Chlorogenic Acid Enhances the Intestinal Health of Weaned Piglets by Inhibiting the TLR4/NF-κB Pathway and Activating the Nrf2 Pathway
Abstract
:1. Introduction
2. Results
2.1. Growth Performance
2.2. Intestinal Morphology
2.3. Expression of Tight Junctions in the Jejunum and Ileum
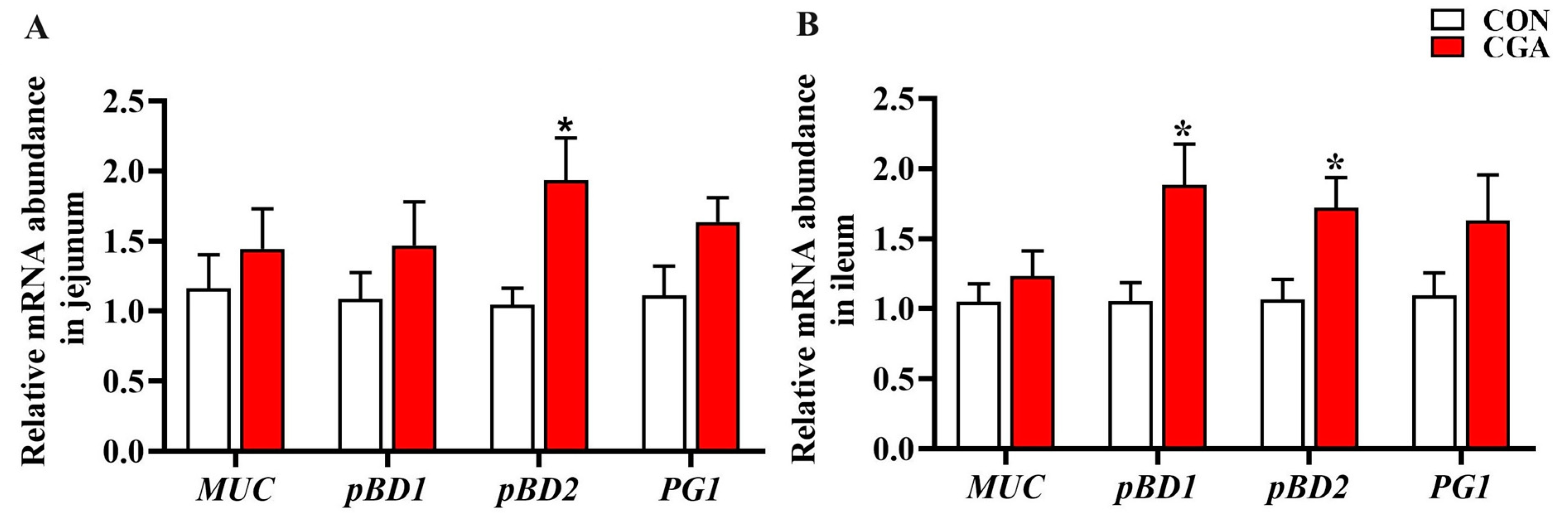
2.4. mRNA Expression of Porcine Beta Defensins and Mucins
2.5. Microbes in the Ileal Contents of the Piglets
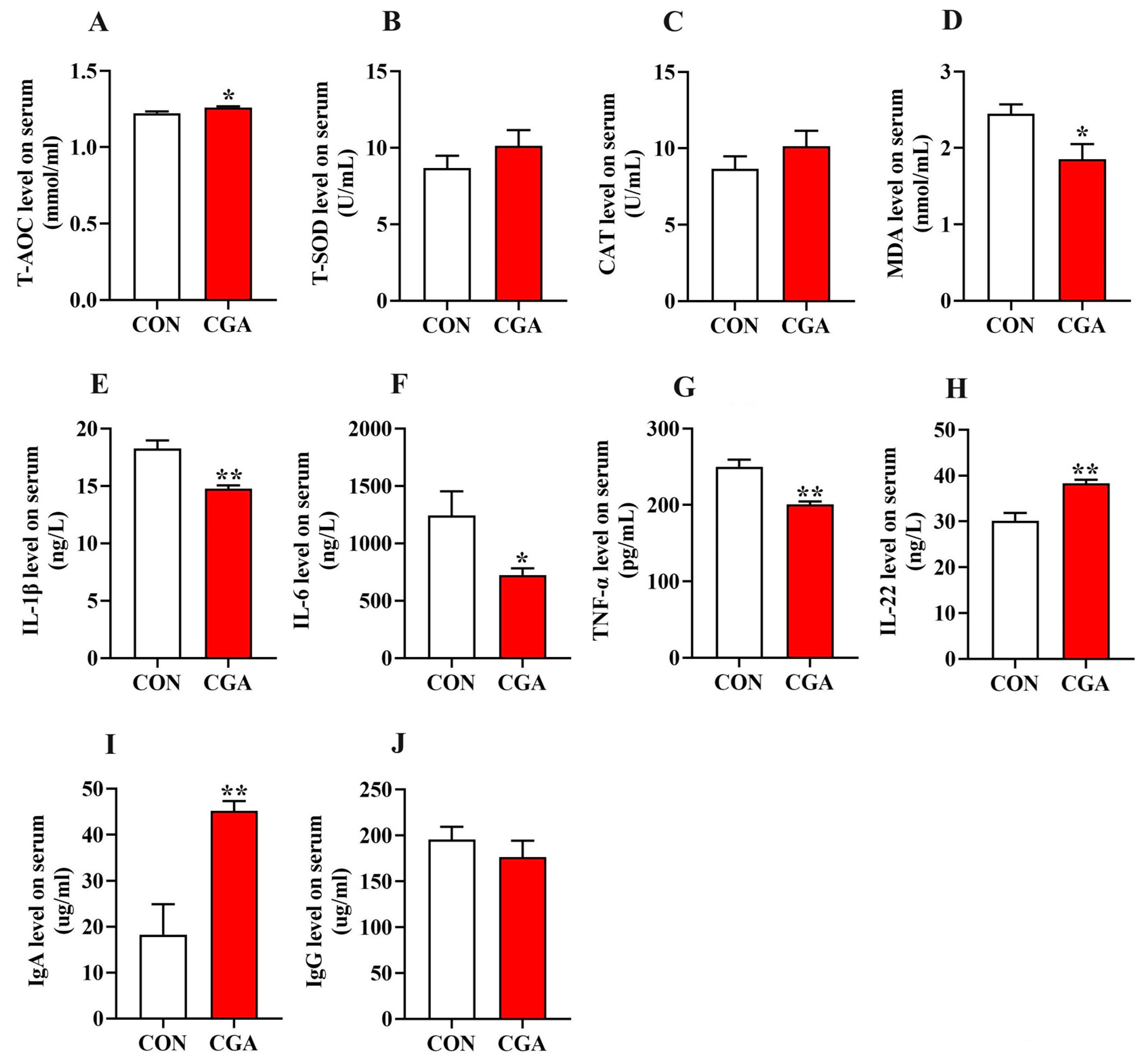
2.6. Antioxidant Status and Immune-Inflammatory Profiles in the Serum and Intestine of Piglets
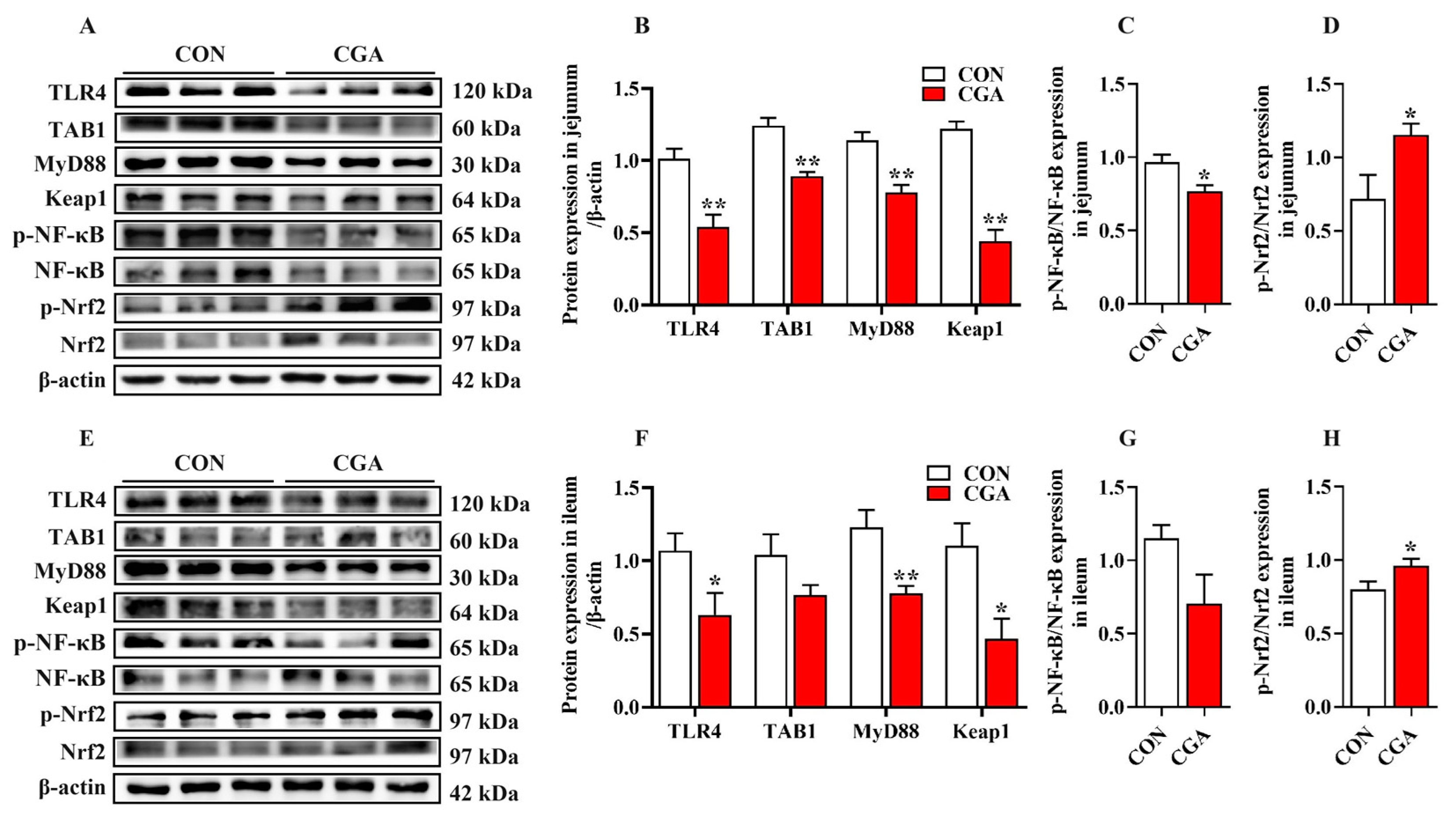
2.7. Activation of the TLR4/NF-κB and Nrf2 Signaling Pathways
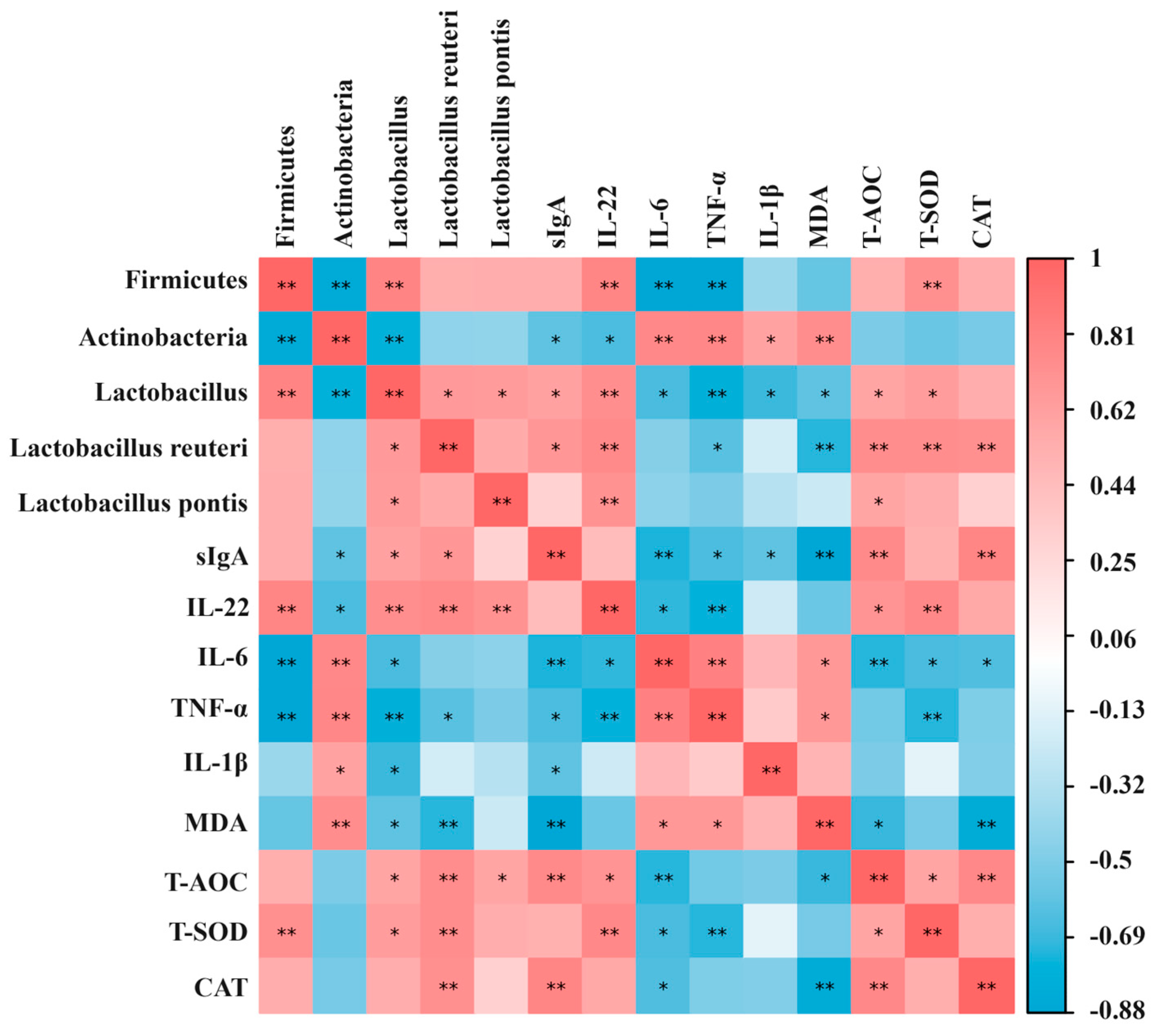
2.8. Correlation Analysis between Gut Microbiota, Oxidative Stress Indicators, and Immune-Inflammatory Factors
2.9. Network Pharmacological Analysis between CGA, Oxidative Stress, and Inflammation
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animals, Diets, and Experimental Design
4.3. Data and Sample Collection
4.4. Intestinal Morphology Analysis
4.5. Chemical Analyses
4.6. Real-Time Quantitative PCR
4.7. Western Blot Analysis
4.8. Immunohistochemical
4.9. 16S rRNA Gene Sequencing of Ileal Microbiota
4.10. Network Pharmacological Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xiong, X.; Wang, K.; Cheng, P.; Zou, L.; Zhou, J.; Qi, M.; Yin, Y. Early weaning leads to the remodeling of lipid profile in piglet jejunal crypt cells during post-weaning days. Anim. Nutr. 2022, 11, 102–111. [Google Scholar] [CrossRef]
