Pterostilbene, a Dimethyl Derivative of Resveratrol, Exerts Cytotoxic Effects on Melanin-Producing Cells through Metabolic Activation by Tyrosinase
Abstract
:1. Introduction
2. Results
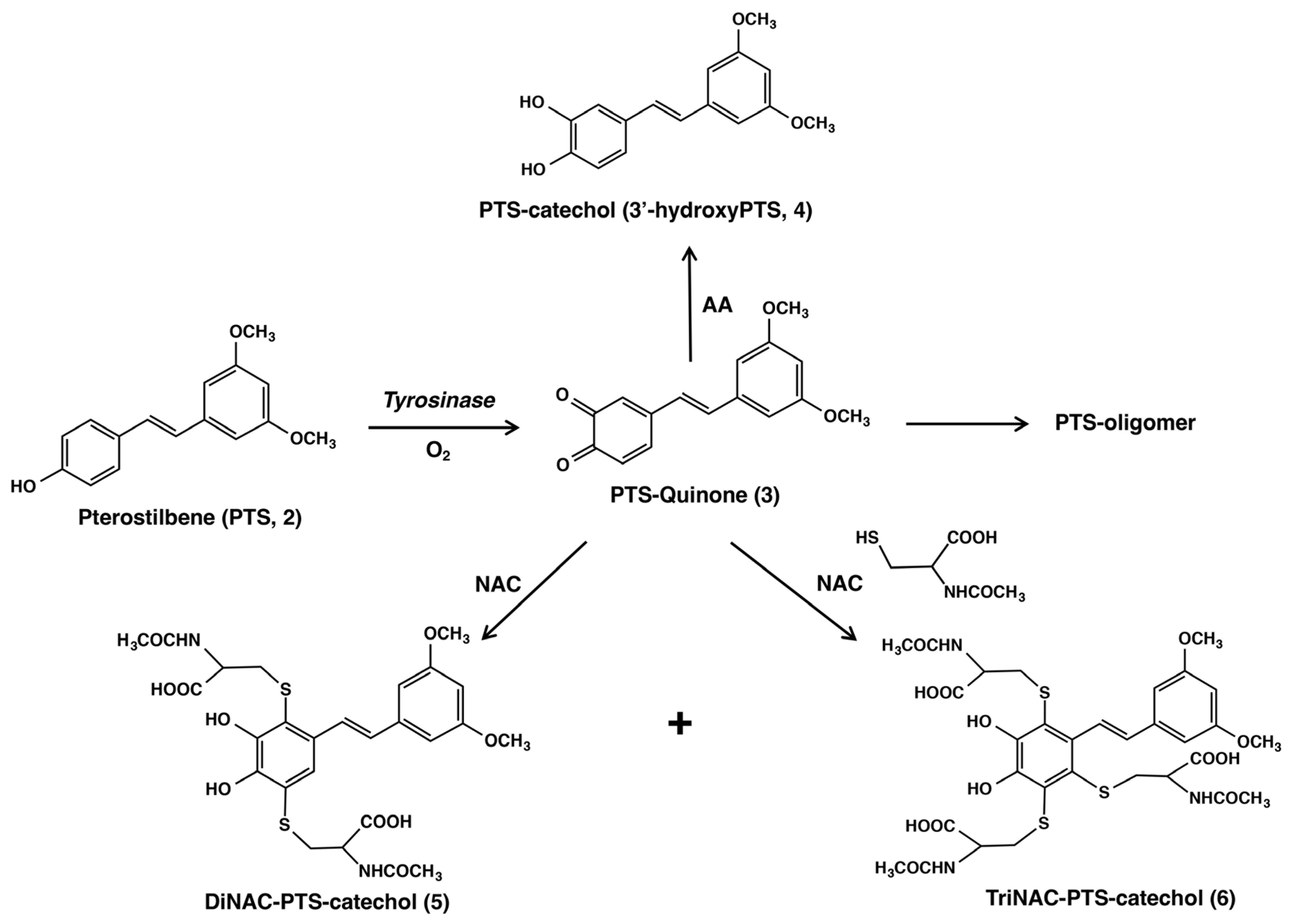
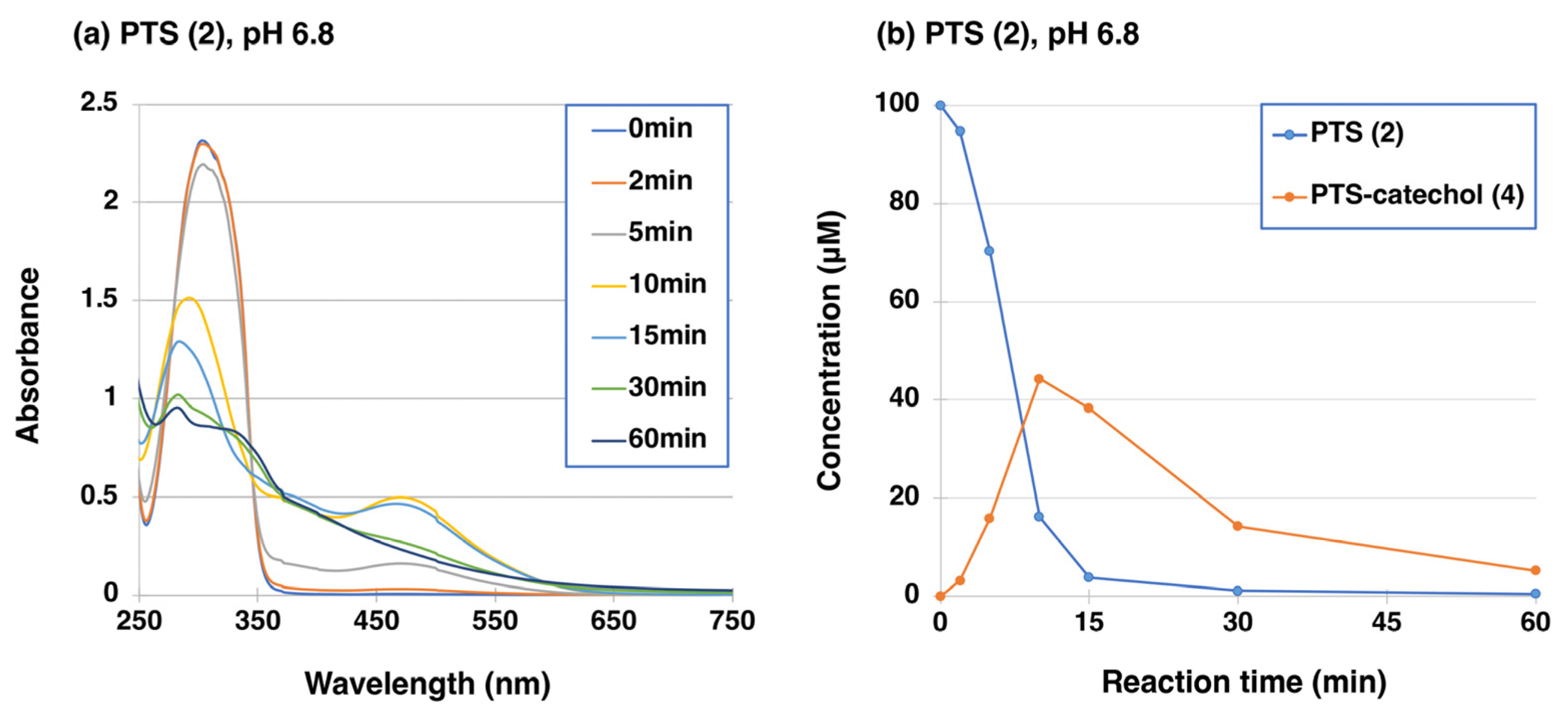
2.1. Tyrosinase-Catalyzed Oxidation of PTS (2)
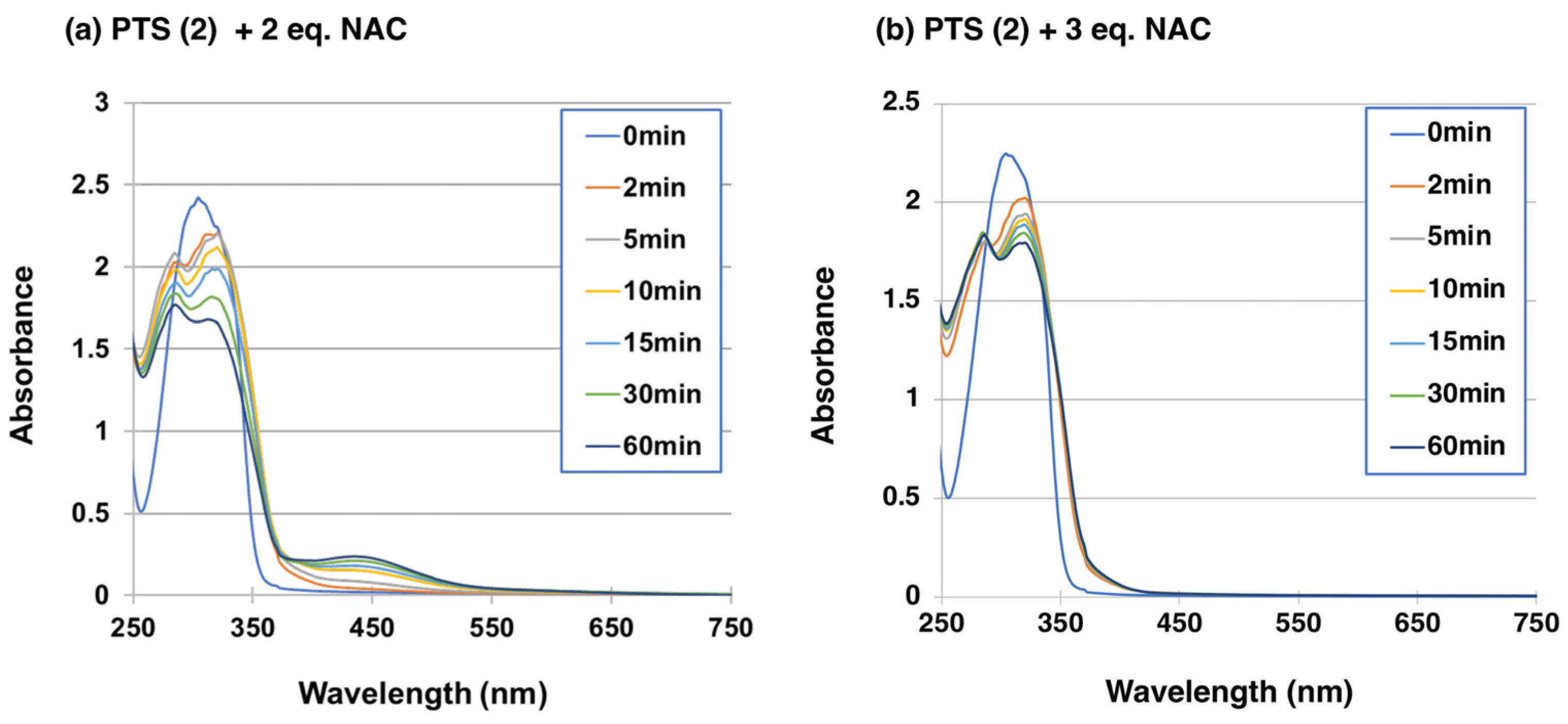
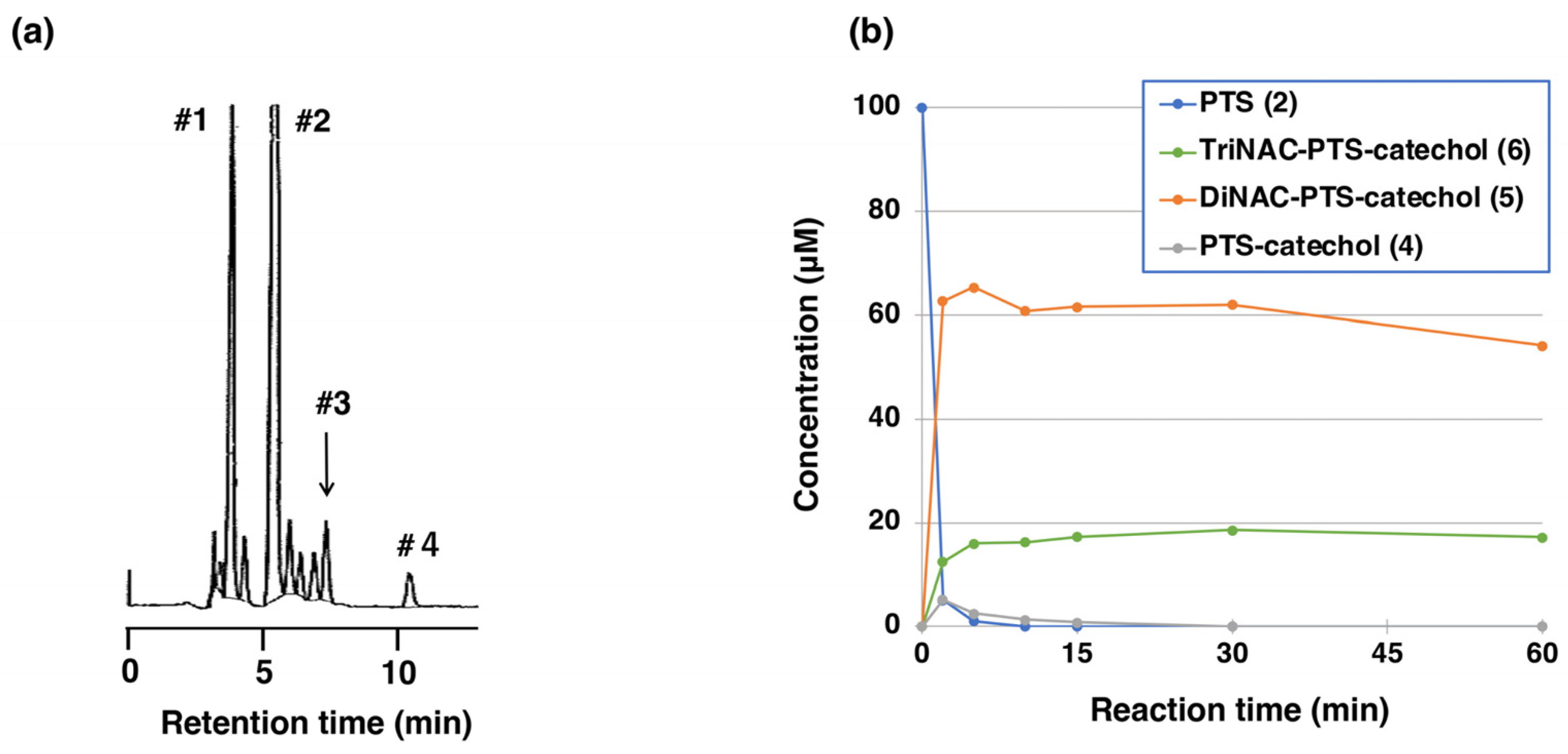
2.2. Reaction of PTS-Quinone (3) with Non-Protein Thiol Compounds Producing Mono- and Di-Adducts
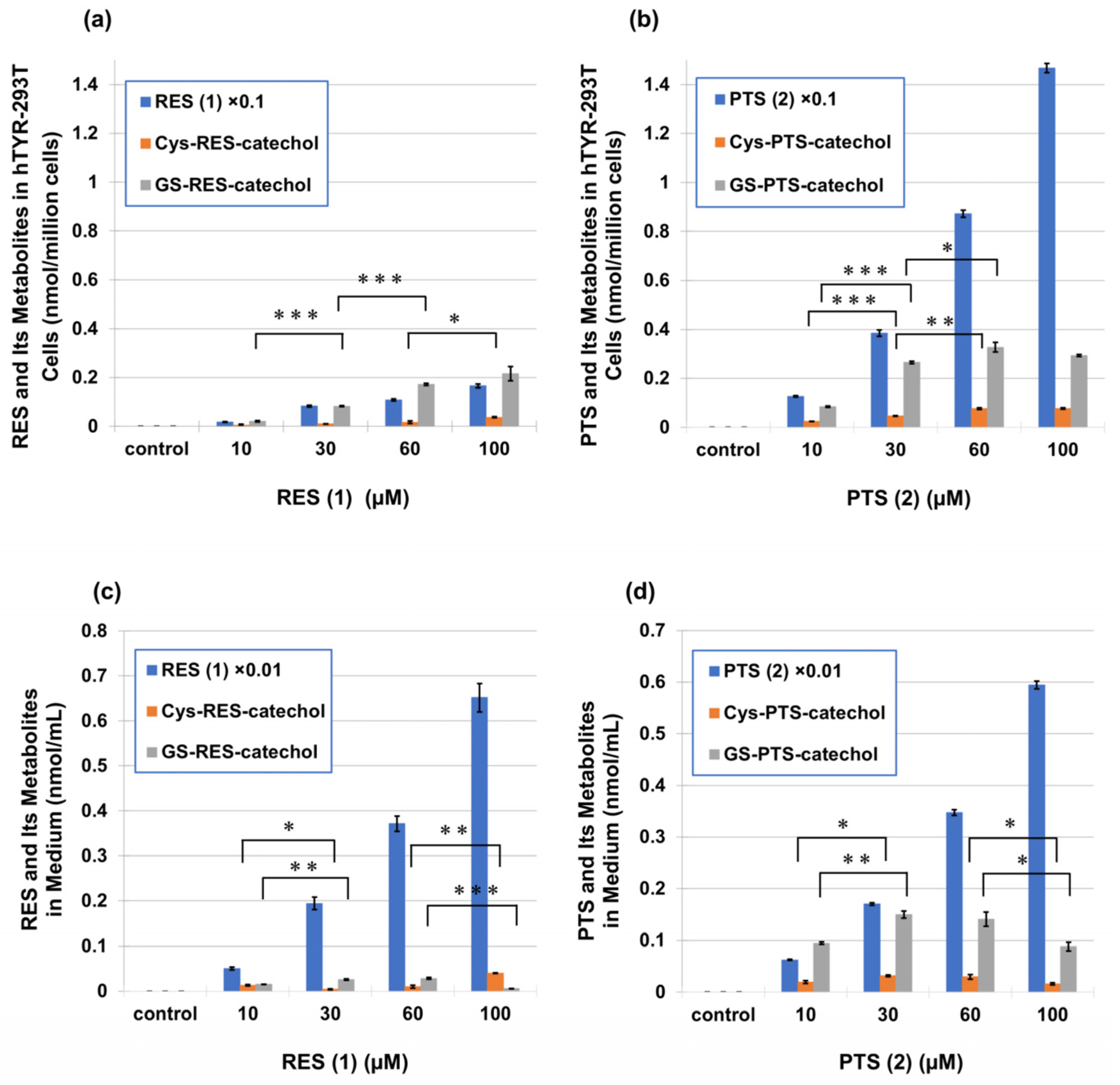
2.3. Metabolism of PTS (2) and RES (1) in Human Tyrosinase-Expressing 293T Cells (hTYR-293T Cells)
2.4. Evaluation of Tyrosinase-Dependent Cytotoxicity in B16BL6 Melanoma Cells
2.5. Inhibition of Melanin Synthesis by PTS (2) and RES (1) in B16BL6 Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. Oxidation of PTS (2) by Tyrosinase in the Absence or Presence of L-Ascorbic Acid or NAC
4.4. Isolation of DiNAC-PTS-Catechol (5) and TriNAC-PTS-Catechol (6)
4.4.1. 3,5-Dimethoxy-3′,4′-hydroxy-trans-stilbene (PTS-catechol (4) or 3′-hydroxyPTS (4))
4.4.2. 2′,5′-Di-S-[(N-acetyl)cysteinyl]-3,5-dimethoxy-3′4′-dihydroxy-trans-stilbene (DiNAC-PTS-catechol (5))
4.4.3. 2′,5′,6′-Tri-S-[(N-acetyl)cysteinyl]-3,5-dimethoxy-3′4′-dihydroxy-trans-stilbene (TriNAC-PTS-catechol (6))
4.5. Metabolism of RES (1) and PTS (2) in Human Tyrosinase-Expressing 293T Cells
4.6. Adduct Formation of RES- and PTS-Quinones with CySH and GSH
4.7. Evaluation of Tyrosinase-Dependent Cytotoxicity in B16BL6 Melanoma Cells
4.8. Evaluation of Tyrosinase-Dependent Cytotoxicity in hTYR-293T Cells
4.9. Measurement of Melanin Levels in B16BL6 Melanoma Cells and in the Culture Medium
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Garcia, A.M.; Meyskens, F.L., Jr. Differences in the glucuronidation of resveratrol and pterostilbene: Altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2014, 29, 112–119. [Google Scholar] [CrossRef]
