Identification of Genomic Regions Associated with Differences in Flowering Time and Inflorescence Architecture between Melastoma candidum and M. normale
Abstract
:1. Introduction
2. Results
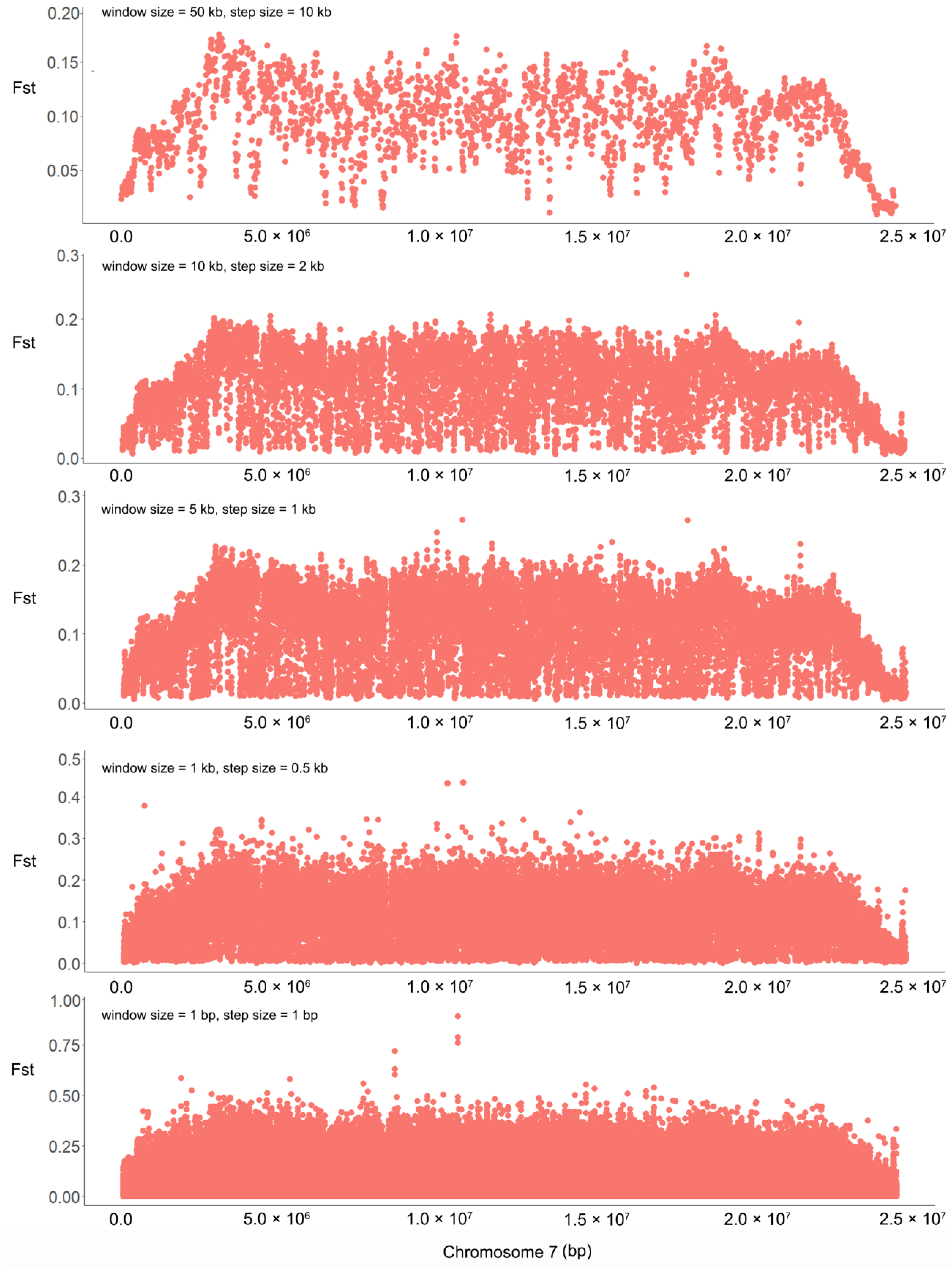
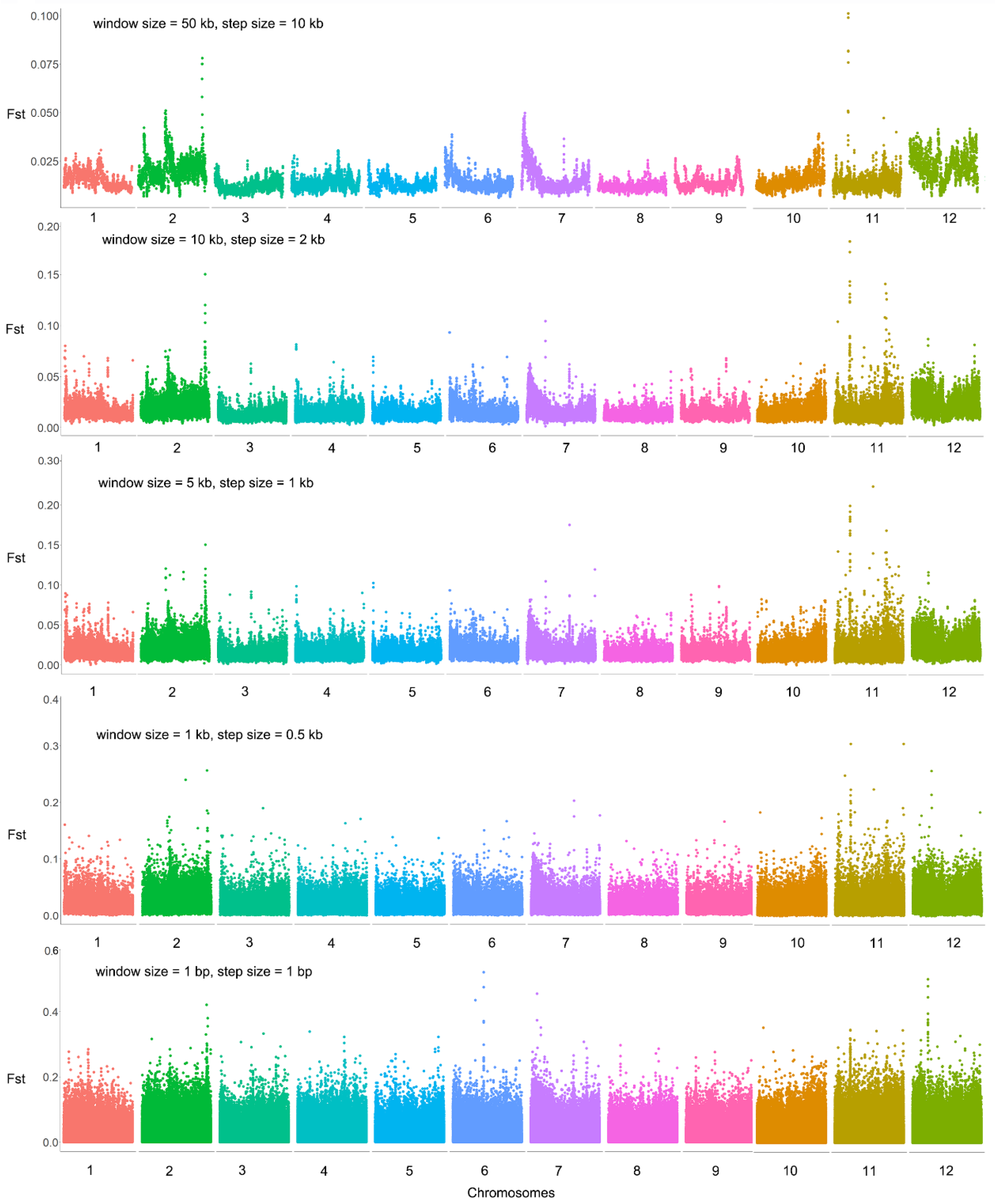
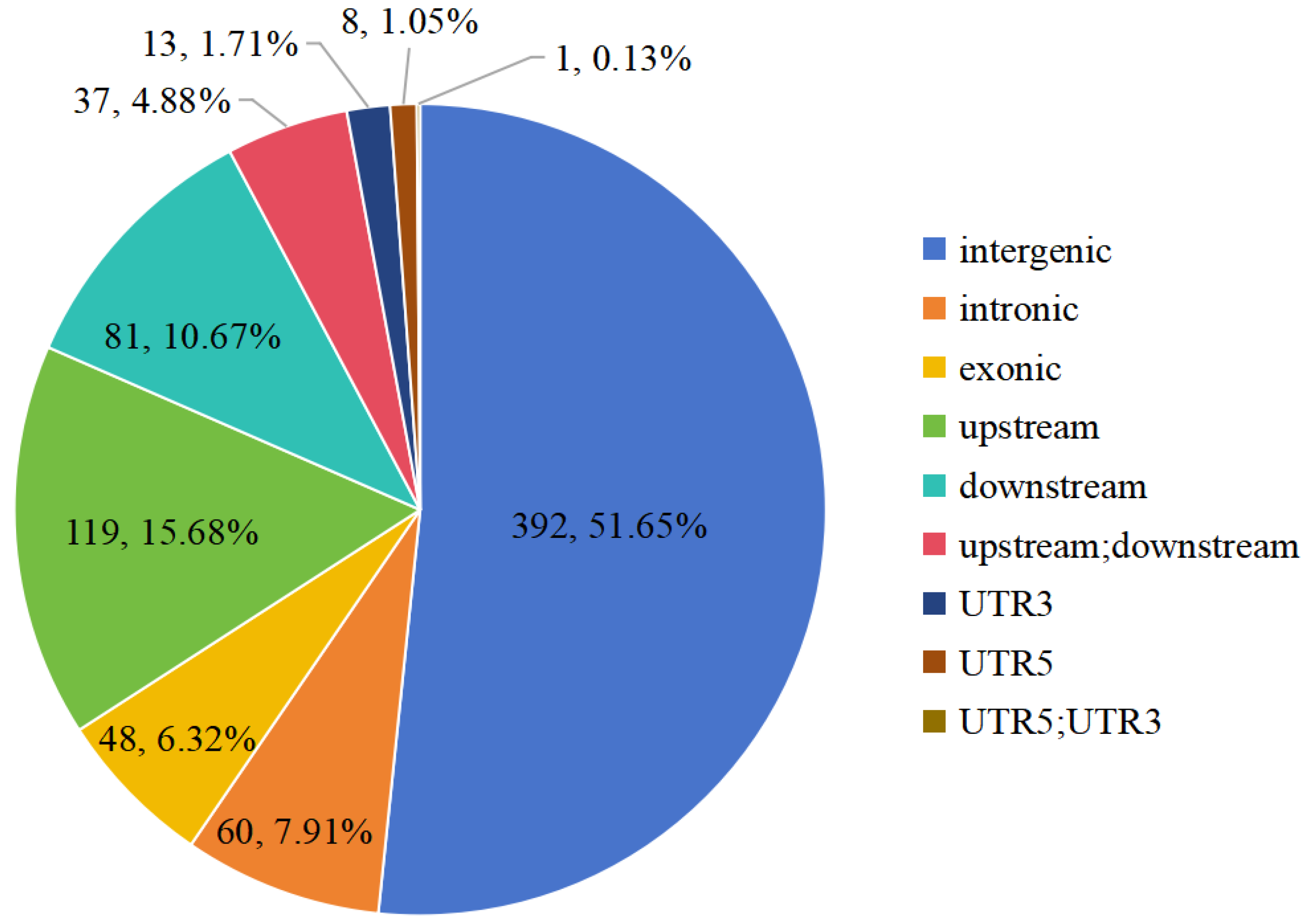
2.1. Highly Differentiated Regions and SNPs between the Two Extreme Bulks for Flowering Time
2.2. Large Inversions on Chromosome 7 between M. candidum and M. normale
2.3. Highly Differentiated Regions and SNPs between the Two Extreme Bulks for Flower Number per Inflorescence
3. Discussion
3.1. Genomic Regions Related to Flowering Time in Melastoma
3.2. Genomic Regions Related to Flower Number per Inflorescence in Melastoma
4. Materials and Methods
4.1. Construction of an F2 Segregating Population and Bulk Sampling
4.2. Library Preparation and Illumina Sequencing
4.3. Bulk Segregation Analysis
4.4. Annotation of Highly Differentiated SNPs between Each Bulk Pair
4.5. GO and KEGG Pathway Enrichment Analyses of Candidate Genes Associated with Flowering Time and Flower Number per Inflorescence
4.6. Detection of Inversions between the Genomes of M. candidun and M. normale
4.7. Identification of Orthologous Proteins between Arabidopsis thaliana and M. candidum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaudinier, A.; Blackman, B.K. Evolutionary processes from the perspective of flowering time diversity. New Phytol. 2020, 225, 1883–1898. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Willis, J.H. Plant speciation. Science 2007, 317, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Yasumoto, A.A.; Nitta, K.; Hirota, S.K.; Yahara, T.; Tachida, H. Difference in flowering time can initiate speciation of nocturnally flowering species. J. Theor. Biol. 2015, 370, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Meng, Q.; Geng, M.; Ren, N.; Zhou, L.; Du, Y.; Cai, Z.; Wang, M.; Wang, X.; Wang, X.; et al. Divergence in flowering time is a major component contributing to reproductive isolation between two wild rice species (Oryza rufipogon and O. nivara). Sci. China Life Sci. 2020, 63, 1714–1724. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, Y.; Lv, T.; Xie, C.; Tian, C. Research progress on the autonomous flowering time pathway in Arabidopsis. Physiol. Mol. Biol. Plants 2017, 23, 477–485. [Google Scholar] [CrossRef]
