Antibacterial Activity and Mechanism of Taxillμs chinensis (DC.) Danser and Its Active Ingredients
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Effect of Different Extracts of Taxillμs chinensis
2.2. The Antibacterial Mechanism of Ethyl Acetate Extract of Taxillμs chinensis
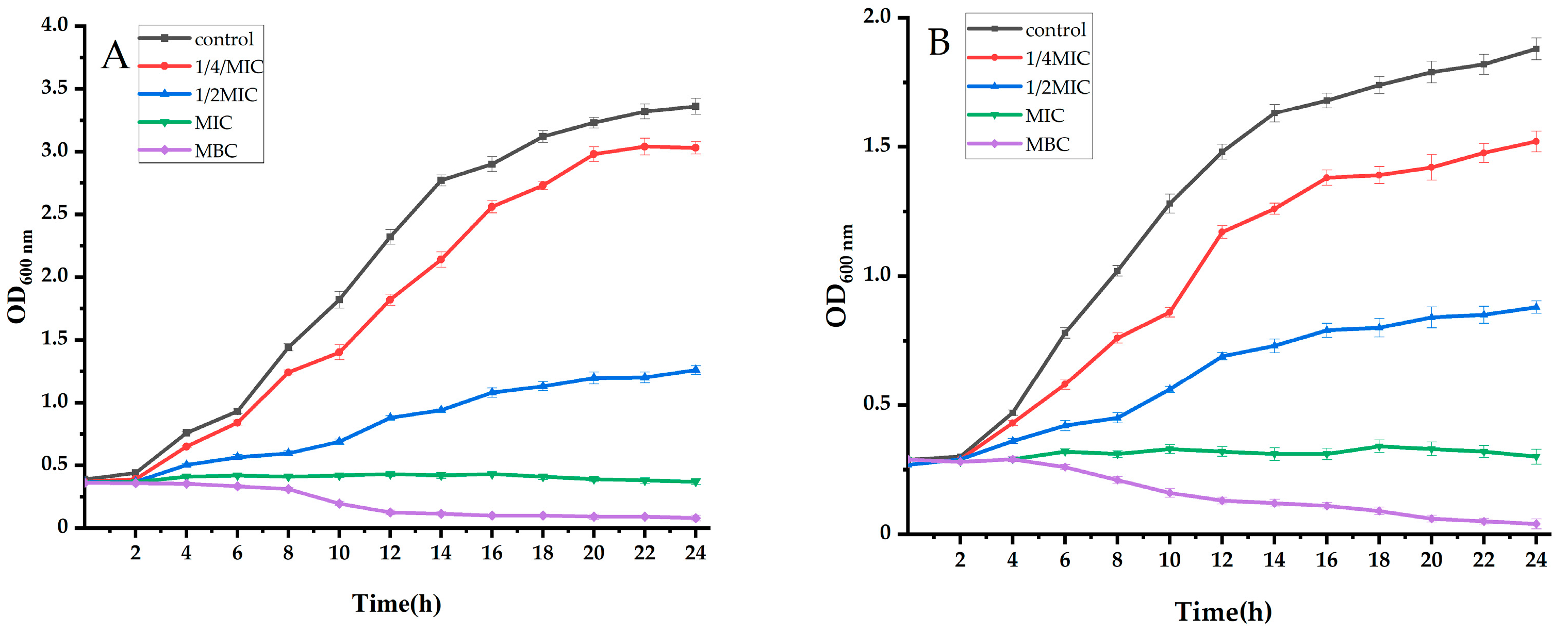
2.2.1. Analysis of the Growth Curve
2.2.2. Analysis of Extracellular Conductivity
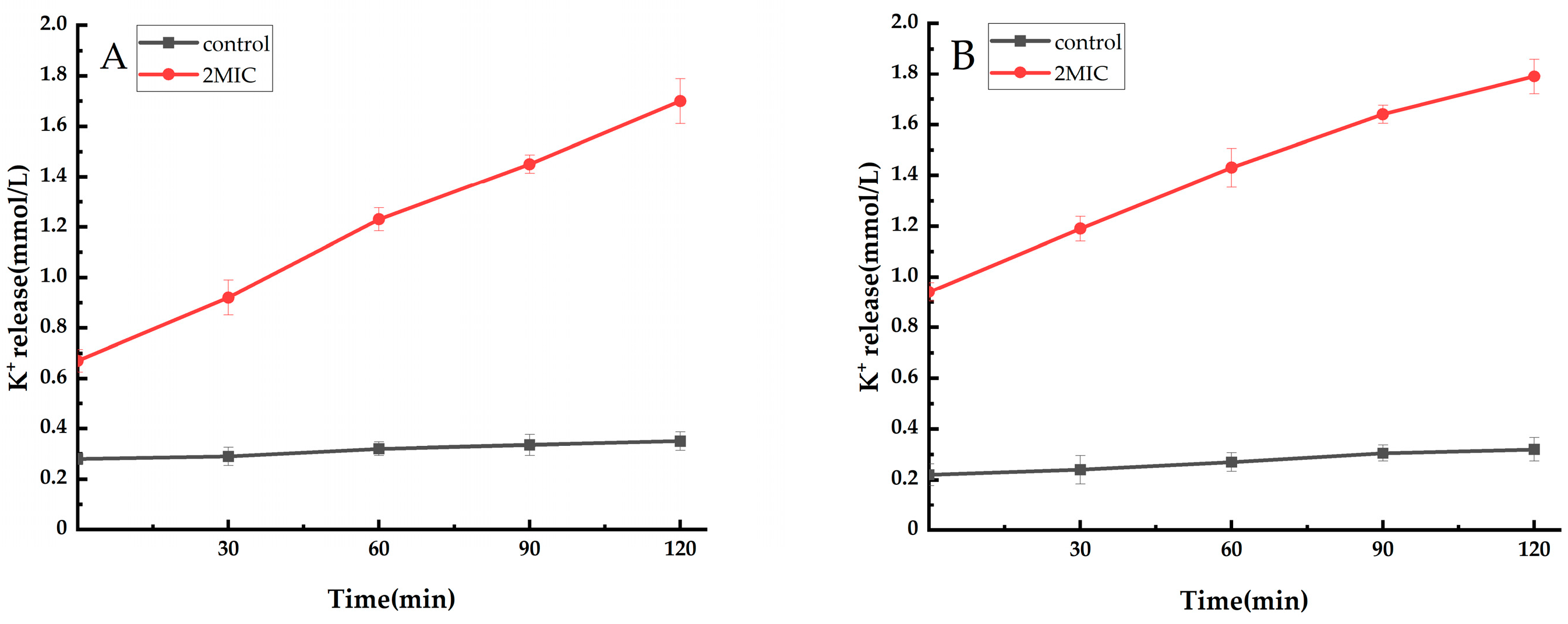
2.2.3. Analysis of Extracellular Potassium Ions (K+)
2.3. UPLC-Q-Orbitrap Was Used to Detect the Active Components of Ethyl Acetate Extract of Taxillμs chinensis
