Polyethylene Glycol/Pullulan-Based Carrier for Silymarin Delivery and Its Potential in Biomedical Applications
Abstract
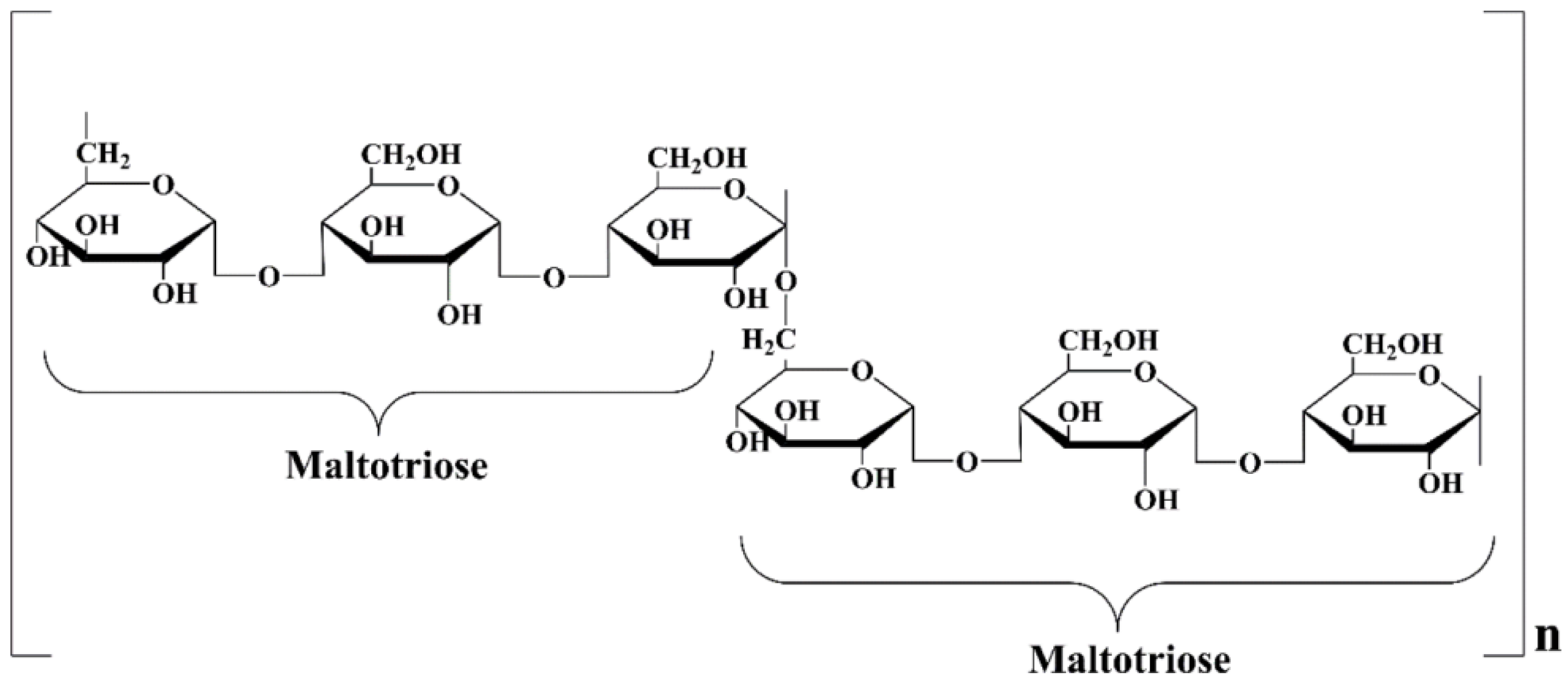
1. Introduction
2. Results
2.1. In Vitro Incubation
2.1.1. Determination of Sorption Capacity
2.1.2. pH Metric Analysis
2.1.3. Conductivity Analysis
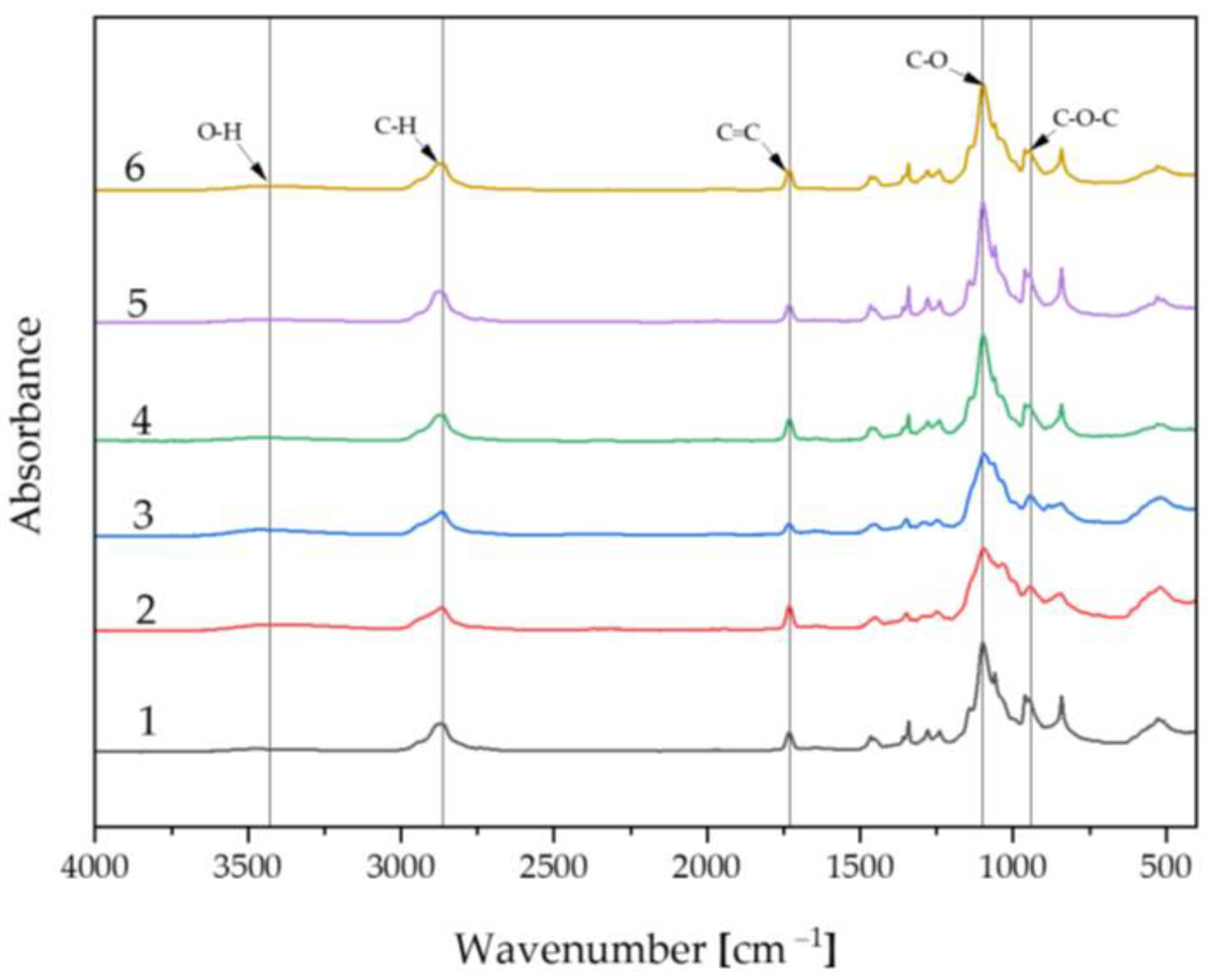
2.2. Fourier-Transform Infrared Spectroscopy Analysis
Spectrum of Biomaterials before Incubation
2.3. Determination of Release Kinetics of Silymarin
Spectrophotometer Analysis
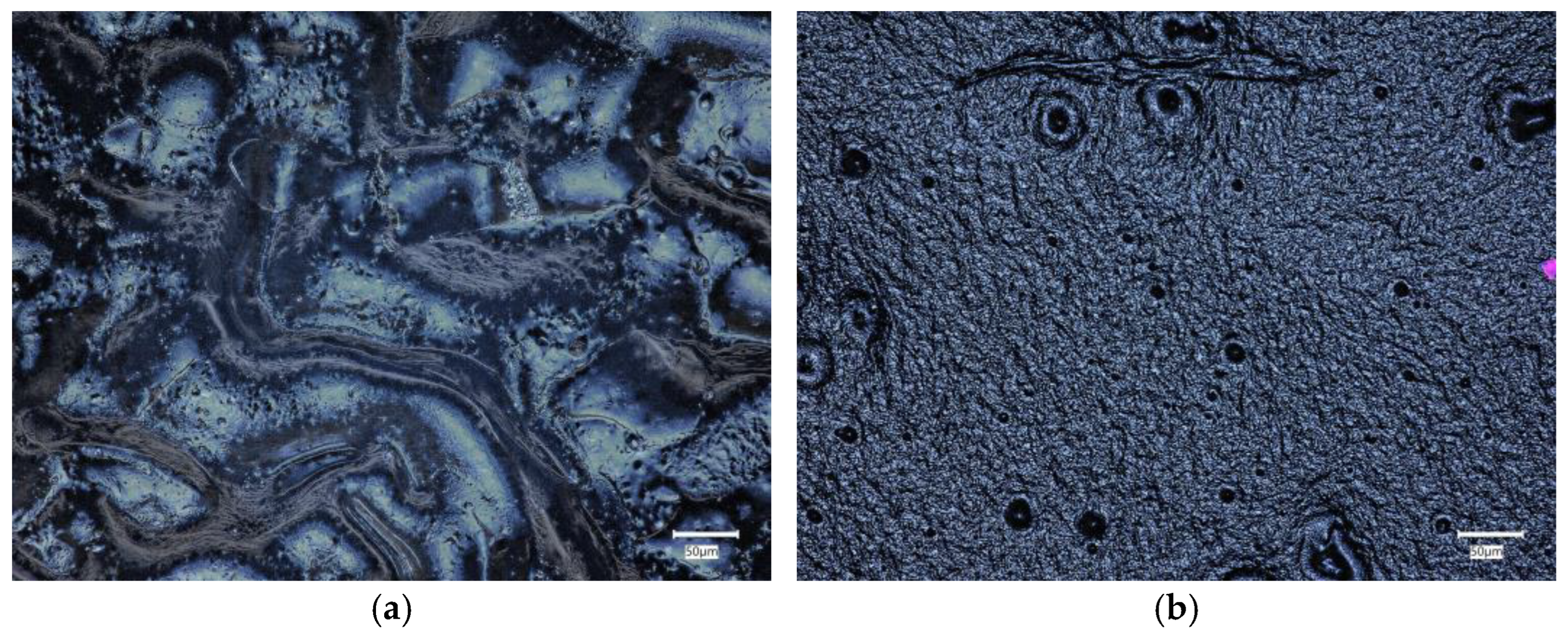
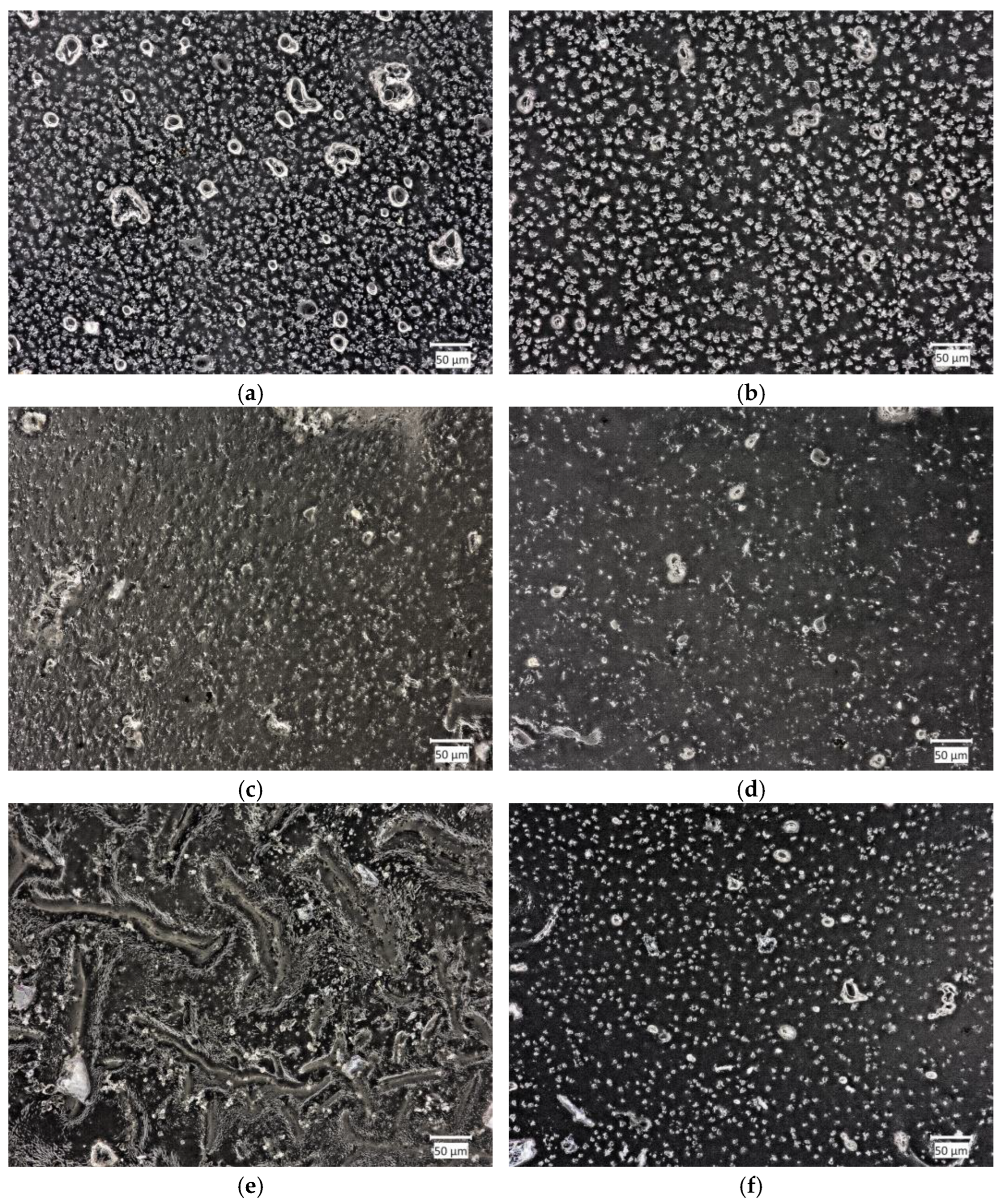
2.4. Morphology Analysis
2.4.1. Material Morphology before Incubation
2.4.2. Material Morphology after Incubation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Biomaterials
4.2.2. In Vitro Incubation
4.2.3. Fourier-Transform Infrared Spectroscopy Analysis
4.2.4. Determination of Release Kinetics of Silymarin
4.2.5. Morphology Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Description |
| PEG | Polyethylene glycol |
| PEGDA | Poly(ethylene glycol) diacrylate |
| SBF | Simulated body fluid |
| FT-IR | Fourier-transform infrared spectroscopy |
References
- Paolino, D.; Sinha, P.; Fresta, M.; Ferrari, M. Drug Delivery Systems. In Encyclopedia of Medical Devices and Instrumentation; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(Lactic Acid) Based Hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze-Thaw Hydrogel Fabrication Method: Basic Principles, Synthesis Parameters, Properties, and Biomedical Applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Erkoc, C.; Yildirim, E.; Yurtsever, M.; Okay, O. Roadmap to Design Mechanically Robust Copolymer Hydrogels Naturally Cross-Linked by Hydrogen Bonds. Macromolecules 2022, 55, 10576–10589. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Sharma, P.; Negi, P.; Mahindroo, N. Recent Advances in Polymeric Drug Delivery Carrier Systems. Adv. Polym. Biomed. Appl. 2018, 10, 369–388. [Google Scholar]
- Heller, A. Integrated Medical Feedback Systems for Drug Delivery. AIChE J. 2005, 51, 1054–1066. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in Biomedical Research and Development—A Review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, J.F. Pullulan in Pharmaceutical and Cosmeceutical Formulations: A Review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef]
- Agrawal, S.; Budhwani, D.; Gurjar, P.; Telange, D.; Lambole, V. Pullulan Based Derivatives: Synthesis, Enhanced Physicochemical Properties, and Applications. Drug Deliv. 2022, 29, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, Production, and Applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.O.; Marinho, E.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Pullulan Hydrogels as Drug Release Platforms in Biomedicine. J. Drug Deliv. Sci. Technol. 2023, 89, 105066. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Zhang, Y.; Wang, G.; Wang, C.; Wang, D.; Wei, G. Efficient Pullulan Production by Aureobasidium Pullulans Using Cost-Effective Substrates. Int. J. Biol. Macromol. 2021, 186, 544–553. [Google Scholar] [CrossRef]
- Ganie, S.A.; Rather, L.J.; Li, Q. A Review on Anticancer Applications of Pullulan and Pullulan Derivative Nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100115. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Majewska, M.; Czeczot, H. Flawonoidy w Profilaktyce i Terapii. Farm. Pol. 2009, 65, 369–377. [Google Scholar]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, P.; Skrzypczak, N. Moda Na Flawonoidy-o Co Tyle Szumu? Tutoring Gedanensis 2021, 6, 55–64. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.D.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Abbas, N. Chemistry of Himalayan Phytochemicals. In Himalayan Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–166. ISBN 9780081022276. [Google Scholar]
- Ekalu, A.; Dama Habila, J. Flavonoids: Isolation, Characterization, and Health Benefits. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A. Oseltamivir Resistance—Disabling Our Influenza Defenses. N. Engl. J. Med. 2005, 353, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Fan, Z.; Yang, Z.; Huang, T.; Tong, Y.; Yang, D.; Mao, X.; Yang, M. Flavonoids—Natural Gifts to Promote Health and Longevity. Int. J. Mol. Sci. 2022, 23, 2176. [Google Scholar] [CrossRef]
- Xing, W.; Gao, W.; Zhao, Z.; Xu, X.; Bu, H.; Su, H.; Mao, G.; Chen, J. Dietary Flavonoids Intake Contributes to Delay Biological Aging Process: Analysis from NHANES Dataset. J. Transl. Med. 2023, 21, 492. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Jiang, N.; Tu, J.; Yang, J.; Zhou, Y. Antioxidant and Anti-Aging Activities of Silybum Marianum Protein Hydrolysate in Mice Treated with D-Galactose. Biomed. Environ. Sci. 2017, 30, 623–631. [Google Scholar] [CrossRef]
- Wellington, K.; Adis, B.J. Silymarin: A Review of Its Clinical Properties in the Management of Hepatic Disorders. BioDrugs 2001, 15, 465–489. [Google Scholar] [CrossRef]
- Ramasamy, K.; Agarwal, R. Multitargeted Therapy of Cancer by Silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef]