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550.e1–550.e2. [Google Scholar] [CrossRef]
- Sakuma, S.; Schnurbusch, T. Of floral fortune: Tinkering with the grain yield potential of cereal crops. New Phytol. 2020, 225, 1873–1882. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Liu, Z.; Guo, Y.; Qiu, L.J. Next-generation sequencing from bulked-segregant analysis accelerates the simultaneous identification of two qualitative genes in soybean. Front. Plant Sci. 2017, 8, 919. [Google Scholar] [CrossRef]
- Tiwari, S.; Sl, K.; Kumar, V.; Singh, B.; Rao, A.R.; Mithra Sv, A.; Rai, V.; Singh, A.K.; Singh, N.K. Mapping QTLs for salt tolerance in rice (Oryza sativa L.) by bulked segregant analysis of recombinant inbred lines using 50K SNP chip. PLoS ONE 2016, 11, e0153610. [Google Scholar] [CrossRef]
- Singh, V.K.; Khan, A.W.; Jaganathan, D.; Thudi, M.; Roorkiwal, M.; Takagi, H.; Garg, V.; Kumar, V.; Chitikineni, A.; Gaur, P.M.; et al. QTL-seq for rapid identification of candidate genes for 100-seed weight and root/total plant dry weight ratio under rainfed conditions in chickpea. Plant Biotechnol. J. 2016, 14, 2110–2119. [Google Scholar] [CrossRef]
- Singh, V.; Sinha, P.; Obala, J.; Khan, A.W.; Chitikineni, A.; Saxena, R.K.; Varshney, R.K. QTL-seq for the identification of candidate genes for days to flowering and leaf shape in pigeonpea. Heredity 2022, 128, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wu, W.; Chen, Y.; Zhi, X.; Zou, P.; Ning, Z.; Fan, Q.; Liu, Y.; Deng, S.; Zeng, K.; et al. Balancing selection on an MYB transcription factor maintains the twig trichome color variation in Melastoma normale. BMC Biol. 2023, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lin, T.; Klein, J.; Wang, S.; Qi, J.; Zhou, Q.; Sun, J.; Zhang, Z.; Weng, Y.; Huang, S. QTL-seq identifies an early flowering QTL located near Flowering Locus T in cucumber. Theor. Appl. Genet. 