- Kluess, J.; Schoenhusen, U.; Souffrant, W.B.; Jones, P.H.; Miller, B.G. Impact of diet composition on ileal digestibility and small intestinal morphology in early-weaned pigs fitted with a T-cannula. Animal 2010, 4, 586–594. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T.; et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 2018, 24, 817–832. [Google Scholar] [CrossRef]
- Pinelli-Saavedra, A. Vitamin E in immunity and reproductive performance in pigs. Reprod. Nutr. Dev. 2003, 43, 397–408. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.H.; Guan, W.T.; Chen, F.; Wang, C.X.; Zhang, Y.Z.; Lv, Y.T.; Lin, G. Selenium and vitamin E in sow diets: II. Effect on selenium status and antioxidant status of the progeny. Anim. Feed Sci. Technol. 2016, 221, 101–110. [Google Scholar] [CrossRef]
- Dai, C.; Li, H.; Zhao, W.; Fu, Y.; Cheng, J. Bioactive functions of chlorogenic acid and its research progress in pig industry. J. Anim. Physiol. Anim. Nutr. 2024, 108, 439–450. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, B.; Chen, D.; Mao, X.; Zheng, P.; Luo, J.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Yan, H.; et al. Chlorogenic acid attenuates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Front. Vet. Sci. 2022, 9, 806253. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tanihara, F.; Do, L.; Sato, Y.; Taniguchi, M.; Takagi, M.; Van Nguyen, T.; Otoi, T. Chlorogenic acid supplementation during in vitro maturation improves maturation, fertilization and developmental competence of porcine oocytes. Reprod. Domest. Anim. 2017, 52, 969–975. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Deng, W.; Li, K.; Liu, H. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides Oliver leaf on growth performance and quality and oxidative status of meat in finishing pigs fed diets containing fresh or oxidized corn oil. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1116–1125. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, S.; Xiao, H.; Wu, Q.; Chi, L.; Zhu, L.; Fang, L.; Li, Y.; Jiang, Z.; Wang, L. Dietary stevia residue extract supplementation improves the performance and antioxidative capacity of growing-finishing pigs. J. Sci. Food Agric. 2022, 102, 4724–4735. [Google Scholar] [CrossRef]
- Huang, S.W.; Wei, J.T.; Zhao, N.; Yang, X.H.; Zhang, W.; Mei, S.Q. Effects of chlorogenic acid and vitamin E on reproductive performance and antioxidant capacity of sows. Chin. J. Anim. Sci. 2015, 51, 79–83. [Google Scholar]
- St-Pierre, B.; Perez Palencia, J.Y.; Samuel, R.S. Impact of early weaning on development of the swine gut microbiome. Microorganisms 2023, 11, 1753. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Edwards, S.A. Review: Improving the performance of neonatal piglets. Animal 2022, 16 (Suppl. 2), 100350. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Zhang, X.; Liu, X.; Wu, Z.; Qi, Y.; Guan, W.; Ren, M.; Zhang, S. Maternal nutrition during late gestation and lactation: Association with immunity and the inflammatory response in the offspring. Front. Immunol. 2021, 12, 758525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Chen, D.; Yu, B.; Zheng, P.; Mao, X.; Luo, Y.; Li, Y.; He, J. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. 2018, 9, 4968–4978. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Zhang, B.; Wu, K.; Yang, A.; Li, C.; Zhang, J.; Zhang, C.; Rajput, S.; Zhang, N. Deoxynivalenol impairs porcine intestinal host defense peptide expression in weaned piglets and IPEC-J2 cells. Toxins 2018, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J.B.; Bai, J.H.; Luo, Y.; Ji, Y.; Xia, B.; Liu, B.; Tan, Z.G.; Lv, X.T.; Liu, J.Y.; et al. Lycopene alleviates DSS-induced colitis and behavioral disorders via mediating microbes-gut-brain axis balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Tang, Y.; Huang, Y. Gut health: The results of microbial and mucosal immune interactions in pigs. Anim. Nutr. 2021, 7, 282–294. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Zheng, P.; Luo, Y.; Huang, Z.; Luo, J.; Mao, X.; Yu, J.; He, J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 2019, 103, 8157–8168. [Google Scholar] [CrossRef]
- Wang, A.N.; Cai, C.J.; Zeng, X.F.; Zhang, F.R.; Zhang, G.L.; Thacker, P.A.; Wang, J.J.; Qiao, S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Microbiol. 2013, 114, 1582–1591. [Google Scholar] [CrossRef]
- Hou, C.; Liu, H.; Zhang, J.; Zhang, S.; Yang, F.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Intestinal microbiota succession and immunomodulatory consequences after introduction of Lactobacillus reuteri I5007 in neonatal piglets. PLoS ONE 2015, 10, e0119505. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Yue, L.Y.; Guan, X.F.; Qiao, S.Y. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 2008, 104, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hou, C.; Wang, G.; Jia, H.; Yu, H.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Lactobacillus reuteri I5007 modulates intestinal host defense peptide expression in the model of IPEC-J2 cells and neonatal piglets. Nutrients 2017, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Liu, S.; Zhou, Y.; Mi, S.; Liu, G.; Wu, X.; Yao, K.; Assaad, H.; Deng, Z.; Hou, Y.; et al. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS ONE 2014, 9, e97815. [Google Scholar] [CrossRef] [PubMed]