2.4. Antimicrobial Effect of Active Compound 4-Indolecarbaldehyde in Taxillμs chinensis
2.4.1. Antibacterial Effect
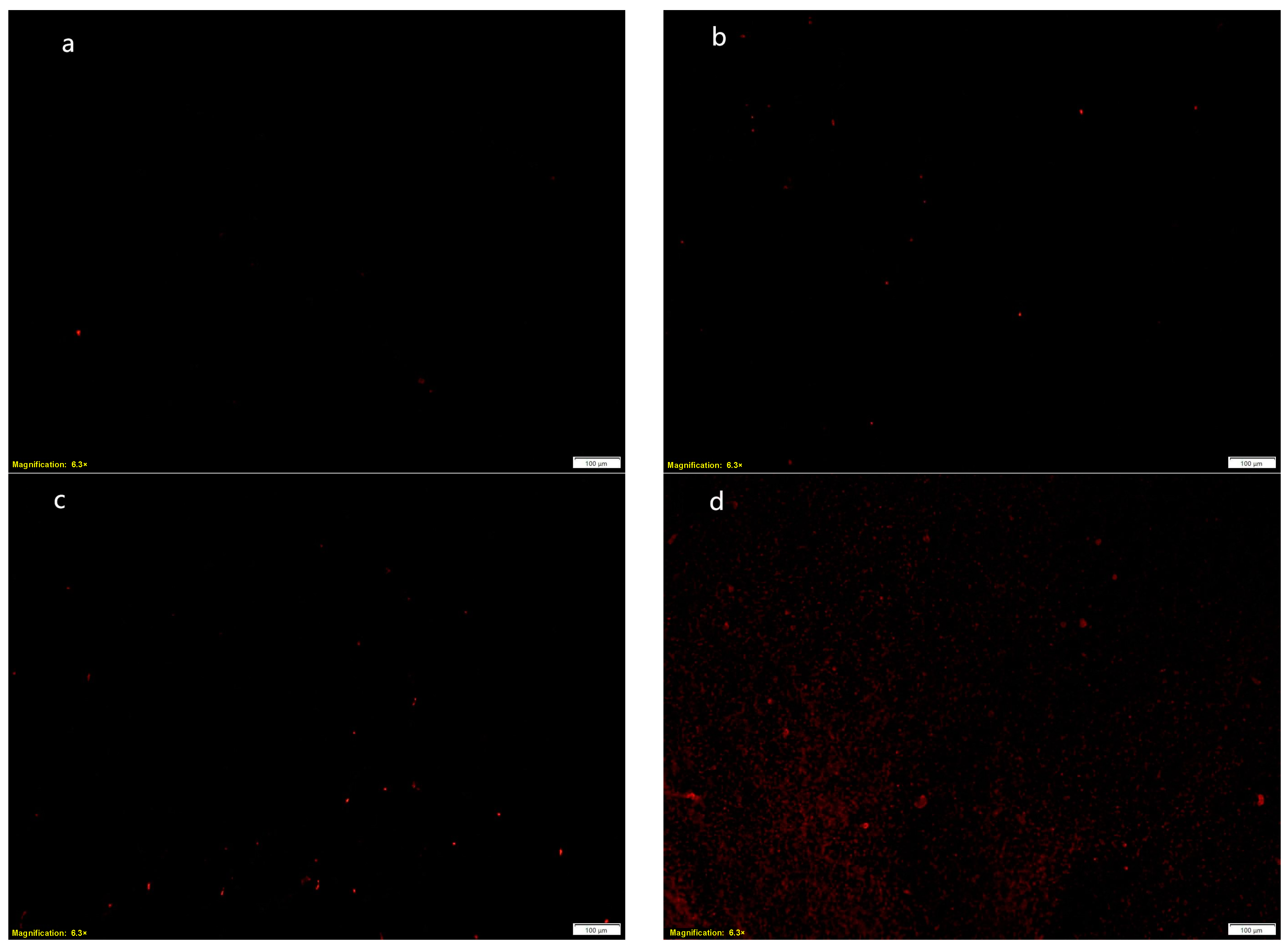
2.4.2. Effect of MRSA Cell Membrane Permeability
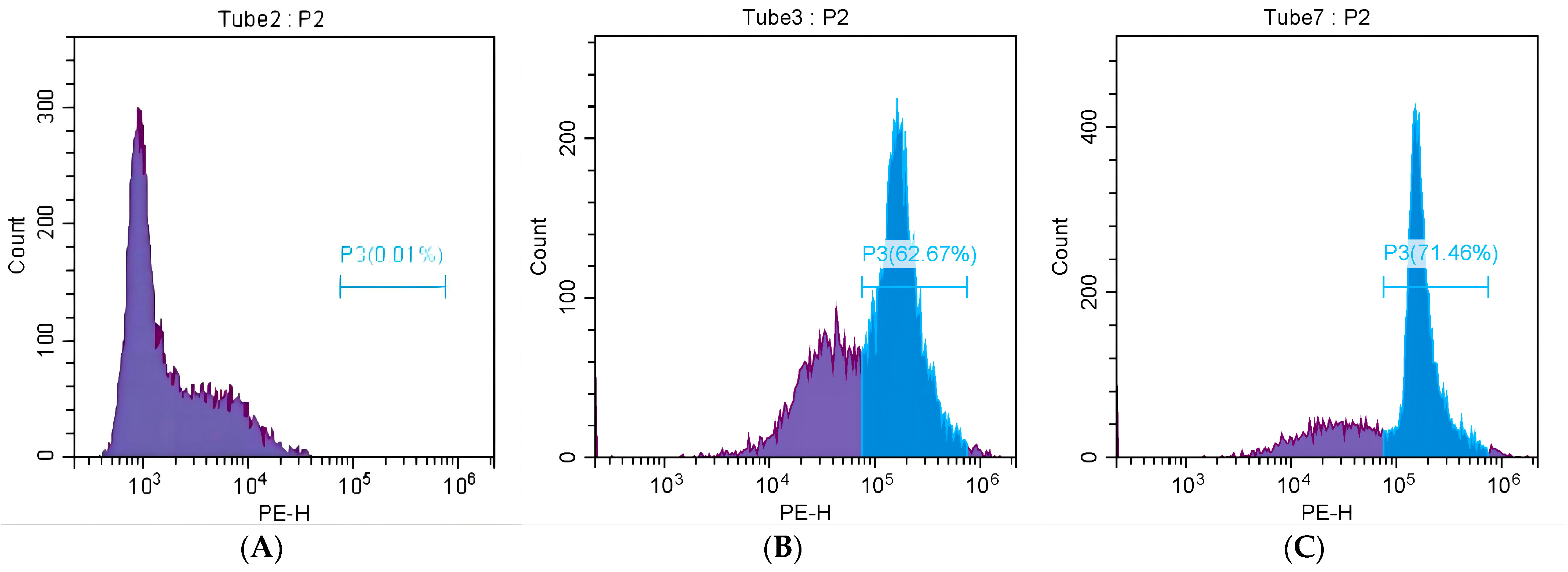
2.4.3. The Effect of MRSA Mortality
3. Materials and Methods
3.1. Plants and Strains
3.2. Activation and Culture of Strains
3.3. Preparation of Ethanol Extract of Taxillμs chinensis and Different Extracts from the Ethanol Extract of Taxillμs chinensis
3.4. Determination of MIC and MBC of Different Extracts of Taxillμs chinensis
3.4.1. Determination of MIC
3.4.2. Determination of MBC
3.5. Antibacterial Effect of Ethyl Acetate Extract of Taxillμs chinensis
3.5.1. Determination of the Growth Curve
3.5.2. Determination of Extracellular Conductivity
3.5.3. Determination of the Potassium Ion Content in Bacterial Suspension
3.6. UPLC-Q-Orbitrap Was Used to Study the Active Components of the Ethyl Acetate Extract of Taxillμs chinensis
3.7. Antibacterial Effect of Active Compounds in Taxillμs chinensis
3.7.1. Determination of MIC, MBC, and IC50 of 4-Indolecarbaldehyde
3.7.2. Detection of MRSA Cell Membrane Permeability
3.7.3. The Mortality of MRSA Was Detected by Flow Cytometry
3.8. Statistical Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Zheng, Y.; Fang, D.; Kamarianakis, Y.; Wilson, J.R. Split bootstrap hierarchical modeling of antibiotics abuse in China. Stat. Med. 2019, 38, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Islam, M.; Wahab, A.; Billah, M. Antimicrobial Resistance Crisis and Combating Approaches. J. Med. 2019, 20, 38–45. [Google Scholar] [CrossRef]
- Manesh, A.; Varghese, G.M.; Collabo, C.I. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe 2021, 2, E419–E420. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO): Antimicrobial Resistance (Key Facts). Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 November 2023).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 21 November 2023).