2014, 127, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, L.; Zhu, B.; Xu, Q.; Qi, X.; Chen, X. QTL mapping of cucumber fruit flesh thickness by SLAF-seq. Sci. Rep. 2015, 5, 15829. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Vegas, J.; Zhang, C.; Song, K.; Lee, S. QTL mapping for downy mildew resistance in cucumber via bulked segregant analysis using next-generation sequencing and conventional methods. Theor. Appl. Genet. 2017, 130, 199–211. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.; Deng, Q.; Wang, H.; Yang, R. Identification of a major-effect QTL associated with pre-harvest sprouting in cucumber (Cucumis sativus L.) using the QTL-seq method. BMC Genom. 2021, 22, 249. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, D.; Tang, W.; Zheng, Y.; Liang, K.; Cutler, A.J.; Wu, W. Mapping of quantitative trait loci underlying cold tolerance in rice seedlings via high-throughput sequencing of pooled extremes. PLoS ONE 2013, 8, e68433. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Y.; Wang, L.; Ma, Z.; Zhao, J.; Wang, P.; Zhang, L.; Liu, Z.; Lu, X. Genetic mapping and molecular marker development for Pi65(t), a novel broad-spectrum resistance gene to rice blast using next-generation sequencing. Theor. Appl. Genet. 2016, 129, 1035–1044. [Google Scholar] [CrossRef]
- Dobbels, A.A.; Michno, J.M.; Campbell, B.W.; Virdi, K.S.; Stec, A.O.; Muehlbauer, G.J.; Naeve, S.L.; Stupar, R.M. An induced chromosomal translocation in soybean disrupts a KASI ortholog and is associated with a high-sucrose and low-oil seed phenotype. G3 Genes Genomes Genet. 2017, 7, 1215–1223. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.H.; Liu, X.; Li, Z.; Lee, S.Y.; Zhou, R.; Tan, G. Lectotypification of the name Melastoma candidum f. albiflorum and its taxonomic status. PhytoKeys 2020, 146, 47–52. [Google Scholar] [CrossRef]
- Zheng, W.; Ren, Y.; Wu, M.; Yang, Y.; Fan, Y.; Piao, X.; Ge, Y.; Wang, S. A review of the traditional uses, phytochemistry and biological activities of the Melastoma genus. J. Ethnopharmacol. 2021, 264, 113322. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, W.; Sun, C.; Zou, P.; Liu, Y.; Dai, S.; Zhou, R. Chromosomal-level genome assembly of Melastoma candidum provides insights into trichome evolution. Front. Plant Sci. 2023, 14, 1126319. