Tear Proteomics in Children and Adolescents with Type 1 Diabetes: A Promising Approach to Biomarker Identification of Diabetes Pathogenesis and Complications
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics and Laboratory Parameters of the Study Population
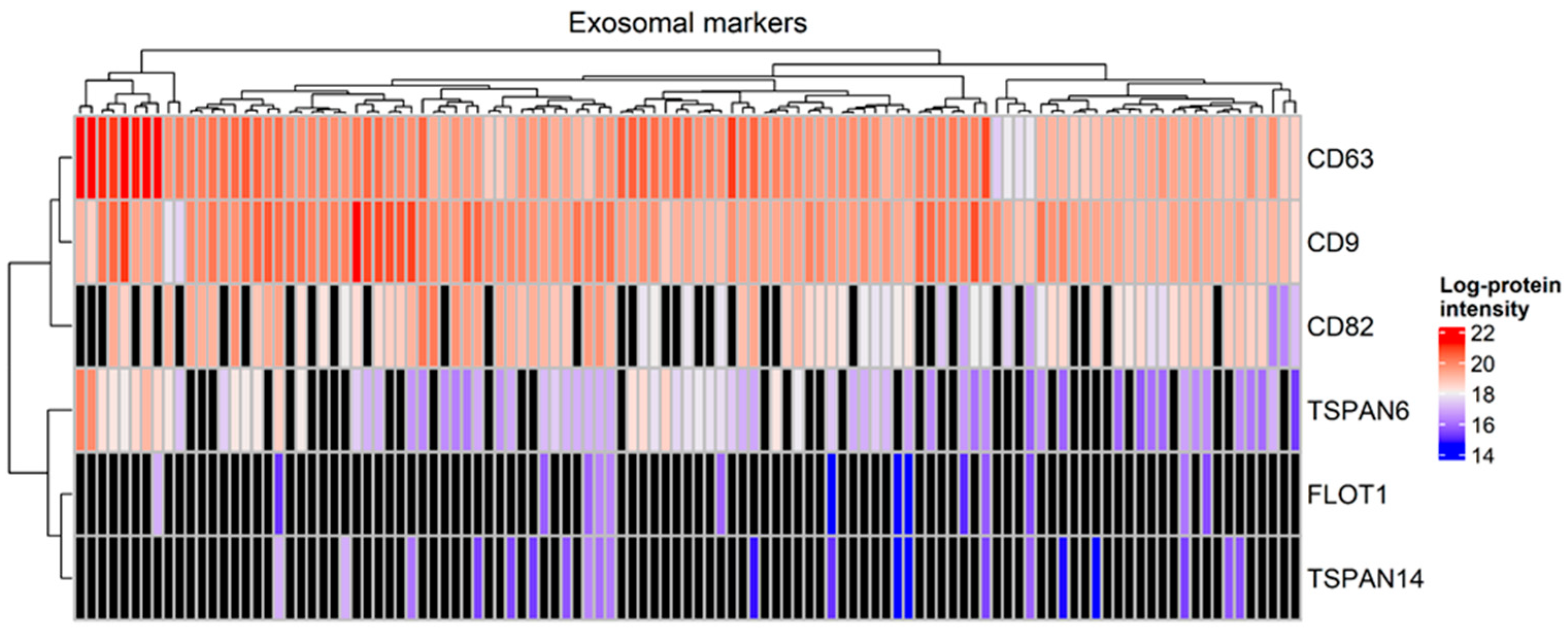
2.2. Identification of Proteins in Total Tear Samples
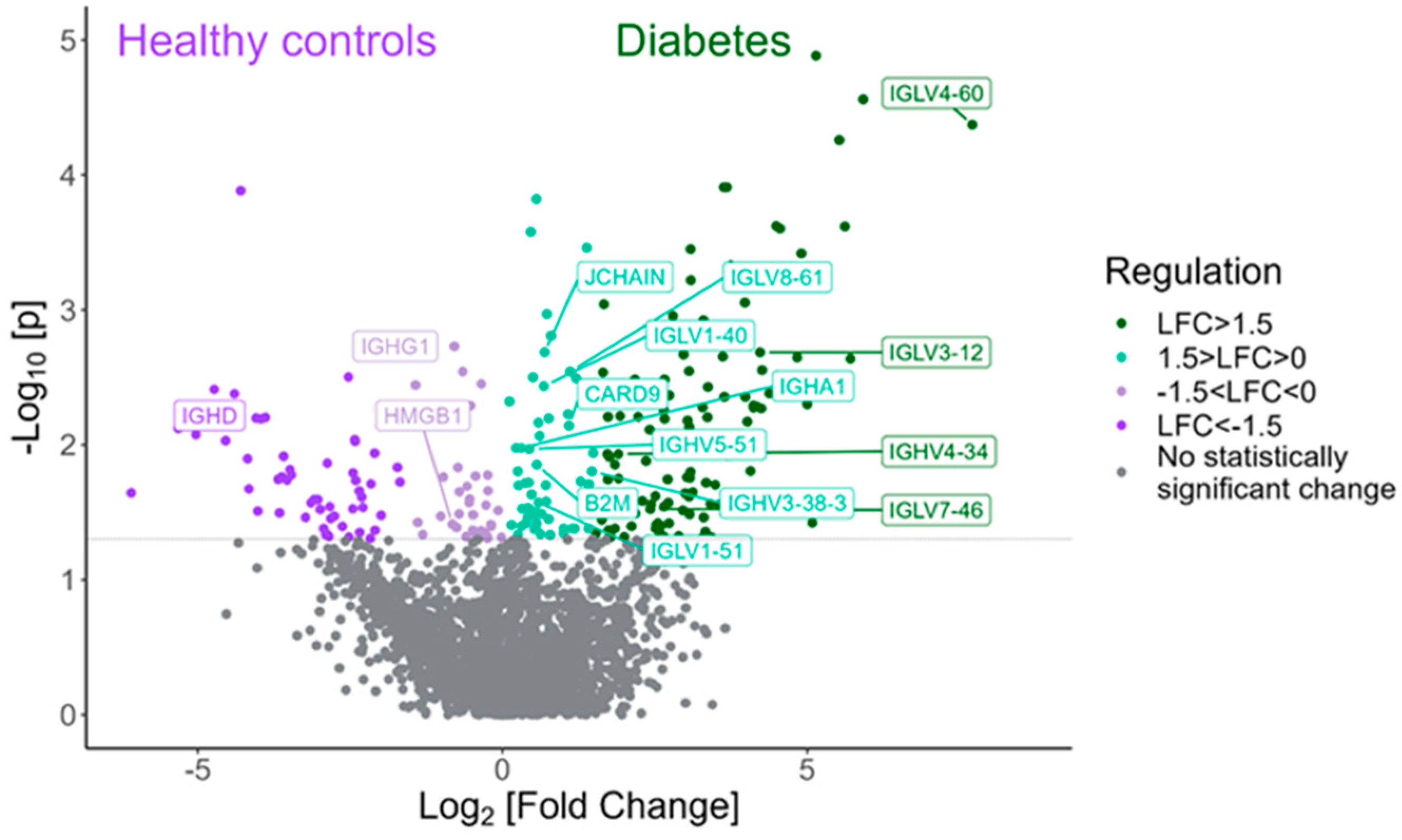
2.3. Differentially Expressed Proteins in Children and Adolescents with T1D Compared to Healthy Subjects
2.4. The Presence of DKA at Diagnosis Influences the Expressed Tear Proteome
2.5. Participants with T1D and Good Glycemic Control Displayed a Distinct Tear Proteomics Profile, Compared to Those with Poor Glycemic Control
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Clinical and Laboratory Parameters
4.3. Assays
4.4. Tear Sample Collection
4.5. Tear Proteomics Protocol
4.6. Statistical Analyses and Graphs
4.7. Pathway Enrichment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, E.; Danne, T.; Ahmad, T.; Ayyavoo, A.; Beran, D.; Ehtisham, S.; Fairchild, J.; Jarosz-Chobot, P.; Ng, S.M.; Paterson, M.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Insulin treatment in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Dart, A.; Donaghue, K.C.; Dost, A.; Feldman, E.L.; Tan, G.S.; Wadwa, R.P.; Zabeen, B.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Microvascular and macrovascular complications in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1432–1450. [Google Scholar] [CrossRef] [PubMed]
- Waernbaum, I.; Lind, T.; Möllsten, A.; Dahlquist, G. The incidence of childhood-onset type 1 diabetes, time trends and association with the population composition in Sweden: A 40-year follow-up. Diabetologia 2023, 66, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Harjutsalo, V.; Rosenbauer, J.; Neu, A.; Cinek, O.; Skrivarhaug, T.; Rami-Merhar, B.; Soltesz, G.; Svensson, J.; Parslow, R.C.; et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: A multicentre prospective registration study. Diabetologia 2019, 62, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, A.; Messaaoui, A.; Tenoutasse, S.; Dorchy, H. Diabetic ketoacidosis in children newly diagnosed with type 1 diabetes mellitus: Role of demographic, clinical, and biochemical features along with genetic and immunological markers as risk factors. A 20-year experience in a tertiary Belgian center. Pediatr. Diabetes 2019, 20, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.J.; Northam, E.A.; Ryan, C.M. The effect of type 1 diabetes on the developing brain. Lancet Child Adolesc. Health 2019, 3, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Mann, M. A beginner’s guide to mass spectrometry–based proteomics. Biochemistry 2020, 42, 64–69. [Google Scholar] [CrossRef]
