0.01 | 2.85 ± 0.02 |
| Euscaphic acid | BQL | 4.93 ± 0.00 |
| Constituent | RIexp a | RIlit b | RLF-CO2 % |
|---|---|---|---|
| Hexanal | 783 | 785 | 0.50 |
| Furfural | 803 | 801 | t |
| (Z)-Hex-3-en-1-ol | 847 | 851 | 0.06 |
| Heptanal | 882 | 882 | 0.10 |
| Benzaldehyde | 932 | 936 | 0.42 |
| α-Pinene | 934 | 936 | 1.68 |
| 6-Methylhept-5-en-2-one | 970 | 972 | 0.36 |
| β-Pinene | 973 | 978 | 0.35 |
| Hexanoic acid | 984 | 983 | 0.27 |
| (E,E)-Hepta-2,4-dienal | 985 | 987 | 0.27 |
| Myrcene | 986 | 987 | t |
| Decane | 1000 | 1000 | 0.34 |
| α-Phellandrene | 1002 | 1002 | 0.19 |
| Benzyl alcohol | 1005 | 1006 | 10.65 |
| Phenylacetaldehyde | 1010 | 1012 | 0.50 |
| p-Cymene | 1013 | 1015 | 0.30 |
| β-Phellandrene | 1020 | 1023 | t |
| 1,8-Cineole | 1021 | 1024 | 0.10 |
| Limonene | 1024 | 1025 | 0.42 |
| (E)-Oct-2-en-1-al | 1033 | 1034 | 0.14 |
| (E)-β-Ocimene | 1039 | 1041 | 0.27 |
| (E,Z)-3,5-Octadien-2-one | 1045 | 1050 | 0.05 |
| p-Cresol | 1057 | 1055 | 0.19 |
| trans-Linalool oxide (f) | 1060 | 1062 | 0.17 |
| (E,E)-octa-3,5-dien-2-one | 1068 | 1070 | 0.05 |
| Methyl benzoate | 1071 | 1072 | 0.23 |
| cis-Linalool oxide (f) | 1074 | 1072 | t |
| 1-Acetyl-2-methylcyclopentene | 1080 | - | t |
| Nonanal | 1084 | 1076 | 0.44 |
| β-Phenylethanol | 1086 | 1085 | 5.20 |
| Benzyl nitrile | 1092 | 1097 | 0.44 |
| Camphor | 1121 | 1123 | 0.35 |
| trans-Pinocarveol | 1125 | 1126 | t |
| Benzyl acetate | 1130 | 1134 | 0.10 |
| Menthone | 1134 | 1136 | 0.07 |
| Ethyl benzoate | 1149 | 1149 | 0.16 |
| Borneol | 1152 | 1150 | 0.16 |
| Terpinen-4-ol | 1163 | 1164 | 0.24 |
| α-Terpineol | 1174 | 1176 | 1.67 |
| Estragole | 1176 | 1179 | 5.80 |
| Myrtenol | 1181 | 1178 | 0.35 |
| Decanal | 1185 | 1180 | 0.10 |
| β-Cyclocitral | 1196 | 1195 | 0.13 |
| Dodecane | 1199 | 1200 | 5.07 |
| Carvone | 1215 | 1214 | 0.33 |
| 2-Methoxybenzyl alcohol | 1227 | 1223 | 0.08 |
| (Z)-Cinnamyl alcohol | 1231 | - | 2.05 |
| (E)-Cinnamaldehyde | 1233 | 1234 | 0.50 |
| Nonanoic acid | 1258 | 1260 | 0.20 |
| Thymol | 1265 | 1267 | 0.28 |
| Bornyl acetate | 1270 | 1270 | t |
| (E)-Cinnamyl alcohol | 1275 | 1275 | 3.77 |
| Carvacrol | 1278 | 1278 | 1.87 |
| Methyl 2-methoxybenzoate | 1297 | 1295 | 1.77 |
| Eugenol | 1331 | 1331 | 7.26 |
| α-Cubebene | 1350 | 1355 | 0.40 |
| Methyleugenol | 1365 | 1369 | 0.64 |
| α-Ylangene | 1371 | 1376 | - |
| a-Copaene | 1377 | 1379 | 0.54 |
| β-Bourbonene | 1385 | 1387 | 0.05 |
| Tetradecane | 1400 | 1400 | 2.92 |
| α-Ionone | 1406 | 1407 | 0.40 |
| α-Gurjunene | 1411 | 1413 | 0.18 |
| β-Caryophyllene | 1419 | 1420 | 3.09 |
| Geranylacetone | 1429 | 1430 | 1.16 |
| β-Copaene | 1430 | 1403 | t |
| Calarene | 1434 | 1437 | 0.93 |
| cis-Muurola-3,5-diene | 1445 | 1447 | 0.20 |
| Selina-4(15),6-diene | 1447 | 1449 | 0.25 |
| α-Humulene | 1452 | 1455 | 0.42 |
| Dodecanol | 1459 | 1460 | 3.01 |
| β-Ionone epoxide | 1462 | 1460 | 0.71 |
| β-Ionone | 1465 | 1467 | 0.48 |
| ar-Curcumene | 1472 | 1473 | 1.91 |
| γ-Curcumene | 1473 | 1474 | 0.32 |
| Germacrene D | 1477 | 1480 | 0.65 |
| Eugenol acetate | 1485 | 1483 | 0.13 |
| α-Zingiberene | 1487 | 1489 | 1.26 |
| Dihydroactinidiolide | 1491 | 1493 | 1.94 |
| α-Selinene | 1492 | 1494 | 0.44 |
| α-Muurolene | 1494 | 1496 | 0.58 |
| δ-Amorphene | 1496 | 1496 | 0.10 |
| Pentadecane | 1550 | 1500 | 0.10 |
| β-Bisabolene | 1501 | 1503 | 0.91 |
| γ-Cadinene | 1507 | 1507 | 1.40 |
| cis-Calamenene | 1510 | 1517 | 0.22 |
| δ-Cadinene | 1515 | 1520 | 3.75 |
| Cadina-1,4-diene | 1523 | 0.13 | |
| α-Calacorene | 1530 | 1527 | 0.10 |
| α-Cadinene | 1533 | 1534 | 0.44 |
| Selina-3,7(11)-diene | 1543 | 1542 | 0.08 |