- Mulakayala, C.; Babajan, B.; Madhusudana, P.; Anuradha, C.; Rao, R.M.; Nune, R.P.; Manna, S.K.; Mulakayala, N.; Kumar, C.S. Synthesis and evaluation of resveratrol derivatives as new chemical entities for cancer. J. Mol. Graph. Model. 2013, 41, 43–54. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Bhat, K.P.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Cytotoxic Mechanism of Resveratrol Probably Involves Mobilization of Endogenous Copper Ions; Portland Press Limited: London, UK, 2007; pp. 1156–1160. [Google Scholar]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lin, Y.-J.; Ho, C.-T.; Yen, G.-C. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Agric. Food Chem. 2013, 61, 602–610. [Google Scholar]
- Li, Y.-R.; Li, S.; Lin, C.-C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Bhullar, K.-S.; Hubbard, B.-P. Lifespan and health-span extension by resveratrol. Biochim. Biophys. Acta 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D.K. Resveratrol: A therapeutic promise for cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liu, K.; Fu, S.; Li, Z.; Yu, X. Pterostilbene attenuates the inflammatory reaction induced by ischemia/reperfusion in rat heart. Mol. Med. Rep. 2015, 11, 724–728. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Ho, C.T.; Wang, Y.J. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol. Nutr. Food Res. 2010, 54, 1819–1832. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Wang, X.; Zhang, J.; Han, W.; Feng, L.; Sun, J.; Jin, H.; Wang, X.J. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am. J. Transl. Res. 2012, 4, 44–51. [Google Scholar] [PubMed]
- Paul, S.; Rimando, A.M.; Lee, H.J.; Ji, Y.; Reddy, B.S.; Suh, N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. 2009, 2, 650–657. [Google Scholar] [CrossRef]
- Lin, H.S.; Yue, B.D.; Ho, P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 1308–1315. [Google Scholar] [CrossRef]
- Rimando, A.M.; Suh, N. Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Planta Med. 2008, 74, 1635–1643. [Google Scholar] [CrossRef]