- Moulder, R.; Bhosale, S.D.; Lahesmaa, R.; Goodlett, D.R. The progress and potential of proteomic biomarkers for type 1 diabetes in children. Expert Rev. Proteom. 2017, 14, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Burch, T.C.; Morris, M.A.; Campbell-Thompson, M.; Pugliese, A.; Nadler, J.L.; Nyalwidhe, J.O. Proteomic analysis of disease stratified human pancreas tissue indicates unique signature of type 1 diabetes. PLoS ONE 2015, 10, e0135663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fillmore, T.L.; Schepmoes, A.A.; Clauss, T.R.; Gritsenko, M.A.; Mueller, P.W.; Rewers, M.; Atkinson, M.A.; Smith, R.D.; Metz, T.O. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. J. Exp. Med. 2013, 210, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Kosteria, I.; Kanaka-Gantenbein, C.; Anagnostopoulos, A.K.; Chrousos, G.P.; Tsangaris, G.T. Pediatric endocrine and metabolic diseases and proteomics. J. Proteom. 2018, 188, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ponzini, E.; Santambrogio, C.; De Palma, A.; Mauri, P.; Tavazzi, S.; Grandori, R. Mass spectrometry-based tear proteomics for noninvasive biomarker discovery. Mass Spectrom. Rev. 2022, 41, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, J.; Guo, Y.; Golubnitschaja, O. Mass spectrometry analysis of human tear fluid biomarkers specific for ocular and systemic diseases in the context of 3P medicine. EPMA J. 2021, 12, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Lépine, M.; Zambito, O.; Sleno, L. Targeted Workflow Investigating Variations in the Tear Proteome by Liquid Chromatography Tandem Mass Spectrometry. ACS Omega 2023, 8, 31168–31177. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.; Martins, B.; Caramelo, F.; Gonçalves, C.; Trindade, G.; Simão, J.; Barreto, P.; Marques, I.; Leal, E.C.; Carvalho, E.; et al. Putative Biomarkers in Tears for Diabetic Retinopathy Diagnosis. Front. Med. 2022, 9, 873483. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Perry, L.; Lowe, J.; Harris, M.; Craig, M.E. ADDN study group. Suboptimal glycemic control in adolescents and young adults with type 1 diabetes from 2011 to 2020 across Australia and New Zealand: Data from the Australasian Diabetes Data Network registry. Pediatr. Diabetes 2022, 23, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Serhan, O.; Faucher, A.; Tran, S.D. Advances in Sjögren’s Syndrome Dry Eye Diagnostics: Biomarkers and Biomolecules beyond Clinical Symptoms. Biomolecules 2024, 14, 80. [Google Scholar] [CrossRef]
- Bajkowska, D.; Szelachowska, M.; Buczyńska, A.; Krętowski, A.J.; Siewko, K. Tears as a Source of Biomarkers in the Diagnosis of Graves’ Orbitopathy. Biomolecules 2022, 12, 1620. [Google Scholar] [CrossRef]
- Tomečková, V.; Tkáčiková, S.; Talian, I.; Fabriciová, G.; Hovan, A.; Kondrakhova, D.; Zakutanská, K.; Skirková, M.; Komanický, V.; Tomašovičová, N. Experimental Analysis of Tear Fluid and Its Processing for the Diagnosis of Multiple Sclerosis. Sensors 2023, 23, 5251. [Google Scholar] [CrossRef]
- Liebner, S.; Czupalla, C.J.; Wolburg, H. Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 2011, 55, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Albavera, L.; Domínguez-Pérez, M.; Medina-Leyte, D.J.; González-Garrido, A.; Villarreal-Molina, T. The Role of the ATP Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef] [PubMed]
- Jongbloets, B.C.; Pasterkamp, R.J. Semaphorin signalling during development. Development 2014, 141, 3292–3297. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Nordengen, K. N-Acetyl-Aspartyl-Glutamate in Brain Health and Disease. Int. J. Mol. Sci. 2022, 23, 1268. [Google Scholar] [CrossRef]
- Sanz, F.J.; Martínez-Carrión, G.; Solana-Manrique, C.; Paricio, N. Evaluation of type 1 diabetes mellitus as a risk factor of Parkinson’s disease in a Drosophila model. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Duca, L.M.; Reboussin, B.A.; Pihoker, C.; Imperatore, G.; Saydah, S.; Mayer-Davis, E.; Rewers, A.; Dabelea, D. Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes and Glycemic Control over Time: The SEARCH for Diabetes in Youth Study. Diabetes 2019, 20, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Xie, K.; Lv, M.; Li, J.; Yao, J.; Yan, K.; Wu, X.; Xu, Y.; Ye, D. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed. Pharmacother. 2021, 133, 110975. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Vitale, M.; Pugliese, G. The Inflammasome in Chronic Complications of Diabetes and Related Metabolic Disorders. Cells 2020, 9, 1812. [Google Scholar] [CrossRef]