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, J.; He, M.; Xiong, Y.; Wang, W.; Liu, W.; Ni, J.; Li, X.; Zhou, R. Two new Melastoma cultivars ‘Tianjiao’ and ‘Xinyuan’. Acta Hortic. Sin. 2016, 43, 1847–1848. [Google Scholar] [CrossRef]
- Chen, J. Melastomataceae. In Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1984; Volume 53, pp. 152–162. [Google Scholar]
- Vasconcelos, T.N.; Proença, C.E. Floral cost vs. floral display: Insights from the megadiverse Myrtales suggest that energetically expensive floral parts are less phylogenetically constrained. Am. J. Bot. 2015, 102, 900–909. [Google Scholar] [CrossRef]
- Atwell, S.; Huang, Y.S.; Vilhjálmsson, B.J.; Willems, G.; Horton, M.; Li, Y.; Meng, D.; Platt, A.; Tarone, A.M.; Hu, T.T. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 2010, 465, 627–631. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Guo, S.; Xu, Y.; Liu, H.; Mao, Z.; Zhang, C.; Ma, Y.; Zhang, Q.; Meng, Z.; Chong, K. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat. Commun. 2013, 4, 1566. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Xu, S.; Zhang, Z.; Xu, Y.; Zhang, J.; Chong, K. OsMADS57 together with OsTB1 coordinates transcription of its target OsWRKY94 and D14 to switch its organogenesis to defense for cold adaptation in rice. New Phytol. 2018, 218, 219–231. [Google Scholar] [CrossRef]
- Ortiz-Barrientos, D.; Engelstädter, J.; Rieseberg, L.H. Recombination rate evolution and the origin of species. Trends Ecol. Evol. 2016, 31, 226–236. [Google Scholar] [CrossRef]
- Wellenreuther, M.; Bernatchez, L. Eco-evolutionary genomics of chromosomal inversions. Trends Ecol. Evol. 2018, 33, 427–440. [Google Scholar] [CrossRef]
- Noor, M.A.F.; Gratos, K.L.; Bertucci, L.A.; Reiland, J. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. USA 2001, 98, 12084–12088. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, M.; Barton, N. Chromosome inversions, local adaptation and speciation. Genetics 2006, 173, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.R.; Carrillo, L.; Lasierra, P.; Nebauer, S.G.; Dominguez-Figueroa, J.; Renau-Morata, B.; Pollmann, S.; Granell, A.; Molina, R.V.; Vicente-Carbajosa, J.; et al. Multifaceted role of cycling DOF factor 3 (CDF3) in the regulation of flowering time and abiotic stress responses in Arabidopsis. Plant Cell Environ. 2017, 40, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G.; Ho, L.A.; Kay, S.A. FKF1 F-Box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef]
- Imaizumi, T.; Tran, H.G.; Swartz, T.E.; Briggs, W.R.; Kay, S.A. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 2003, 426, 302–306. [Google Scholar] [CrossRef]
- Balanzà, V.; Martínez-Fernández, I.; Ferrándiz, C. Sequential action of FRUITFULL as a modulator of the activity of the floral regulators SVP and SOC1. J. Exp. Bot. 2014, 65, 1193–1203. [Google Scholar] [CrossRef]
- Sheldon, C.C.; Rouse, D.T.; Finnegan, E.J.; Peacock, W.J.; Dennis, E.S. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc. Natl. Acad. Sci. USA 2000, 97, 3753–3758. [Google Scholar] [CrossRef]
- Macknight, R.; Bancroft, I.; Page, T.; Lister, C.; Schmidt, R.; Love, K.; Westphal, L.; Murphy, G.; Sherson, S.; Cobbett, C.; et al. FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 1997, 89, 737–745. [Google Scholar] [CrossRef]
- Simpson, G.G.; Dijkwel, P.P.; Quesada, V.; Henderson, I.; Dean, C. FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 2003, 113, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Marquardt, S.; Lister, C.; Swiezewski, S.; Dean, C. Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 2010, 327, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Laubinger, S.; Hoecker, U. The SPA1-like proteins SPA3 and SPA4 repress photomorphogenesis in the light. Plant J. 2003, 35, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, Z. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.J.; Lehti-Shiu, M.D.; Fernandez, D.E. The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J. 2007, 50, 1007–1019. [Google Scholar] [CrossRef]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef]
