Navigating the Intersection of Glycemic Control and Fertility: A Network Perspective
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Eades, C.E.; France, E.F.; Evans, J.M.M. Prevalence of Impaired Glucose Regulation in Europe: A Meta-Analysis. Eur. J. Public Health 2016, 26, 699–706. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.E3. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic Regulation of Glucose Homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.; Cutting, R.; Tipton, A.; Skull, J.; Ledger, W.L.; Li, T.C. Effect of Increased Body Mass Index on Oocyte and Embryo Quality in IVF Patients. Reprod. Biomed. Online 2007, 15, 532–538. [Google Scholar] [CrossRef]
- van der Steeg, J.W.; Steures, P.; Eijkemans, M.J.C.; Habbema, J.D.F.; Hompes, P.G.A.; Burggraaff, J.M.; Oosterhuis, G.J.E.; Bossuyt, P.M.M.; van der Veen, F.; Mol, B.W.J. Obesity Affects Spontaneous Pregnancy Chances in Subfertile, Ovulatory Women. Hum. Reprod. 2008, 23, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Livadas, S.; Anagnostis, P.; Bosdou, J.K.; Bantouna, D.; Paparodis, R. Polycystic Ovary Syndrome and Type 2 Diabetes Mellitus: A State-of-the-Art Review. World J. Diabetes 2022, 13, 5–26. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Lamb, D.J. The Biology of Infertility: Research Advances and Clinical Challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Dhindsa, S. Update: Hypogonadotropic Hypogonadism in Type 2 Diabetes and Obesity. J. Clin. Endocrinol. Metab. 2011, 96, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Advanced Protein Glycosylation in Diabetes and Aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced Glycation End-Products: A Review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Bierhaus, A.; Hofmann, M.A.; Ziegler, R.; Nawroth, P.P. AGEs and Their Interaction with AGE-Receptors in Vascular Disease and Diabetes Mellitus. I. The AGE Concept. Cardiovasc. Res. 1998, 37, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Hartog, J.W.L.; Voors, A.A.; Bakker, S.J.L.; Smit, A.J.; van Veldhuisen, D.J. Advanced Glycation End-Products (AGEs) and Heart Failure: Pathophysiology and Clinical Implications. Eur. J. Heart Fail. 2007, 9, 1146–1155. [Google Scholar] [CrossRef]