- Yeo, S.C.; Ho, P.C.; Lin, H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: The impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013, 57, 1015–1025. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethyl ether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.-H.; Tsai, Y.-C.; Lin, J.-N.; Fan, L.-L.; Pan, M.-H.; Ho, C.-T.; Wu, J.-Y.; Way, T.-D. Activation of AMPK by pterostilbene suppresses lipogenesis and cell-cycle progression in p53 positive and negative human prostate cancer cell. J. Agric. Food Chem. 2012, 60, 63399–63407. [Google Scholar] [CrossRef]
- Cheng, T.-C.; Lai, C.-S.; Chung, M.-C.; Kalyanam, N.; Majeed, M.; Ho, C.-T.; Ho, Y.-S.; Pan, M.-H. Potent anti-cancer effect of 3′-hydroxypterostilbebe in human colon xenograft tumors. PLoS ONE 2014, 9, e111814. [Google Scholar] [CrossRef]
- Melzoch, K.; Hanzlíková, I.; Filip, V.; Buckiová, D.; Šmidrkal, J. Resveratrol in parts of vine and wine originating from Bohemian and Moravian vineyard regions. Agric. Conspect. Sci. 2001, 66, 53–57. [Google Scholar]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Shao, X.; Chen, X.; Badmaev, V.; Ho, C.T.; Sang, S. Structural identification of mouse urinary metabolites pf pterostilbene usingnliquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1770–1778. [Google Scholar] [CrossRef]
- Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Roberti, M.; Pizzirani, D.; Meli, M.; Dusonchet, L.; Gebbia, N.; Abbadessa, V.; Crosta, L.; et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int. J. Biochem. Cell Biol. 2005, 37, 1709–1726. [Google Scholar] [CrossRef]
- Ma, Z.J.; Li, X.; Li, N.; Wang, J.H. Stilbenes from Sphaerophysa salsula. Fitoterapia 2002, 73, 313–315. [Google Scholar] [CrossRef]
- Mak, K.K.; Wu, A.T.; Lee, W.H.; Chang, T.C.; Chiou, J.F.; Wamg, L.S.; Wu, C.H.; Huang, C.Y.; Shieh, Y.S.; Chao, T.Y.; et al. Pterostilbene, a bioactive componet of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment andmetstasis via modulating NF-kappaB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef]