- Camini, F.C.; Costa, D.C. Silymarin: Not Just Another Antioxidant. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190206. [Google Scholar] [CrossRef]
- Adetuyi, B.O.; Omolabi, F.K.; Olajide, P.A.; Oloke, J.K. Pharmacological, Biochemical and Therapeutic Potential of Milk Thistle (Silymarin): A Review. World News Nat. Sci. 2021, 37, 75–91. [Google Scholar]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, R.; Shahidi, Z.; Eyvari-Brooshghalan, S. Silymarin and Neurodegenerative Diseases: Therapeutic Potential and Basic Molecular Mechanisms. Phytomedicine 2020, 79, 153320. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Meier, R.; Brignoli, R. The Use of Silymarin in the Treatment of Liver Diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef]
- Surai, P.F.; Surai, A.; Earle-Payne, K. Silymarin and Inflammation: Food for Thoughts. Antioxidants 2024, 13, 98. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Jabeen, N.; Sohail, M.; Shah, S.A.; Mahmood, A.; Khan, S.; ur Rehman Kashif, M.; Khaliq, T. Silymarin Nanocrystals-Laden Chondroitin Sulphate-Based Thermoreversible Hydrogels; A Promising Approach for Bioavailability Enhancement. Int. J. Biol. Macromol. 2022, 218, 456–472. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Reddy, V.R.M.; Kim, B.; Patro, B.; Park, C.; Kim, W.K.; Sharma, P. Fabrication of Cu2ZnSnS4 Light Absorber Using a Cost-Effective Mechanochemical Method for Photovoltaic Applications. Materials 2022, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Callaghan, M.J.; Longaker, M.T. Progress and Potential for Regenerative Medicine. Annu. Rev. Med. 2007, 58, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Słota, D.; Florkiewicz, W.; Piętak, K.; Szwed, A.; Włodarczyk, M.; Siwińska, M.; Rudnicka, K.; Sobczak-Kupiec, A. Preparation, Characterization, and Biocompatibility Assessment of Polymer-Ceramic Composites Loaded with Salvia Officinalis Extract. Materials 2021, 14, 6000. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Drug Delivery Systems Using Pullulan, a Biocompatible Polysaccharide Produced by Fungal Fermentation of Starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Atala, A. Regenerative Medicine Strategies. J. Pediatr. Surg. 2012, 47, 17–28. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K. Chapter 15 Pullulan as Th Erapeutic Tool in Biomedical Applications. In Advances in Industrial Biotechnology; IK International Publishing House Pvt. Ltd.: Delhi, India, 2016; Volume 8. [Google Scholar]
- Xu, M.; Liu, J.; Sun, J.; Xu, X.; Hu, Y.; Liu, B. Optical Microscopy and Electron Microscopy for the Morphological Evaluation of Tendons: A Mini Review. Orthop. Surg. 2020, 12, 366–371. [Google Scholar] [CrossRef]





| Sample | 20% (w/w) PEG Solution [mL] | 20% (w/w) Pullulan Solution [mL] | Silymarin [mg] | PEG 400 [mL] | PEGDA 700 [mL] | Photoinitiator [μL] |
|---|---|---|---|---|---|---|
| 1. | 10 | - | - | 1 | 2 | 50 |
| 2. | - | 10 | 1 | |||
| 3. | - | - | 11 | |||
| 4. | 5 | 5 | 1 | |||
| 5. | 7.5 | 2.5 | 1 | |||
| 6. | 2.5 | 7.5 | 1 | |||
| 1.1 | 10 | - | 5 mg | 1 | ||
| 2.1 | - | 10 | 1 | |||
| 3.1 | - | - | 11 | |||
| 4.1 | 5 | 5 | 1 | |||
| 5.1 | 7.5 | 2.5 | 1 | |||
| 6.1 | 2.5 | 7.5 | 1 |
| Ringer’s Fluid | |
|---|---|
| Component | Amount [g/L] |
| NaCl | 8.600 |
| KCl | 0.300 |
| CaCl2·H2O | 0.480 |
| SBF | |
|---|---|
| Component | Amount [g/L] |
| NaCl | 8.035 |
| NaHCO3 | 0.355 |
| KCl | 0.225 |
| K2HPO4·3H2O | 0.231 |