- Vicente Miranda, H.; El-Agnaf, O.M.A.; Outeiro, T.F. Glycation in Parkinson’s Disease and Alzheimer’s Disease. Mov. Disord. 2016, 31, 782–790. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, Y.; Long, S.; Chen, Z.; Mo, Z. The Role of Advanced Glycation End Products in Human Infertility. Life Sci. 2020, 255, 117830. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical Localization of Advanced Glycation End-Products (AGEs) and Their Receptor (RAGE) in Polycystic and Normal Ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Receptor for AGE (RAGE) and Its Ligands—Cast into Leading Roles in Diabetes and the Inflammatory Response. J. Mol. Med. 2009, 87, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Momeni, Z.; Theaker, M.; Jagadeeshan, S.; Yamamoto, Y.; Ianowski, J.P.; Campanucci, V.A. RAGE-Dependent Potentiation of TRPV1 Currents in Sensory Neurons Exposed to High Glucose. PLoS ONE 2018, 13, e0193312. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological Pain and the Neuroimmune Interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef]
- Ramal-Sanchez, M.; Bernabò, N.; Valbonetti, L.; Cimini, C.; Taraschi, A.; Capacchietti, G.; Machado-Simoes, J.; Barboni, B. Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review. Int. J. Mol. Sci. 2021, 22, 4306. [Google Scholar] [CrossRef]
- Rossato, M.; Ion Popa, F.; Ferigo, M.; Clari, G.; Foresta, C. Human Sperm Express Cannabinoid Receptor Cb1, the Activation of Which Inhibits Motility, Acrosome Reaction, and Mitochondrial Function. J. Clin. Endocrinol. Metab. 2005, 90, 984–991. [Google Scholar] [CrossRef]
- Barabási, A.-L.; Oltvai, Z.N. Network Biology: Understanding the Cell’s Functional Organization. Nat. Rev. Genet. 2004, 5, 101–113. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Grayson, B.E.; Seeley, R.J.; Sandoval, D.A. Wired on Sugar: The Role of the CNS in the Regulation of Glucose Homeostasis. Nat. Rev. Neurosci. 2013, 14, 24–37. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Faber, C.L.; Schwartz, M.W. Central Nervous System Control of Glucose Homeostasis: A Therapeutic Target for Type 2 Diabetes? Annu. Rev. Pharmacol. Toxicol. 2022, 62, 55–84. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging Insights into Hypothalamic-Pituitary-Gonadal Axis Regulation and Interaction with Stress Signalling. J. Neuroendocr. 2018, 30, e12590. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, G.E. Advanced Glycation Endproducts in Diabetes and Diabetic Complications. Endocrinol. Metab. Clin. North Am. 2013, 42, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Poretsky, L. Looking beyond Overnutrition for Causes of Epidemic Metabolic Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 15537–15538. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced Glycoxidation and Lipoxidation End Products (AGEs and ALEs): An Overview of Their Mechanisms of Formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Arena, S.; Salzano, A.M.; Renzone, G.; D’Ambrosio, C.; Scaloni, A. Non-Enzymatic Glycation and Glycoxidation Protein Products in Foods and Diseases: An Interconnected, Complex Scenario Fully Open to Innovative Proteomic Studies. Mass Spectrom. Rev. 2014, 33, 49–77. [Google Scholar] [CrossRef]