- Hiltbrunner, A.; Tscheuschler, A.; Viczián, A.; Kunkel, T.; Kircher, S.; Schäfer, E. FHY1 and FHL act together to mediate nuclear accumulation of the Phytochrome A photoreceptor. Plant Cell Physiol. 2006, 47, 1023–1034. [Google Scholar] [CrossRef]
- Genoud, T.; Schweizer, F.; Tscheuschler, A.; Debrieux, D.; Casal, J.J.; Schäfer, E.; Hiltbrunner, A.; Fankhauser, C. FHY1 mediates nuclear import of the light-activated Phytochrome A photoreceptor. PLoS Genet. 2008, 4, e1000143. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Rieseberg, L.H. Revisiting the impact of inversions in evolution: From population genetic markers to drivers of adaptive shifts and speciation? Annu. Rev. Ecol. Evol. Syst. 2008, 39, 21–42. [Google Scholar] [CrossRef]
- Harringmeyer, O.S.; Hoekstra, H.E. Chromosomal inversion polymorphisms shape the genomic landscape of deer mice. Nat. Ecol. Evol. 2022, 6, 1965–1979. [Google Scholar] [CrossRef]
- Sanchez-Donoso, I.; Ravagni, S.; Rodríguez-Teijeiro, J.D.; Christmas, M.J.; Huang, Y.; Maldonado-Linares, A.; Puigcerver, M.; Jiménez-Blasco, I.; Andrade, P.; Gonçalves, D.; et al. Massive genome inversion drives coexistence of divergent morphs in common quails. Curr. Biol. 2022, 32, 462–469.e6. [Google Scholar] [CrossRef] [PubMed]
- Lindtke, D.; Lucek, K.; Soria-Carrasco, V.; Villoutreix, R.; Farkas, T.E.; Riesch, R.; Dennis, S.R.; Gompert, Z.; Nosil, P. Long-term balancing selection on chromosomal variants associated with crypsis in a stick insect. Mol. Ecol. 2017, 26, 6189–6205. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Wang, B.; Mojica, J.P.; Mandáková, T.; Prasad, K.V.S.K.; Goicoechea, J.L.; Perera, N.; Hellsten, U.; Hundley, H.N.; Johnson, J.; et al. Young inversion with multiple linked QTLs under selection in a hybrid zone. Nat. Ecol. Evol. 2017, 1, 0119. [Google Scholar] [CrossRef] [PubMed]
- Kanrar, S.; Onguka, O.; Smith, H.M.S. Arabidopsis inflorescence architecture requires the activities of KNOX-BELL homeodomain heterodimers. Planta 2006, 224, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Sawa, S.; Ito, T.; Shimura, Y.; Okada, K. FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant Cell 1999, 11, 69–86. [Google Scholar] [CrossRef]