- Abdyeva, A.; Kurtova, E.; Savinkova, I.; Galkov, M.; Gorbacheva, L. Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation. Int. J. Mol. Sci. 2024, 25, 1122. [Google Scholar] [CrossRef]
- Grohová, A.; Dáňová, K.; Špíšek, R.; Palová-Jelínková, L. Cell Based Therapy for Type 1 Diabetes: Should We Take Hyperglycemia Into Account? Front Immunol. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Hypothesis: The “metabolic memory”, the new challenge of diabetes. Diabetes Res. Clin. Pract. 2009, 86 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Ihnat, M.A.; Thorpe, J.E.; Kamat, C.D.; Szabó, C.; Green, D.E.; Warnke, L.A.; Lacza, Z.; Cselenyák, A.; Ross, K.; Shakir, S.; et al. Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia 2007, 50, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, H.J.; Fages, C.; Rauvala, H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappa B require the cytoplasmic domain of the receptor but different downstream signaling pathways. J. Biol. Chem. 1999, 274, 19919–19924. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Jash, K.; Gondaliya, P.; Kirave, P.; Kulkarni, B.; Sunkaria, A.; Kalia, K. Cognitive dysfunction: A growing link between diabetes and Alzheimer’s disease. Drug Dev. Res. 2020, 81, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Shalimova, A.; Graff, B.; Gąsecki, D.; Wolf, J.; Sabisz, A.; Szurowska, E.; Jodzio, K.; Narkiewicz, K. Cognitive Dysfunction in Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Song, J. The importance of BDNF and RAGE in diabetes-induced dementia. Pharmacol. Res. 2020, 160, 105083. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Zhao, R.X.; Xin, Z.; Hou, Z.J.; Wang, H.; Xie, R.R.; Li, D.M.; Yang, J.K. Tear-derived exosomal biomarkers of Graves’ ophthalmopathy. Front Immunol. 2022, 13, 1088606. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, S.; Zhao, X.; Zhao, P.; Jia, Q.; Ma, H.; Lin, Q. Role of tear exosomes in the spread of herpes simplex virus type 1 in recurrent herpes simplex keratitis. Eye 2023, 37, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Stergioti, E.M.; Manolakou, T.; Sentis, G.; Samiotaki, M.; Kapsala, N.; Fanouriakis, A.; Boumpas, D.T.; Banos, A. Transcriptomic and proteomic profiling reveals distinct pathogenic features of peripheral non-classical monocytes in systemic lupus erythematosus. Clin. Immunol. 2023, 255, 109765. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A Hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 19 November 2021).