- Cengiz, E.; Tamborlane, W.V. A Tale of Two Compartments: Interstitial versus Blood Glucose Monitoring. Diabetes Technol. Ther. 2009, 11 (Suppl. S1), S11–S16. [Google Scholar] [CrossRef]
- Cimini, C.; Moussa, F.; Taraschi, A.; Ramal-Sanchez, M.; Colosimo, A.; Capacchietti, G.; Mokh, S.; Valbonetti, L.; Tagaram, I.; Bernabò, N.; et al. Pre-Treatment of Swine Oviductal Epithelial Cells with Progesterone Increases the Sperm Fertilizing Ability in an IVF Model. Animals 2022, 12, 1191. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Ramos-Ibeas, P.; Acuña, O.S.; Avilés, M.; Coy, P.; Rizos, D.; Gutiérrez-Adán, A. The Oviduct: From Sperm Selection to the Epigenetic Landscape of the Embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L. Factors Regulating Sperm Capacitation. Syst. Biol. Reprod. Med. 2010, 56, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.P.; Gadella, B.M. Bicarbonate-Induced Membrane Processing in Sperm Capacitation. Theriogenology 2005, 63, 342–351. [Google Scholar] [CrossRef]
- Pertynska-Marczewska, M.; Cypryk, K. The Possible Impact of Advanced Glycation End Products on Pregnancy Outcome in Women with Diabetes Mellitus Type 1. Minerva Endocrinol. 2017, 42, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 Structures in Distinct Conformations Reveal Activation Mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.S.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological Blockade of the Vanilloid Receptor TRPV1 Elicits Marked Hyperthermia in Humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) Receptors and Mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of Advanced Glycation End Products in Diabetic Neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef]
- Bernabò, N.; Pistilli, M.G.; Mattioli, M.; Barboni, B. Role of TRPV1 Channels in Boar Spermatozoa Acquisition of Fertilizing Ability. Mol. Cell Endocrinol. 2010, 323, 224–231. [Google Scholar] [CrossRef]
- De Toni, L.; Garolla, A.; Menegazzo, M.; Magagna, S.; Di Nisio, A.; Šabović, I.; Rocca, M.S.; Scattolini, V.; Filippi, A.; Foresta, C. Heat Sensing Receptor TRPV1 Is a Mediator of Thermotaxis in Human Spermatozoa. PLoS ONE 2016, 11, e0167622. [Google Scholar] [CrossRef]