- Takemoto, J.K.; Remsberg, C.M.; Davies, N.M. Pharmacologic activities of 3′-hydroxypterostilbene: Cytotoxic, anti-oxidant, anti-adipogenic, anti-inflammatory, histone deacetylase and sirtuin 1 inhibitory activity. J. Pharm. Pharm. Sci. 2015, 18, 713–727. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Smita, S.; Lange, F.; Wolkenhauer, O.; Köhling, R. Deciphering hallmark processes of aging from interaction networks. Biochim. Biophys. Acta 2016, 1860, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of resveratrol in clinical management of chronic diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef]
- Agrawal, M. Natural polyphenols based new therapeutic avenues for advanced biomedical applications. Drug Metab. Rev. 2015, 47, 420–430. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Cucciolla, V.; Borriello, A.; Oliva, A.; Galletti, P.; Zappia, V.; Della Ragione, F. Resveratrol: From basic science to the clinic. Cell Cycle 2007, 6, 2495–2510. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, X.P.; Chen, T.S. Resveratrol protects rabbit articular chondrocyte against sodium nitroprusside-induced apoptosis via scavenging ROS. Apoptosis 2014, 19, 1354–1363. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Springo, Z.; Tucsek, Z.; Gautam, T.; Giles, C.B.; Wren, J.D.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: Role of resveratrol treatment in vasoprotection. Aging Cell 2015, 14, 400–408. [Google Scholar] [CrossRef]
- Ito, S.; Fujiki, Y.; Matsui, N.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of resveratrol produces a highly reactive ortho-quinone: Implications for melanocyte toxicity. Pigment Cell Melanoma Res. 2019, 32, 766–776. [Google Scholar] [CrossRef]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in guinea pig skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef]