- Greb, T.; Clarenz, O.; Schafer, E.; Muller, D.; Herrero, R.; Schmitz, G.; Theres, K. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003, 17, 1175–1187. [Google Scholar] [CrossRef]
- Siefers, N.; Dang, K.K.; Kumimoto, R.W.; Bynum Iv, W.E.; Tayrose, G.; Holt Iii, B.F. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 2009, 149, 625–641. [Google Scholar] [CrossRef]
- Liepman, A.H.; Olsen, L.J. Genomic analysis of aminotransferases in Arabidopsis thaliana. Crit. Rev. Plant Sci. 2004, 23, 73–89. [Google Scholar] [CrossRef]
- Feng, C.; Xiang, Q.; Franks, R.G. Phylogeny-based developmental analyses illuminate evolution of inflorescence architectures in dogwoods (Cornus s. l., Cornaceae). New Phytol. 2011, 191, 850–869. [Google Scholar] [CrossRef]
- Zhou, R.; Ling, S.; Zhao, W.; Osada, N.; Chen, S.; Zhang, M.; He, Z.; Bao, H.; Zhong, C.; Zhang, B.; et al. Population genetics in nonmodel organisms: II. Natural selection in marginal habitats revealed by deep sequencing on dual platforms. Mol. Biol. Evol. 2011, 28, 2833–2842. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Kofler, R.; Pandey, R.V.; Schlötterer, C. PoPoolation2: Identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics 2011, 27, 3435–3436. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.; Hao, M.; Leidos, L.; Jiao, X.; Baseler, M.; Lane, H.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Rausch, T.; Zichner, T.; Schlattl, A.; Stütz, A.M.; Benes, V.; Korbel, J.O. DELLY: Structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 2012, 28, i333–i339. [Google Scholar] [CrossRef]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Franks, R.G.; Xiang, Q. Alterations of CorTFL1 and CorAP1 expression correlate with major evolutionary shifts of inflorescence architecture in Cornus (Cornaceae)—A proposed model for variation of closed inflorescence forms. New Phytol. 2017, 216, 519–535. [Google Scholar] [CrossRef]
- Chen, D.; Yan, W.; Fu, L.; Kaufmann, K. Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat. Commun. 2018, 9, 4534. [Google Scholar] [CrossRef] [PubMed]