- Darszon, A.; Sánchez-Cárdenas, C.; Orta, G.; Sánchez-Tusie, A.A.; Beltrán, C.; López-González, I.; Granados-González, G.; Treviño, C.L. Are TRP Channels Involved in Sperm Development and Function? Cell Tissue Res. 2012, 349, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.F.; Schust, D.J. Manifestations of Immune Tolerance in the Human Female Reproductive Tract. Front. Immunol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Wellner, A.; Gadermaier, G.; Ilchmann, A.; Briza, P.; Krause, M.; Nagai, R.; Burgdorf, S.; Scheurer, S.; Vieths, S.; et al. Ovalbumin Modified with Pyrraline, a Maillard Reaction Product, Shows Enhanced T-Cell Immunogenicity. J. Biol. Chem. 2014, 289, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yao, T.; Zhou, Z.; Zhu, J.; Zhang, S.; Hu, W.; Shen, C. Advanced Glycation End Products Enhance Macrophages Polarization into M1 Phenotype through Activating RAGE/NF-ΚB Pathway. Biomed. Res. Int. 2015, 2015, 732450. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T. Effect of Diet-Derived Advanced Glycation End Products on Inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Archana, S.S.; Selvaraju, S.; Binsila, B.K.; Arangasamy, A.; Krawetz, S.A. Immune Regulatory Molecules as Modifiers of Semen and Fertility: A Review. Mol. Reprod. Dev. 2019, 86, 1485–1504. [Google Scholar] [CrossRef]
- Taylor, U.; Rath, D.; Zerbe, H.; Schuberth, H.J. Interaction of Intact Porcine Spermatozoa with Epithelial Cells and Neutrophilic Granulocytes during Uterine Passage. Reprod. Domest. Anim. 2008, 43, 166–175. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm Transport in the Female Reproductive Tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Nicander, L.; Viring, S.; Einarsson, S.; Larsson, K. Ultrastructure of the Uterotubal Junction in Preovulatory Pigs. Anat. Histol. Embryol. 1990, 19, 16–36. [Google Scholar] [CrossRef]
- Yousef, M.S.; Marey, M.A.; Hambruch, N.; Hayakawa, H.; Shimizu, T.; Hussien, H.A.; Abdel-Razek, A.R.K.; Pfarrer, C.; Miyamoto, A. Sperm Binding to Oviduct Epithelial Cells Enhances TGFB1 and IL10 Expressions in Epithelial Cells as Well as Neutrophils In Vitro: Prostaglandin E2 As a Main Regulator of Anti-Inflammatory Response in the Bovine Oviduct. PLoS ONE 2016, 11, e0162309. [Google Scholar] [CrossRef]
- Martínez, P.; Proverbio, F.; Camejo, M.I. Sperm Lipid Peroxidation and Pro-Inflammatory Cytokines. Asian J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- John Aitken, R. Free Radicals, Lipid Peroxidation and Sperm Function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef]