- Lei, M.-J.; Dong, Y.; Sun, C.-X.; Zhang, X.-H. Resveratrol inhibits proliferation, promotes differentiation and melanogenesis in HT-144 melanoma cells through inhibition of MEK/ERK kinase pathway. Microb. Pathog. 2017, 111, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yamashita, T.; Ishi-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef]
- Acharya, J.D.; Ghaskadbi, S.S. Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complement. Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Yue, L.; Li, X.; Zhao, L.; Yang, X.; Wang, X.; Yang, Y.; Qu, Y. Neuroprotective effects of pterostilbene against oxidative stress injury: Involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Res. 2016, 1643, 70–79. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jing, S.; Li, Y.; Yang, Z.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Kosuru, R.; Singh, S. Pterostilbene ameliorates insulin sensitivity, glycemic control and oxidative stress in fructose-fed diabetic rats. Life Sci. 2017, 182, 112–121. [Google Scholar] [CrossRef]
- Jin, J.H.; Jiang, Y.Y.; Wang, Y.; Meng, Z.W.; Li, D.H.; Zhang, L.; Wang, H.; Zhang, Y.-J. Oxyresveratrol-induced activation of Nrf2/HO-1 signaling pathway enhances ability of resveratrol to inhibit UVB-induced melanin. Int. J. Dermatol. Venereol. 2021, 4, 152–162. [Google Scholar]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent Raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef]
- Ito, S.; Prota, G. A facile one-step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1975, 33, 1118–1119. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Oxidative conjugation of chlorogenic acid with glutathione: Structural characterization of addition products and a new nitrite-promoted pathway. Bioorgan. Med. Chem. 2003, 11, 4797–4805. [Google Scholar] [CrossRef]