- Palócz, O.; Pászti-Gere, E.; Gálfi, P.; Farkas, O. Chlorogenic acid combined with Lactobacillus plantarum 2142 reduced LPS-induced intestinal inflammation and oxidative stress in IPEC-J2 cells. PLoS ONE 2016, 11, e0166642. [Google Scholar] [CrossRef]
- Wozniak, A.; Drewa, G.; Wozniak, B.; Schachtschabel, D.O. Activity of antioxidant enzymes and concentration of lipid peroxidation products in selected tissues of mice of different ages, both healthy and melanoma-bearing. Z. Für Gerontol. Und Geriatr. 2004, 37, 184–189. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Cochet, F.; Peri, F. The role of carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signalling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef]
- Becker, C.E.; O’Neill, L.A. Inflammasomes in inflammatory disorders: The role of TLRs and their interactions with NLRs. Semin. Immunopathol. 2007, 29, 239–248. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef]
- Ye, H.Y.; Jin, J.; Jin, L.W.; Chen, Y.; Zhou, Z.H.; Li, Z.Y. Chlorogenic acid attenuates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/NF-κB signal pathway. Inflammation 2017, 40, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Zou, T.; You, J.; Guan, W. Plant-derived polyphenols in sow nutrition: An update. Anim. Nutr. 2023, 12, 96–107. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Chen, X.; Wei, S.Y.; Li, J.S.; Zhang, Q.F.; Wang, Y.X.; Zhao, S.L.; Yu, J.; Wang, C.; Qin, Y.; Wei, Q.J.; et al. Overexpression of heme oxygenase-1 prevents renal interstitial inflammation and fibrosis induced by unilateral ureter obstruction. PLoS ONE 2016, 11, e0147084. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Chen, J.L.; Zheng, P.; Zhang, C.; Yu, B.; He, J.; Yu, J.; Luo, J.Q.; Mao, X.B.; Huang, Z.Q.; Chen, D.W. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Phys. Anim. Nutr. 2017, 101, 1137–1146. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.M.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Damian, S.; Gable, A.L.; Nastou, K.C.; David, L.; Rebecca, K.; Sampo, P.; Doncheva, N.T.; Marc, L.; Tao, F.; Peer, B. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]





| Item | CON | CGA | SEM | p-Value |
|---|---|---|---|---|
| Initial BW, kg | 6.85 | 6.73 | 0.19 | 0.836 |
| Final BW, kg | 13.21 | 13.96 | 0.46 | 0.131 |
| ADG, g/d | 302.70 | 372.00 | 12.17 | 0.096 |
| ADFI, g/d | 454.82 | 497.11 | 15.86 | 0.164 |
| G/F | 0.67 | 0.75 | 0.01 | 0.567 |
| Diarrhea rate, % | 29.17 | 17.78 | 2.78 | 0.016 |
| Ingredients | % | Nutrient Levels 2 | |
|---|---|---|---|
| Corn | 41.21 | NE, MJ/kg | 10.88 |
| Enzyme-treated soybean meal | 19.46 | CP, % | 22.73 |
| Expanded soybean | 11.00 | SID CP, % | 18.43 |
| Low-protein whey powder | 10.00 | SID Lys, % | 1.43 |
| Whey protein concentrate | 4.00 | SID Met + Cys, % | 0.78 |
| Fishmeal | 3.00 | SID Thr, % | 0.84 |
| Soybean oil | 2.82 | SID Trp, % | 0.26 |
| Sucrose | 2.00 | SID Ile, % | 0.85 |
| Soybean hulls | 2.00 | SID Val, % | 0.91 |
| Lysine HCl | 0.33 | SID Leu, % | 1.64 |
| DL-Methionine | 0.14 | SID Lys/ME, g/MJ | 5.49 |
| L-Threonine | 0.09 | Ca, % | 0.79 |
| Calcium hydrophosphate | 0.80 | STTD P, % | 0.62 |
| NaCl | 0.35 | Na, % | 0.31 |