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Li, X.L.; Cai, Y.Q.; Xia, Q.C.; Liao, Y.Q.; Qin, R.X. Antibacterial sensitizers from natural plants: A powerful weapon against methicillin-resistant Staphylococcus aureus. Front. Pharmacol. 2023, 14, 1118793. [Google Scholar] [CrossRef]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, L.; Shen, J.Z. Antibacterial Effect and Mode of Action of Flavonoids from Licorice Against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol 2019, 10, 2489. [Google Scholar] [CrossRef]
- Chatterjee, R.; Singh, D.; Aggarwal, M.L.; Varma, A.; Chauhan, A.; Tripathi, S. Assessment of Murraya koenigii Leaf Extract against New Multiple Drug Resistance Human Enteric Pathogens. Asian J. Chem. 2020, 32, 2647–2652. [Google Scholar] [CrossRef]
- Dedieu, L.; Brunel, J.M.; Lorenzi, V.; Muselli, A.; Berti, L.; Bolla, J.M. Antibacterial Mode of Action of the Daucus carota Essential Oil Active Compounds against Campylobacter jejuni and Efflux-Mediated Drug Resistance in Gram-Negative Bacteria. Molecules 2020, 25, 5448. [Google Scholar] [CrossRef]
- Hou, L.; Ye, M.; Wang, X.; Zhu, Y.; Sun, X.; Gu, R.; Gu, R.; Chen, L.; Fang, B. Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus Aureus. Molecules 2024, 29, 1528. [Google Scholar] [CrossRef]
- Zhang, W.-T.; Miao, R.-P.; Zhao, Q.-H.; Sun, Y.; Han, S.-J.; Yang, H.W.; Xiong, M.; Yu, G.Y.; Wang, Y.X. Clinical and fundamental research Yinhua Miyanling Tablets in treating urinary tract infection. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2019, 44, 2403–2410. [Google Scholar]
- Sai, J.; Xiong, L.; Zheng, J.; Liu, C.; Lu, Y.; Wang, G.; Wang, Y.; Wang, T.; Guan, X.; Chen, F.; et al. Protective Effect of Yinhua Miyanling Tablet on Lipopolysaccharide-Induced Inflammation through Suppression of NLRP3/Caspase-1 Inflammasome in Human Peripheral Blood Mononuclear Cells. Evid. Based Complement. Altern. Med. Ecam 2016, 2016, 2758140. [Google Scholar] [CrossRef] [PubMed]
- Na, L. Effects of Yinhua Miyanling Tablet on Diuresis Mechanism by Renin–Angiotensin-Aldosterone System and Expression of Aquaporins. Master’s Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
- Li, W.; Zheng, Y.J.; Hu, G.; Yu, J. Clinical study on Yinhua Miyanling Tablets combined with norfloxacin in treatment of type Ⅲ prostatitis. Drugs Clin. 2024, 39, 466–470. [Google Scholar]
- Sai, J.Y.; Hu, Y.; Zhang, C. Influence of ethanol extract of Yinhuamiyanling Tablets in ultrastructure of Escherichia coli and its antibacterial mechanism. J. Jilin Univ. 2014, 40, 117–120. [Google Scholar]
- Xinlei, W.; Jie, H.; Xiaotian, Z.; Zipeng, D.; Huaidong, W.; Liu, C.; Zheng, J.; Wang, F. Anti-Lipopolysaccharide effect of antibacterialtraditional chinese medicine Yinhuamiyanling tablet in vivo and in vitro. J. Jilin Univ. 2014, 40, 276–280. [Google Scholar]
- Li, Y.; Lv, Y.; Liu, J.; Xue, F.; Yang, W.; Zhang, J. In vitro activity of Yinhua Miyan Ling. Chin. J. Clin. Pharmacol. 2015, 31, 279–282. [Google Scholar]
- Wang, C.; Yu, H.; Li, Z.; Wu, J.; Gao, P.; He, S.; Tang, D.; Wang, Q.; Liu, H.; Lv, H.; et al. Novel applications of Yinhua Miyanling tablets in ulcerative colitis treatment based on metabolomics and network pharmacology. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 128, 155366. [Google Scholar] [CrossRef]