- Nishimaki-Mogami, T.; Ito, S.; Cui, H.; Akiyama, T.; Tamehiro, N.; Adachi, R.; Wakamatsu, K.; Ikarashi, Y.; Kondo, K. A cell-based evaluation of human tyrosinase-mediated metabolic activation of leukoderma-inducing phenolic compounds. J. Dermatol. Sci. 2022, 108, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.H.; Suh, H.-J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S.; Rees, J.L. The usefulness of 4-amino-3-hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Melanoma Res. 2002, 15, 225–232. [Google Scholar] [CrossRef]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzela, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- Cummings, M.P.; Nordlund, J.J. Chemical leukoderma. Am. J. Contact Dermat. 1995, 6, 122–127. [Google Scholar]
- Boissy, R.E.; Manga, P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004, 17, 208–214. [Google Scholar] [CrossRef]
- Ishii-Osai, Y.; Yamashita, T.; Tamura, Y.; Sato, N.; Ito, A.; Honda, H.; Wakamatsu, K.; Ito, S.; Nakayama, E.; Okura, M.; et al. N-propionyl-4-S-cysteaminylphenol induces apoptosis in B16F1 cells and mediates tumor-specific T-cell immune responses in a mouse melanoma model. J. Dermatol. Sci. 2012, 67, 51–60. [Google Scholar] [CrossRef]
- Prezioso, J.A.; Epperly, M.W.; Wang, N.; Bloomer, W.D. Effect of tyrosinase activity on the cytotoxicity of 4-S-cysteaminylphenol and N-acetyl-4-S-cysteaminylphenol in melanoma cells. Cancer Lett. 1992, 63, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yamada, I.; Seki, S.; Ito, S.; Suzuki, S.; Matsubara, O.; Kasuga, T. The killing effect of 4-S-cysteaminylphenol, a newly synthesized melanin precursor, on B16 melanoma cell lines. Br. J. Cancer 1991, 63, 187–190. [Google Scholar] [CrossRef]
- Ito, S.; Okura, M.; Nakanishi, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T. Tyrosinase-catalyzed metabolism of rhododendrol (RD) in B16 melanoma cells: Production of RD-pheomelanin and covalent binding with thiol proteins. Pigment Cell Melanoma Res. 2015, 28, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, S.; Hachiya, A.; Nakamura, S.; Yasuda, Y.; Fujimori, T.; Takano, K.; Morikawa, S.; Hase, T.; Suzuki, T.; Matsunaga, K. Depigmentation