| MgCl2·6H2O | 0.311 |
| HCl 1M | 39 mL |
| CaCl2 | 0.292 |
| Na2SO4 | 0.072 |
| Tris | 6.118 |
| Artificial Saliva | |
|---|---|
| Component | Amount [g/L] |
| NaCl | 0.400 |
| KCl | 0.400 |
| CaCl2·2H2O | 0.795 |
| Na2HPO4·H2O | 0.780 |
| Na2S·9H2O | 0.005 |
| CH4N2O | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaniec, J.; Niziołek, K.; Polanowski, P.; Słota, D.; Kosińska, E.; Sadlik, J.; Miernik, K.; Jampilek, J.; Sobczak-Kupiec, A. Polyethylene Glycol/Pullulan-Based Carrier for Silymarin Delivery and Its Potential in Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 9972. https://doi.org/10.3390/ijms25189972
Iwaniec J, Niziołek K, Polanowski P, Słota D, Kosińska E, Sadlik J, Miernik K, Jampilek J, Sobczak-Kupiec A. Polyethylene Glycol/Pullulan-Based Carrier for Silymarin Delivery and Its Potential in Biomedical Applications. International Journal of Molecular Sciences. 2024; 25(18):9972. https://doi.org/10.3390/ijms25189972
Chicago/Turabian StyleIwaniec, Julia, Karina Niziołek, Patryk Polanowski, Dagmara Słota, Edyta Kosińska, Julia Sadlik, Krzysztof Miernik, Josef Jampilek, and Agnieszka Sobczak-Kupiec. 2024. "Polyethylene Glycol/Pullulan-Based Carrier for Silymarin Delivery and Its Potential in Biomedical Applications" International Journal of Molecular Sciences 25, no. 18: 9972. https://doi.org/10.3390/ijms25189972
APA StyleIwaniec, J., Niziołek, K., Polanowski, P., Słota, D., Kosińska, E., Sadlik, J., Miernik, K., Jampilek, J., & Sobczak-Kupiec, A. (2024). Polyethylene Glycol/Pullulan-Based Carrier for Silymarin Delivery and Its Potential in Biomedical Applications. International Journal of Molecular Sciences, 25(18), 9972. https://doi.org/10.3390/ijms25189972