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]


| T1D Group (n = 56) | Control Group (n = 56) | p-Value | |
|---|---|---|---|
| Mean age (years) | 11.5 ± 2.4 | 11.5 ± 2.5 | 0.900 |
| Sex (male/female) | 25/31 | 25/31 | 1.000 |
| Pubertal/ prepubertal status | 37/19 | 32/21 (3 with no data) | 0.557 |
| Median BMI SDS | 0.68 (−0.22, 1.47) | 0.58 (−0.04, 1.80) | 0.932 |
| Median HbA1c (mmol/mol, %) | 60 (54,66), 7.6% (7.1%, 8.2%) | 36 (33, 37), 5.4% (5.2%, 5.5%) | <0.001 |
| Median glucose (mg/dL) | 170 (136, 200) | 83 (80, 89) | <0.001 |
| Median total cholesterol (mg/dL) | 168 (144, 187) | 155 (144,165) | 0.021 |
| Median LDL (mg/dL) | 86 (70,111) | 84 (72, 93) | 0.324 |
| Median triglycerides (mg/dL) | 54 (42,64) | 54 (47,74) | 0.313 |
| T1D Group (n = 56) | |
|---|---|
| Median disease duration (years) | 2.2 (1.5, 4.8) |
| Mean age at T1D onset (years) | 8.0 ± 3.3 |
| Presence of DKA at diagnosis (yes/no) | 25/29 (2 with no data) |
| No DKA/mild DKA/ moderate–severe DKA at diagnosis | 29/13/12 (2 with no data) |
| Episodes of severe hypoglycemia (n) | 3 |
| FGM-CGM use/ no FGM-CGM use | 45/11 |
| Use of Insulin pump therapy/ multiple daily injections | 7/49 |
| Comorbidities | 1 child with hypercholesterolemia |
| Gene Name | Protein Name | Biological Function | p-Value | Log2(Fold Change) (T1D-HCs) |
|---|---|---|---|---|
| TFF1 | Trefoil factor 1 | Product of mucous epithelium, supporting its role as a physical barrier and expressed in inflammation occurring in various pathologies. | <0.001 | 8.61 |
| IGLV4-60 | Immunoglobulin lambda variable 4–60 | Adaptive immunity. | <0.001 | 7.71 |
| AGRN | Agrin | Proteoglycan, component of the basement membrane in different tissues. Central role in neuromuscular junction and undetermined role in brain synapses and neuron homeostasis. | <0.001 | 5.92 |
| ABCA1 | Phospholipid-transporting ATPase ABCA1 | Transmembrane protein expressed in various tissues, essential for cholesterol homeostasis associated with neurological pathologies such as Alzheimer’s disease. | 0.002 | 5.71 |
| LRG1 | Leucine-rich alpha-2-glycoprotein | Secreted glycoprotein, expressed during granulocyte differentiation and involved in cellular homeostasis, immunity, inflammation response and neovascularization. | 0.0002 | 5.61 |
| LIPH | Lipase member H | Hydrolase with a possible role in lipid metabolism. High expression is described in a variety of cancers. | <0.001 | 5.52 |
| SEMA3E | Semaphorin 3E | Secreted protein involved in neurodevelopment and angiogenesis. Plays an important role in the formation of long axon tracts in the brain. | <0.001 | 5.14 |
| LORICRIN | Loricrin | Major keratinocyte protein that contributes to the barrier function of the epidermal cornified cell envelope. | 0.03 | 5.08 |
| FOLH1 | Glutamate carboxypeptidase 2 | Metallopeptidase responsible for the conversion of NAAG to NAA and glutamate, resulting in increased levels of glutamate in the brain. Higher expression is associated with neurocognitive disorders. | 0.005 | 5.00 |
| MAN2B1 | Lysosomal alpha-mannosidase | Lysosome protein implicated in the catabolism of N-linked carbohydrates and mainly expressed in the lung, pancreas, brain and leukocytes. | 0.0006 | 4.95 |