caused by application of the active brightening material, rhododendrol, is related to tyrosinase activity at a certain threshold. J. Dermatol. Sci. 2014, 76, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ojika, M.; Yamashita, T.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of rhododendrol produces 2-methylchromane-6,7-dione, the putative ultimate toxic metabolite: Implications for melanocyte toxicity. Pigment Cell Melanoma Res. 2014, 27, 744–753. [Google Scholar] [CrossRef]
- Ito, S.; Gerwat, W.; Kolbe, L.; Yamashita, T.; Ojika, M.; Wakamatsu, K. Human tyrosinase is able to oxidize both enantiomers of rhododendrol. Pigment Cell Melanoma Res. 2014, 27, 1149–1153. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical reactivities of ortho-quinones produced in living organisms: Fate of quinonoid products formed by tyrosinase and phenoloxidase action on phenol and catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Graham, D.G.; Tiffany, S.M.; Bell, W.R., Jr.; Gutknecht, W.F. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol. Pharmacol. 1978, 14, 644–653. [Google Scholar] [PubMed]
- Hasegawa, K.; Ito, S.; Inoue, S.; Wakamatsu, K.; Ozeki, H.; Ishiguro, I. Dihydro-1,4-benzothiazine-6,7-dione, the ultimate toxic metabolite of 4-S-cysteaminylphenol and 4-S-cysteaminylcatechol. Biochem. Pharmacol. 1997, 53, 1435–1444. [Google Scholar] [CrossRef]
- Na, J.-I.; Shin, J.-W.; Choi, H.-R.; Kwon, S.-H.; Park, K.-C. Resveratrol as a multifunctional topical hypopigmenting agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Lv, J.; Wang, F. Pterostilbene inhibits melanogenesis, melanocyte dendricity and melanosome transport through cAMP/PKA/CREB pathway. Eur. J. Pharmacol. 2022, 932, 175231. [Google Scholar] [CrossRef] [PubMed]