| Limestone powder | 1.80 | ||
| Premix 1 | 1.00 | ||
| Total | 100.00 |
| Genes | Prime Sequence (5′-3′) | GenBank | Product Size (bp) |
|---|---|---|---|
| MUC | F: CTGCTCCGGGTCCTGTGGGA | XM_021082584.1 | 101 |
| R: CCCGCTGGCTGGTGCGATAC | |||
| pBD-1 | F: ACCGCCTCCTCCTTGTATTC | NM_213838.1 | 150 |
| R: CACAGGTGCCGATCTGTTTC | |||
| pBD-2 | F: CCAGAGGTCCGACCACTACA | NM_214442.2 | 88 |
| R: GGTCCCTTCAATCCTGTTGAA | |||
| PG1 | F: GTAGGTTCTGCGTCTGTGTCG | NM_001123149.2 | 166 |
| R: CAAATCCTTCACCGTCTACCA | |||
| β-actin | F: TGCGGGACATCAAGGAGAAGC | XM_021086047 | 273 |
| R: ACAGCACCGTGTTGGCGTAGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Tian, M.; Wu, J.; Qiu, Y.; Xu, X.; Tian, C.; Hou, J.; Wang, L.; Gao, K.; Yang, X.; et al. Chlorogenic Acid Enhances the Intestinal Health of Weaned Piglets by Inhibiting the TLR4/NF-κB Pathway and Activating the Nrf2 Pathway. Int. J. Mol. Sci. 2024, 25, 9954. https://doi.org/10.3390/ijms25189954
Zhang B, Tian M, Wu J, Qiu Y, Xu X, Tian C, Hou J, Wang L, Gao K, Yang X, et al. Chlorogenic Acid Enhances the Intestinal Health of Weaned Piglets by Inhibiting the TLR4/NF-κB Pathway and Activating the Nrf2 Pathway. International Journal of Molecular Sciences. 2024; 25(18):9954. https://doi.org/10.3390/ijms25189954
Chicago/Turabian StyleZhang, Beibei, Min Tian, Jing Wu, Yueqin Qiu, Xiaoming Xu, Chaoyang Tian, Jing Hou, Li Wang, Kaiguo Gao, Xuefen Yang, and et al. 2024. "Chlorogenic Acid Enhances the Intestinal Health of Weaned Piglets by Inhibiting the TLR4/NF-κB Pathway and Activating the Nrf2 Pathway" International Journal of Molecular Sciences 25, no. 18: 9954. https://doi.org/10.3390/ijms25189954