- Shang, X.F.; Pan, H.; Li, M.X.; Miao, X.L.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Sato, Y.; Suzaki, S.; Nishikawa, T.; Kihara, M.; Shibata, H.; Higuti, T. Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2000, 72, 483–488. [Google Scholar] [CrossRef]
- Uçar, E. Polygonum aviculare L.’s biological Activities: Investigating its Anti-Proliferative, Antioxidant, chemical properties supported by molecular docking study. Inorg. Chem. Commun. 2024, 162, 112228. [Google Scholar] [CrossRef]
- Millar, B.C.; Rao, J.R.; Moore, J.E. Fighting antimicrobial resistance (AMR): Chinese herbal medicine as a source of novel antimicrobials—An update. Lett. Appl. Microbiol. 2021, 73, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.Y.; Wei, B.Y.; Ma, J.Q.; Wang, L.; Chen, Y.Y. Phytochemicals, Biological Activities, Molecular Mechanisms, and Future Prospects of Plantago asiatica L. J. Agric. Food Chem. 2023, 71, 143–173. [Google Scholar] [CrossRef] [PubMed]
- Thuerig, B.; Ramseyer, J.; Hamburger, M.; Oberhänsli, T.; Potterat, O.; Schärer, H.J.; Tamm, L. Efficacy of a Juncus effusus extract on grapevine and apple plants against Plasmopara viticola and Venturia inaequalis, and identification of the major active constituent. Pest. Manag. Sci. 2016, 72, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia of the people’s Republic of China: 2020; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 312.
- Shanshan, Y.; ‘An, C.Y.; He, B.; Yang, Y. Research progress on chemical constituents and pharmacological activities of Taxilli Herba. Chin. Med. Bull. 2024, 30, 199–202. [Google Scholar]
- Bandian, L.; Moghaddam, M.; Bahreini, M.; Vatankhah, E. Antibacterial characteristics and mechanisms of some herbal extracts and ϵ-polylysine against two spoilage bacterial. Food Biosci. 2022, 50, 102060. [Google Scholar] [CrossRef]
- Wang, F.; Wei, F.; Song, C.; Jiang, B.; Tian, S.; Yi, J.; Yu, C.; Song, Z.; Sun, L.; Bao, Y.; et al. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind. Crops Prod. 2017, 109, 358–366. [Google Scholar] [CrossRef]
- Kang, M.-C.; Ding, Y.; Kim, E.-A.; Choi, Y.K.; De Araujo, T.; Heo, S.J.; Lee, S.H. Indole Derivatives Isolated from Brown Alga Sargassum thunbergii Inhibit Adipogenesis through AMPK Activation in 3T3-L1 Preadipocytes. Mar. Drugs 2017, 15, 119. [Google Scholar] [CrossRef]
- Cha, S.H.; Hwang, Y.; Heo, S.J.; Jun, H.S. Indole-4-carboxaldehyde Isolated from Seaweed, Sargassum thunbergii, Attenuates Methylglyoxal-Induced Hepatic Inflammation. Mar. Drugs 2019, 17, 486. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Seepersaud, M.; Maxwell, A.; Jayaraman, J.; Ramsubhag, A. Isolation and Antibacterial Activity of Indole Alkaloids from Pseudomonas aeruginosa UWI-1. Molecules 2020, 25, 3744. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Chen, D.; Yu, Z.; Huang, L.; Yu, H.; Yao, W.; Xie, Y. Inactivation effect of Staphylococcus aureus and application on fresh-cut pineapples by plasma-activated tartaric acid. Food Biosci. 2023, 54, 102789. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.; Lin, J.; Liu, X.; Qi, D.; Aheyeli-Kai, Y.; Ma, H. Separation, structural identification and antibacterial activity of pectin oligosaccharides derived from seed melon. Food Biosci. 2023, 53, 102616. [Google Scholar] [CrossRef]