| FAM110A | Protein FAM110A | Located in the cytoplasm and involved in cell proliferation and differentiation. Its expression depends on the cell cycle and is higher when CD4 lymphocytes are stimulated. | 0.02 | −6.09 |
| TM9SF3 | Transmembrane 9 superfamily member 3 | Predicted to be involved in protein localization to the membrane. Mainly unknown biological functions of TM9 family proteins (possible adhesion in immune response, tumor progression). | 0.007 | −5.32 |
| NOL12 | Nucleolar protein 12 | Enables identical protein binding activity. Predicted to be active in nucleolus, regulating its structure. Possible association with aging. | <0.001 | −5.29 |
| ODAD4 | Outer dynein arm-docking complex subunit 4 | Localizes to ciliary axonemes and plays a role in the docking of the outer dynein arm to cilia. Mutations in this gene cause severely reduced ciliary motility and the disorder CILD35. | 0.008 | −5.03 |
| MFN1 | Mitofusin-1 | Mitochondrial outer membrane GTPase that mediates mitochondrial clustering and fusion. | 0.003 | −4.7 |
| ACTN2 | Alpha-actinin-4; alpha-actinin-2 | Actin binding, bundling protein linked to several forms of cardiomyopathy and myopathy. | 0.009 | −4.54 |
| SLC25A1 | Tricarboxylate transport protein, mitochondrial | Transport protein which regulates the movement of citrate across the inner membranes of the mitochondria. | 0.004 | −4.39 |
| MARK2 | Serine/threonine-protein kinase MARK2 | Developmental protein involved in axon guidance, neuronal migration and activation of the Wnt signaling pathway. | 0.0001 | −4.29 |
| EFL1 | Elongation factor-like GTPase 1 | Elongation factor implicated in protein biosynthesis and involved in Shwachman–Diamond syndrome 2. | 0.01 | −4.18 |
| TRIR | Telomerase RNA component interacting RNase | Exonuclease activity, implicated in RNA binding. | 0.006 | −4.04 |
| Gene Name | Protein Name | Biological Function | p-Value | Log2(Fold Change) (with DKA–without DKA) |
|---|---|---|---|---|
| KIAA1217 | Sickle tail protein homolog | Developmental protein necessary for skeletal system development at the embryonic stage. | 0.01 | 5.37 |
| PTK7 | Inactive tyrosine-protein kinase 7 | Transmembrane receptor involved in multiple cellular processes regarding tissue homeostasis, and regulating the Wnt signaling pathway. | 0.006 | 5.21 |
| FAM3C | Protein FAM3C | Developmental protein involved in retinal laminar formation. | 0.01 | 4.90 |
| INCENP | Inner centromere protein | Part of the CPC, with essential function in mitosis and cell division. | 0.03 | 4.80 |
| IRF3 | Interferon regulatory factor 3 | Activator of IFN-dependent immune responses playing a role in immune processes against viruses. | 0.02 | 4.52 |
| RP2 | Protein XRP2 | GTPase activation with a role in protein transport between the Golgi apparatus and the ciliary membrane. It has been associated with retinitis pigmentosa. | 0.03 | 4.31 |
| ARRB2 | Beta-arrestin-2 | Signal transduction inhibitor with high expression in the brain. | 0.02 | 4.29 |
| ERAP2 | Endoplasmic reticulum aminopeptidase 2 | Aminopeptidase involved in appropriate antigen presentation by MHC class I molecules and playing a role in immune processes. | 0.007 | 4.21 |
| PRH2 | Salivary acidic proline-rich phosphoprotein 1/2 | Mainly salivary protein that acts as inhibitor of crystal growth of calcium phosphates. | 0.02 | 4.14 |