- Basri, D.F.; Lew, L.C.; Muralitharan, R.V.; Nagapan, T.S.; Ghazali, A.R. Pterostilibene inhibits the melanogenesis activity in UVB-irradiated B164A5 cells. Dose-Response 2021, 19, 1–8. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Hyun, C.-G.; Lee, N.-H.; Park, S.-S.; Shin, D.-B. Comparative depigmentation effects of resveratrol and its two methyl analogues in α-melanocyte stimulating hormone-triggered B16/F10 murine melanoma cells. Prev. Nutr. Food. Sci. 2016, 21, 155–159. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Gowrisankar, Y.V.; Wang, L.-W.; Zhang, Y.-Z.; Chen, X.-Z.; Huang, P.-J.; Yen, H.-R.; Yang, H.-L. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol. 2021, 44, 102007. [Google Scholar] [CrossRef]
- Sugumaran, M.; Umit, K.; Evans, J.; Muriph, R.; Ito, S.; Wakamatsu, K. Oxidative oligomerization of DBL catechol, a potent cytotoxic compound for melanocytes, reveals the occurrence of novel ionic Diels-Alder type additions. Int. J. Mol. Sci. 2020, 21, 6774. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, H.; Nishimaki-Mogami, T.; Tamehiro, N.; Shibata, N.; Mandai, H.; Ito, S.; Wakamatsu, K. Pterostilbene, a Dimethyl Derivative of Resveratrol, Exerts Cytotoxic Effects on Melanin-Producing Cells through Metabolic Activation by Tyrosinase. Int. J. Mol. Sci. 2024, 25, 9990. https://doi.org/10.3390/ijms25189990
Tanaka H, Nishimaki-Mogami T, Tamehiro N, Shibata N, Mandai H, Ito S, Wakamatsu K. Pterostilbene, a Dimethyl Derivative of Resveratrol, Exerts Cytotoxic Effects on Melanin-Producing Cells through Metabolic Activation by Tyrosinase. International Journal of Molecular Sciences. 2024; 25(18):9990. https://doi.org/10.3390/ijms25189990
Chicago/Turabian StyleTanaka, Hitomi, Tomoko Nishimaki-Mogami, Norimasa Tamehiro, Norihito Shibata, Hiroki Mandai, Shosuke Ito, and Kazumasa Wakamatsu. 2024. "Pterostilbene, a Dimethyl Derivative of Resveratrol, Exerts Cytotoxic Effects on Melanin-Producing Cells through Metabolic Activation by Tyrosinase" International Journal of Molecular Sciences 25, no. 18: 9990. https://doi.org/10.3390/ijms25189990