- Lampiao, F.; du Plessis, S.S. TNF-α and IL-6 Affect Human Sperm Function by Elevating Nitric Oxide Production. Reprod. Biomed. Online 2008, 17, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tsuneyama, K.; Kominami, R.; Shinohara, H.; Sakurai, S.; Yonekura, H.; Watanabe, T.; Takano, Y.; Yamamoto, H.; Yamamoto, Y. Expression Profiling of Endogenous Secretory Receptor for Advanced Glycation End Products in Human Organs. Mod. Pathol. 2005, 18, 1385–1396. [Google Scholar] [CrossRef]
- Katsuoka, F.; Kawakami, Y.; Arai, T.; Imuta, H.; Fujiwara, M.; Kanma, H.; Yamashita, K. Type II Alveolar Epithelial Cells in Lung Express Receptor for Advanced Glycation End Products (RAGE) Gene. Biochem. Biophys. Res. Commun. 1997, 238, 512–516. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Crandall, J.; Goldberg, T.; Oberstein, R.; Dardaine, V.; Peppa, M.; Rayfield, E.J. Inflammatory Mediators Are Induced by Dietary Glycotoxins, a Major Risk Factor for Diabetic Angiopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 15596. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and Molecular Aspects of Ovarian Follicle Ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95. [Google Scholar] [CrossRef]
- Taraschi, A.; Cimini, C.; Capacchietti, G.; Ramal-Sanchez, M.; Valbonetti, L.; Machado-Simoes, J.; Moussa, F.; Tagaram, I.; Mokh, S.; Al Iskandarani, M.; et al. Two-Player Game in a Complex Landscape: 26S Proteasome, PKA, and Intracellular Calcium Concentration Modulate Mammalian Sperm Capacitation by Creating an Integrated Dialogue—A Computational Analysis. Int. J. Mol. Sci. 2020, 21, 6256. [Google Scholar] [CrossRef]
- Bernabò, N.; Barboni, B.; Maccarrone, M. Systems Biology Analysis of the Endocannabinoid System Reveals a Scale-Free Network with Distinct Roles for Anandamide and 2-Arachidonoylglycerol. OMICS 2013, 17, 646–654. [Google Scholar] [CrossRef]
- Bernabò, N.; Greco, L.; Ordinelli, A.; Mattioli, M.; Barboni, B. Capacitation-Related Lipid Remodeling of Mammalian Spermatozoa Membrane Determines the Final Fate of Male Gametes: A Computational Biology Study. OMICS 2015, 19, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, N.; Mattioli, M.; Barboni, B. Signal Transduction in the Activation of Spermatozoa Compared to Other Signalling Pathways: A Biological Networks Study. Int. J. Data Min. Bioinform. 2015, 12, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef] [PubMed]
- Pržulj, N.; Wigle, D.A.; Jurisica, I. Functional Topology in a Network of Protein Interactions. Bioinformatics 2004, 20, 340–348. [Google Scholar] [CrossRef]
- Yao, F.; Coquery, J.; Lê Cao, K.-A. Independent Principal Component Analysis for Biologically Meaningful Dimension Reduction of Large Biological Data Sets. BMC Bioinform. 2012, 13, 24. [Google Scholar] [CrossRef]