- Dai, J.M.; Bai, M.; Li, C.Z.; San Cheang, W.; Cui, H.Y.; Lin, L. Antibacterial properties of citral against Staphylococcus aureus: From membrane damage to metabolic inhibition. Food Biosci. 2023, 53, 102770. [Google Scholar] [CrossRef]
- Figueiredo, C.G.F.; Santos, M.S.D.; Santos, A.S.; Silva, E.D.; Lima, B.; de Lucca Junior, W.; de Araujo, Y.L.F.M.; de Aragão Batista, M.V. In vitro evaluation of the antibacterial effect of Brazilian red propolis ethanol extract in the prevention of periodontal disease in dogs. Comp. Immunol. Microbiol. Infect. Dis. 2023, 92, 101924. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, P.; Szymańska-Majchrzak, J.; Koliński, M.; Kmiecik, S.; Wrzosek, M.; Struga, M.; Szulczyk, D. Novel Tetrazole-Based Antimicrobial Agents Targeting Clinical Bacteria Strains: Exploring the Inhibition of Staphylococcus aureus DNA Topoisomerase IV and Gyrase. Int. J. Mol. Sci. 2022, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Benramdane, E.; Chougui, N.; Ramos, P.A.B.; Makhloufi, N.; Tamendjari, A.; Silvestre, A.J.; Santos, S.A. Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria. Int. J. Mol. Sci. 2022, 23, 11161. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2002, 49, 1049. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Romanowski, E.G.; Mah, F.S.; Shanks, R.M.Q.; Gordon, Y.J. Topical levofloxacin 1.5% overcomes in vitro resistance in rabbit keratitis models. Acta Ophthalmol. 2010, 88, e120–e125. [Google Scholar] [CrossRef]
- Yuan, W.; Yuk, H.-G. Antimicrobial efficacy of Syzygium antisepticum plant extract against Staphylococcus aureus and methicillin-resistant S. aureus and its application potential with cooked chicken. Food Microbiol. 2018, 72, 176–184. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Zeng, L.; Bi, Y.; Kong, F.; Wang, Z.; Tan, S. Characterization, in-vitro digestion, antioxidant, anti-hyperlipidemic and antibacterial activities of Zanthoxylum bungeanum Maxim essential oil nano-emulsion. Food Biosci. 2023, 56, 103082. [Google Scholar] [CrossRef]
- Cao, J.R.; Fu, H.J.; Gao, L.H.; Zheng, Y. Antibacterial activity and mechanism of lactobionic acid against Staphylococcus aureus. Folia Microbiol. 2019, 64, 899–906. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Song, L.; Zhang, X.; Liu, D.; Hu, Y.; Guo, M. Inactivation of Staphylococcus aureus using ultrasound in combination with thyme essential oil nanoemulsions and its synergistic mechanism. LWT 2021, 147, 111574. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tang, L.H.; Wu, M.R.; Shu, L.X.; Xu, Y.Y.; Yao, Y.; Li, Y. A practical method for rapid discrimination of constituents in Psoraleae Fructus by UPLC-Q-Orbitrap MS. J. Mass Spectrom. 2023, 58, e4966. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Cai, Y.Y.; Ma, L.T.; Li, F.T.; Zhang, M.Y.; Wang, Y.; Zheng, F.; Pi, Z.; Yue, H. Identification and chemical profiling of anti-alcoholic liver disease biomarkers of ginseng Huang jiu using UPLC-Q-Orbitrap-HRMS and network pharmacology-based analyses. Front. Nutr. 2022, 9, 978122. [Google Scholar] [CrossRef]