| Spathulenol | 1565 | 1572 | 0.17 |
| Caryophyllene oxide | 1572 | 1578 | 0.22 |
| Unknown 1 | 1583 | - | 4.23 |
| Ledol | 1598 | 1600 | 0.19 |
| Hexadecane | 1597 | 1600 | 0.22 |
| Unknown 2 | 1616 | - | 1.88 |
| T-cadinol | 1627 | 1633 | 0.11 |
| α-Cadinol | 1639 | 1642 | 0.15 |
| Tetradecanol | 1659 | 1661 | 0.61 |
| Heptadecane | 1697 | 1700 | 2.25 |
| Benzyl salicylate | 1833 | 1847 | 0.05 |
| Total identified | 89.80 |
| Compound | Anti-Inflammatory Activity | Reference |
|---|---|---|
| Benzyl alcohol | LPS-stimulated inflammatory response suppression through the regulation of NF-κB and AP-1 activity. It is used as an excipient in injectable forms alongside diclofenac sodium. The presence of benzyl alcohol helps enhance the overall anti-inflammatory effects of such formulations. | [49,50] |
| Eugenol | Inhibiting COX-2, reducing leukocyte migration and rolling in response to chemotactic stimuli. Inhibiting pro-inflammatory mediators, such as IL-1β and TNF-α. Blocking NF-κB activation, which is a key regulator in the inflammatory response. Inhibiting both the COX-2 and 5-LOX pathways. | [45,51] |
| Estragole | Reducing paw edema induced by carrageenan, dextran, histamine and arachidonic acid. Inhibiting vascular permeability, leukocyte migration and protein extravasation. | [44] |
| β-Caryophyllene | Inhibiting IL-1β-induced nitric oxide (NO) production in human chondrocytes. Reduces the expression of inflammatory mediators such as iNOS, MMP-1 and MMP-13 and activates the CB2 receptor pathway, contributing to its effects in osteoarthritis models. | [52] |
| (E)- and (Z)-Cinnamyl alcohol | Inhibition of the lipoxygenase (LOX) pathway, reducing the production of leukotrienes and other pro-inflammatory mediators. In sepsis-induced models, it reduced the levels of pro-inflammatory cytokines IL-1β and IL-18 in the circulatory system by targeting the NLRP3 inflammasome pathway. | [53,54] |
| Dihydroactinidiolide | Reducing IL-8 production in Cutibacterium acnes-stimulated THP-1 cells. | [55] |
| Sample | Hyaluronidase IC50 (μg of DE/mL) | LOX IC50 (mg of DE/mL) | XO IC50 (mg of DE/mL) | ORAC (µg of Trolox/mg of DE) |
|---|---|---|---|---|
| RLF-CO2 | 106.98 ± 4.45 | 0.67 ± 0.03 | 4.50 ± 0.15 | 119.92 ± 7.33 |
| RLF-CO2 + 30%EtOH | 23.75 ± 1.44 | 0.50 ± 0.02 | 2.36 ± 0.09 | 338.99 ± 8.6 |
| OA | 500.88 ± 10.28 | 0.68 ± 0,03 | 2.08 ± 0.09 | 54.39 ± 2.81 |
| Quercetin | - | 9.37 ± 0.00 | - | - |
| Allopurinol | - | - | 0.03 ± 0.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyko, L.; Olech, M.; Gawlik, U.; Krajewska, A.; Kalemba, D.; Tyśkiewicz, K.; Piórecki, N.; Prokopiv, A.; Nowak, R. Rhododendron luteum Sweet Flower Supercritical CO2 Extracts: Terpenes Composition, Pro-Inflammatory Enzymes Inhibition and Antioxidant Activity. Int. J. Mol. Sci. 2024, 25, 9952. https://doi.org/10.3390/ijms25189952
Łyko L, Olech M, Gawlik U, Krajewska A, Kalemba D, Tyśkiewicz K, Piórecki N, Prokopiv A, Nowak R. Rhododendron luteum Sweet Flower Supercritical CO2 Extracts: Terpenes Composition, Pro-Inflammatory Enzymes Inhibition and Antioxidant Activity. International Journal of Molecular Sciences. 2024; 25(18):9952. https://doi.org/10.3390/ijms25189952
Chicago/Turabian StyleŁyko, Lena, Marta Olech, Urszula Gawlik, Agnieszka Krajewska, Danuta Kalemba, Katarzyna Tyśkiewicz, Narcyz Piórecki, Andriy Prokopiv, and Renata Nowak. 2024. "Rhododendron luteum Sweet Flower Supercritical CO2 Extracts: Terpenes Composition, Pro-Inflammatory Enzymes Inhibition and Antioxidant Activity" International Journal of Molecular Sciences 25, no. 18: 9952. https://doi.org/10.3390/ijms25189952