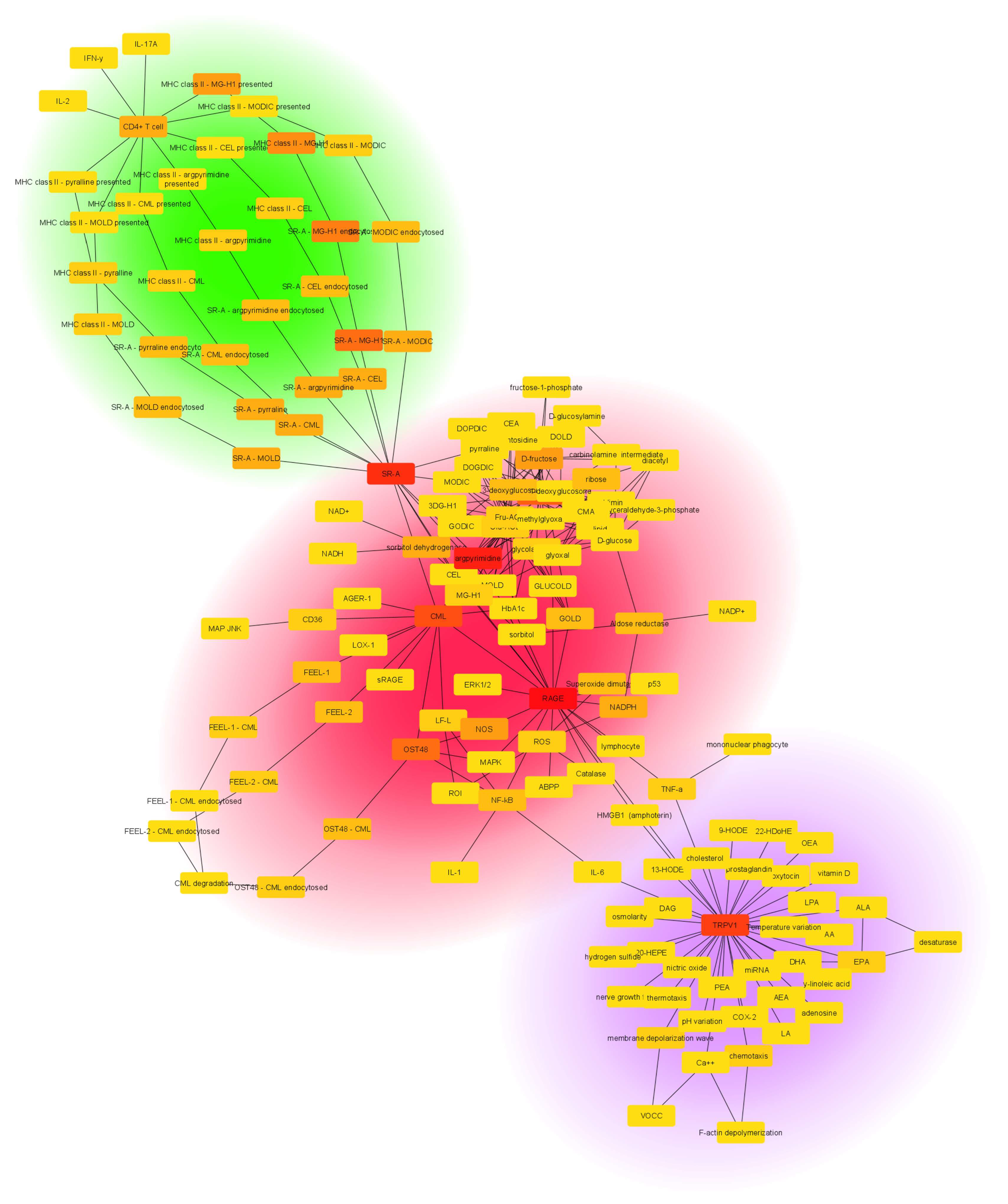
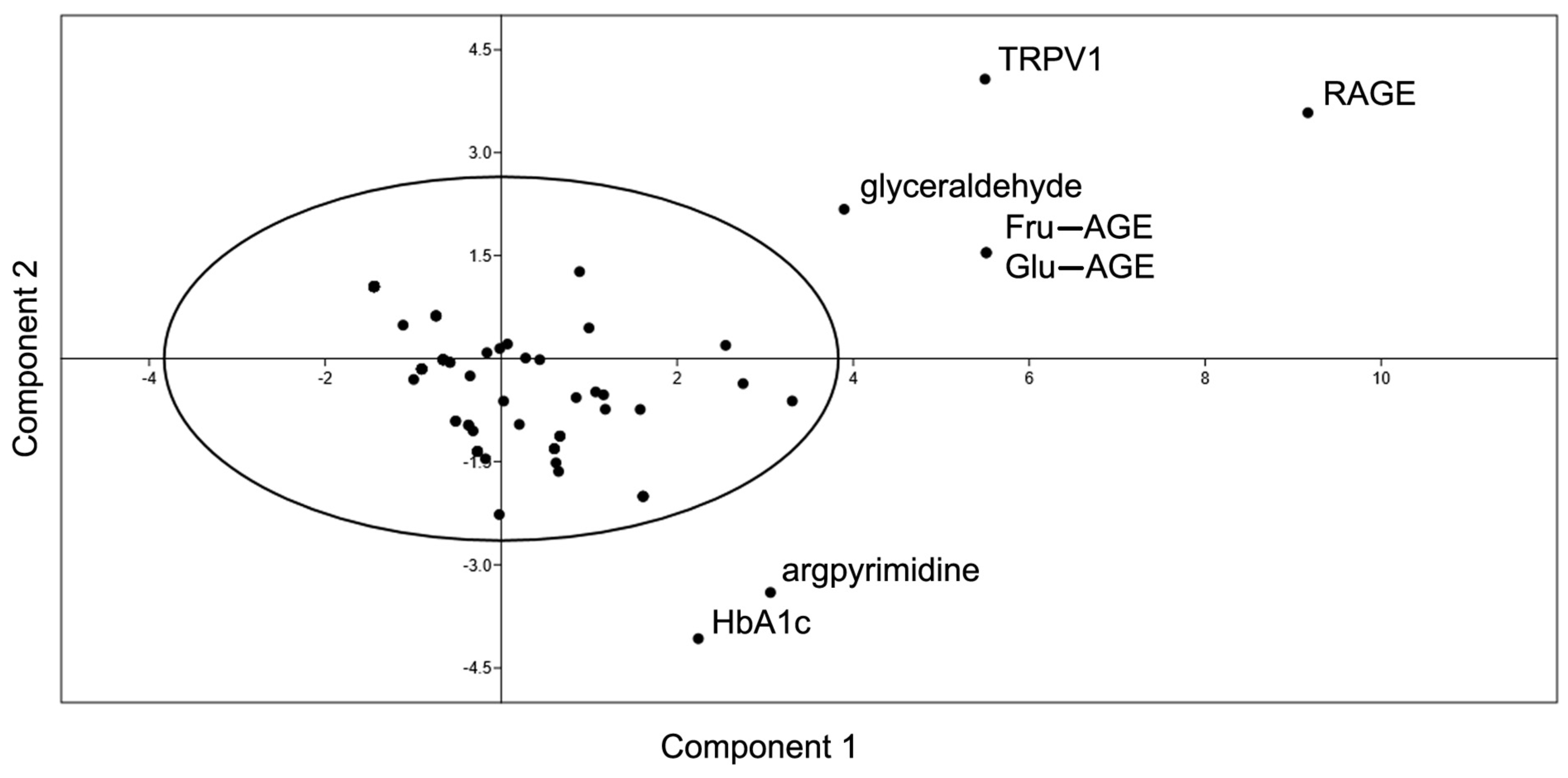

| Parameter | Value | |
|---|---|---|
| Connected components | 1 | |
| Number of nodes | 145 | |
| Number of edges | 262 | |
| Averaged number of neighbors | 3.586 | |
| Clustering coefficient | 0.023 | |
| Network diameter | 16 | |
| Characteristic path length | 5.453 | |
| Averaged number of neighbors | 3.586 | |
| Node degree | ɣ r R2 | −1.276 |
| 0.8303 | ||
| 0.6894 | ||
| Parameter | Definition |
|---|---|
| Connected components | The number of networks in which any two vertices are connected to each other by links and which are connected to no additional vertices in the network |
| Number of nodes | The total number of molecules involved |
| Number of edges | The total number of interactions found |
| Clustering coefficient | Calculated as CI = 2nI/kI(kI − 1), where nI is the number of links connecting the kI neighbors of node I to each other. It is a measure of how the nodes tend to form clusters |
| Network diameter | The longest of all the calculated shortest paths in a network |
| Characteristic path length | The expected distance between two connected nodes |
| Average number of neighbors | The mean number of connections of each node |
| Node degree | The number of interactions of each node |
| Node degree distribution | Represent the probability that a selected node has k links |
| ɣ | Exponent of node degree equation |
| R | Pearson correlation coefficient of node degree vs. number of nodes on logarithmized data |
| R2 | Coefficient of determination of node degree vs. number of nodes on logarithmized data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Carlo, C.; Cimini, C.; Belda-Perez, R.; Valbonetti, L.; Bernabò, N.; Barboni, B. Navigating the Intersection of Glycemic Control and Fertility: A Network Perspective. Int. J. Mol. Sci. 2024, 25, 9967. https://doi.org/10.3390/ijms25189967
Di Carlo C, Cimini C, Belda-Perez R, Valbonetti L, Bernabò N, Barboni B. Navigating the Intersection of Glycemic Control and Fertility: A Network Perspective. International Journal of Molecular Sciences. 2024; 25(18):9967. https://doi.org/10.3390/ijms25189967
Chicago/Turabian StyleDi Carlo, Carlo, Costanza Cimini, Ramses Belda-Perez, Luca Valbonetti, Nicola Bernabò, and Barbara Barboni. 2024. "Navigating the Intersection of Glycemic Control and Fertility: A Network Perspective" International Journal of Molecular Sciences 25, no. 18: 9967. https://doi.org/10.3390/ijms25189967