| CPN2 | Carboxypeptidase N subunit 2 | Located in blood microparticles and extracellular exosomes and involved in protein stabilization. | 0.01 | 4.09 |
| AKR1E2 | 1,5-anhydro-D-fructose reductase | NADPH-dependent reductase which, in animal experiments, prevents the formation of AGEs. | 0.01 | −7.20 |
| MYH11 | Myosin 7B, myosin-9 | Actin-binding muscle protein that plays a role in muscle contraction through ATP hydrolysis. | 0.02 | −5.29 |
| AGT | Angiotensinogen | Protein with vasoactive molecular functions, that constitutes an essential component of the renin–angiotensin system (RAS). | 0.02 | −4.86 |
| ACTN2 | Alpha-actinin-4, alpha-actinin-2 | Bundling protein with actin-binding molecular functions in different structures in the cells. | 0.03 | −4.84 |
| ATP1A3 | Sodium/potassium-transporting ATPase subunit alpha-1, sodium/potassium-transporting ATPase subunit alpha-3 | ATPase responsible for the ion transport through the plasma membrane, with high expression in the brain. | 0.02 | −4.37 |
| CKB | Creatine kinase B-type | Transferase involved in energy homeostasis. | 0.009 | −4.33 |
| HGD | Homogentisate 1,2-dioxygenase | Enzyme involved in the catabolism of tyrosine and phenylalanine and related to alkaptonuria. | 0.04 | −4.27 |
| CNP | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | One of the most abundant proteins in the central nervous system’s myelin that is implicated in neurodegeneration. | 0.02 | −4.23 |
| GSDMB | Gasdermin-B | Precursor of a pore forming protein involved in cytolysis and pyroptosis. | 0.01 | −3.27 |
| DDAH1 | N(G)-dimethylarginine dimethylaminohydrolase 1 | Hydrolase playing a role in the regulation of nitric oxide production. | 0.04 | −2.57 |
| Gene Name | Protein Name | Biological Function | p-Value | Log2(Fold Change) (Poor Glycemic Control–Good Glycemic Control) |
|---|---|---|---|---|
| MCM6 | DNA replication licensing factor MCM6 | DNA Hydrolase implicated in DNA replication and cell cycle. | 0.001 | 7.89 |
| AKR1E2 | 1,5-anhydro-D-fructose reductase | NADPH-dependent reductase which, in animal experiments, prevents the formation of AGEs. | 0.02 | 7.74 |
| FAM110A | Protein FAM110A | Located in the cytoplasm and involved in cell proliferation and differentiation. Its expression depends on the cell cycle and is higher when CD4 lymphocytes are stimulated. | 0.02 | 6.70 |
| RPLP0 | 60S acidic ribosomal protein P0 | Ribosomal protein implicated in protein synthesis with a possible role in cellular apoptosis. | 0.01 | 6.31 |
| COL18A1 | Collagen alpha-1 (XVIII) chain | Secreted proteoglycan that probably plays a major role in determining the retinal structure as well as in the closure of the neural tube. Its C-terminal fragment, endostatin, has antiangiogenic functions. | 0.01 | 4.88 |
| C4B_2 | Complement C4-A; complement C4-B | Non-enzymatic component of the C3 and C5 convertases involved in the activation of the classical complement pathway. | 0.01 | 4.86 |
| HPR | Haptoglobin; haptoglobin-related protein | Secreted acute phase plasma protein with antioxidant and anti-inflammatory effects. | 0.01 | 4.69 |
| SURF4 | Surfeit locus protein 4 | A cargo receptor regulating the export of proteins, mainly lipoproteins, to the Golgi system. | 0.01 | 4.48 |
| RPL23A | 60S ribosomal protein L23a | Ribosomal protein having a structural activity in the cell. | 0.03 | 4.38 |