- Li, M.X.; Xie, J.; Bai, X.; Du, Z.Z. Anti-aging potential, anti-tyrosinase and antibacterial activities of extracts and compounds isolated from Rosa chinensis cv. ‘JinBian’. Ind. Crops Prod. 2021, 159, 113059. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Sun, Y.; Xu, J.; Yu, Z.; Huang, L.; Yao, W.; Xie, Y. Inactivation action of ultrasound-assisted cinnamaldehyde on planktonic and biofilm methicillin-resistant Staphylococcus aureus and its application in beef system. Food Biosci. 2023, 55, 103031. [Google Scholar] [CrossRef]
- Sawada, T.; Katayama, M.; Takatani, S.; Ohiro, Y. Early detection of drug-resistant Streptococcus pneumoniae and Haemophilus influenzae by quantitative flow cytometry. Sci. Rep. 2021, 11, 2873. [Google Scholar] [CrossRef]


| Microbe Species | Concentration (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ethyl Acetate Extract | Acetone Extract | n-Butanol Extract | Chloroform Extract | Petroleum Ether Extract | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Staphylococcus aureus | 2.5 | 5 | 2.5 | 10 | 5 | 10 | 10 | 10 | >20 | - |
| Bacillus spizizenii | 2.5 | 5 | 2.5 | 10 | 5 | 10 | 10 | 20 | >20 | - |
| Escherichia coli | 5 | 10 | 5 | 10 | 5 | 10 | 10 | 20 | >20 | - |
| Aspergillus niger | 2.5 | 5 | 5 | 5 | 5 | 10 | 10 | 10 | >20 | - |
| Penicillium italicum | 5 | 10 | 5 | 10 | 5 | 10 | 10 | 20 | >20 | - |
| Structure | Name | Formula | RT [min] | Molecular Weight | mzCloud Best Match |
|---|---|---|---|---|---|
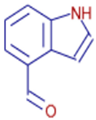 | 4-Indolecarbaldehyde | C9H7NO | 11.83 | 145.0528 | 86.0 |
| Microbe Species | Concentration (μg/mL) | ||
|---|---|---|---|
| 4-Indolecarbaldehyde | |||
| MIC | MBC | IC50 | |
| Escherichia coli | 256 | 1024 | 147.22 |
| Bacillus spizizenii | 128 | 512 | 72.74 |
| Staphylococcus aureus | 128 | 512 | 69.35 |
| MRSA | 128 | 512 | 67.07 |
| Aspergillus niger | 256 | 1024 | 165.35 |
| Aspergillus flavus | 256 | 1024 | 173.26 |
| Penicillium | 256 | 1024 | 183.53 |
| Botrytis cinerea | 256 | 512 | 144.68 |
| Rhizopus sp. | 256 | 512 | 153.49 |
| Rhizopus oryzae | 128 | 1024 | 76.23 |
| Geotrichum candidum | 128 | 512 | 64.49 |
| Mucor racemosus | 64 | 512 | 40.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Huang, S.; Zhu, S.; Gao, B. Antibacterial Activity and Mechanism of Taxillμs chinensis (DC.) Danser and Its Active Ingredients. Int. J. Mol. Sci. 2024, 25, 10246. https://doi.org/10.3390/ijms251910246
Feng Y, Huang S, Zhu S, Gao B. Antibacterial Activity and Mechanism of Taxillμs chinensis (DC.) Danser and Its Active Ingredients. International Journal of Molecular Sciences. 2024; 25(19):10246. https://doi.org/10.3390/ijms251910246
Chicago/Turabian StyleFeng, Yanjing, Silu Huang, Shengying Zhu, and Bo Gao. 2024. "Antibacterial Activity and Mechanism of Taxillμs chinensis (DC.) Danser and Its Active Ingredients" International Journal of Molecular Sciences 25, no. 19: 10246. https://doi.org/10.3390/ijms251910246
APA StyleFeng, Y., Huang, S., Zhu, S., & Gao, B. (2024). Antibacterial Activity and Mechanism of Taxillμs chinensis (DC.) Danser and Its Active Ingredients. International Journal of Molecular Sciences, 25(19), 10246. https://doi.org/10.3390/ijms251910246