| CASP6 | Caspase-6 | Protease implicated in apoptosis and programmed cell death, with a role also in axonal degeneration and the immune response. | 0.04 | 4.13 |
| GSDMA | Gasdermin-A | Pore-forming protein involved in pyroptosis. | 0.001 | −7.21 |
| CALML5 | Calmodulin-like protein 5 | Calcium-binding protein involved in the differentiation of keratinocytes. | 0.003 | −6.96 |
| EHD4 | EH domain containing protein 4 | ATP- and membrane-binding protein that regulates membrane reorganization. | 0.03 | −6.64 |
| UPF1 | Regulator of nonsense transcripts 1 | Part of a multiprotein complex involved in mRNA nuclear export and mRNA surveillance. | 0.015 | −6.46 |
| CRK | Adapter molecule crk | Proto-oncogene involved in several signaling pathways playing a role in cell adhesion and branching. | 0.004 | −6.29 |
| MYD88 | Myeloid differentiation primary response protein MyD88 | Cytosolic adapter protein involved in inflammatory processes and the immune response. | 0.01 | −6.18 |
| LCMT1 | Leucine carboxyl methyltransferase 1 | Enzyme which, in an opposing way, together with PPME1 (protein phosphatase methylesterase 1) regulates the methylation of protein PPA2 that is expressed in the brain and implicated in neuronal signal transduction. | 0.009 | −6.17 |
| PPME1 | Protein phosphatase methylesterase 1 | Enzyme which, in an opposing way, with LCMT1 (leucine carboxyl methyltransferase 1) regulates the methylation of protein PPA2 that is expressed in the brain and implicated in neuronal signal transduction. | 0.005 | −5.98 |
| COL1A2 | Collagen alpha-2 (I) chain | Pro-alpha2 chain of type I collagen playing a role in diseases such as the Ehler–Danlos syndrome and osteogenesis imperfecta. | 0.005 | −5.91 |
| CARS1 | Cysteine–tRNA ligase, cytoplasmic | Aminoacyl-tRNA synthetase involved in protein biosynthesis in the cytoplasm, abundant in the exocrine pancreas and neuronal cells. | 0.04 | −5.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, E.; Kitani, R.-A.; Stroggilos, R.; Lygirou, V.; Vasilakis, I.-A.; Letsou, K.; Vlahou, A.; Zoidakis, J.; Samiotaki, M.; Kanaka-Gantenbein, C.; et al. Tear Proteomics in Children and Adolescents with Type 1 Diabetes: A Promising Approach to Biomarker Identification of Diabetes Pathogenesis and Complications. Int. J. Mol. Sci. 2024, 25, 9994. https://doi.org/10.3390/ijms25189994
Angelopoulou E, Kitani R-A, Stroggilos R, Lygirou V, Vasilakis I-A, Letsou K, Vlahou A, Zoidakis J, Samiotaki M, Kanaka-Gantenbein C, et al. Tear Proteomics in Children and Adolescents with Type 1 Diabetes: A Promising Approach to Biomarker Identification of Diabetes Pathogenesis and Complications. International Journal of Molecular Sciences. 2024; 25(18):9994. https://doi.org/10.3390/ijms25189994
Chicago/Turabian StyleAngelopoulou, Eleni, Rosa-Anna Kitani, Rafael Stroggilos, Vasiliki Lygirou, Ioannis-Anargyros Vasilakis, Konstantina Letsou, Antonia Vlahou, Jerome Zoidakis, Martina Samiotaki, Christina Kanaka-Gantenbein, and et al. 2024. "Tear Proteomics in Children and Adolescents with Type 1 Diabetes: A Promising Approach to Biomarker Identification of Diabetes Pathogenesis and Complications" International Journal of Molecular Sciences 25, no. 18: 9994. https://doi.org/10.3390/ijms25189994