- Wils, C.R.; Kaufmann, K. Gene-regulatory networks controlling inflorescence and flower development in Arabidopsis thaliana. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2017, 1860, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, X.; Lai, H.; Zhang, X.; Zhong, J. The diversification of the shoot branching system: A quantitative and comparative perspective in meristem determinacy. Curr. Opin. Plant Biol. 2024, 81, 102574. [Google Scholar] [CrossRef] [PubMed]





| Gene_ID | Chromosome | Gene Name | Number of Highly Differentiated SNPs | Fst of Highly Differentiated SNP(s) | Arabidopsis Orthologs | Pathways of Flowering Time in Arabidopsis to Which the Gene Belongs |
|---|---|---|---|---|---|---|
| MLD38_026337 | 7 | CSTF77 | 5 | 0.30–0.36 | AT1G17760 | - |
| MLD38_024073 | 7 | FY | 2 | 0.33, 0.35 | AT5G13480 | - |
| MLD38_027099 | 7 | SPA3 | 1 | 0.31 | AT1G53090, AT3G15354 | - |
| MLD38_026174 | 7 | CDF3 | 2 | 0.38, 0.43 | AT3G47500, AT5G62430 | Photoperiod pathway |
| MLD38_026194 | 7 | AGL8 | 1 | 0.47 | AT5G60910 | Age pathway |
| MLD38_024433 | 7 | FUS3 | 9 | 0.31–0.40 | AT3G26790 | - |
| MLD38_024058 | 7 | AGL15 | 1 | 0.35 | AT5G13790 | - |
| MLD38_026516 | 7 | FHY1 | 2 | 0.31, 0.32 | AT2G37678, AT5G02200 | - |
| MLD38_025118 | 7 | EZA1 | 1 | 0.32 | AT1G02580, AT4G02020 | - |
| MLD38_026475 | 7 | COL9 | 6 | 0.33–0.41 | AT3G07650, AT5G48250 | |
| MLD38_027028 | 7 | CIB1 | 2 | 0.30, 0.38 | AT4G34530 | Photoperiod pathway |
| MLD38_025534 | 7 | FKF1 | 6 | 0.32–0.45 | AT1G68050 | Photoperiod pathway |
| MLD38_023961 | 7 | FAR1 | 5 | 0.32–0.40 | AT4G15090, AT4G19990 | - |
| MLD38_025645 | 7 | PGM | 12 | 0.30–0.40 | AT5G51820 | - |
| MLD38_025363 | 7 | HAP2A | 4 | 0.32–0.34 | AT5G12840 | - |
| Start Position | End Position | Inversion Size (bp) | Flowering-Related Genes Contained within the Inversion |
|---|---|---|---|
| 757,425 | 3,760,509 | 3,003,084 | FY, AGL15, FAR1 |
| 6,342,111 | 19,027,720 | 12,685,609 | CSTF77, CDF3, AGL8, EZA1, COL9, PGMP, HAP2A, FKF1 |
| 10,533,038 | 17,653,689 | 7,120,651 | AGL8, PGM, FKF1 |
| 10,896,857 | 12,709,275 | 1,812,418 | FKF1 |
| 11,415,949 | 14,960,373 | 3,544,424 | FKF1 |
| Gene_ID | Chromosome | Gene Name | Number of Highly Differentiated SNPs | Fst of Highly Differentiated SNP(s) | Arabidopsis Orthologs |
|---|---|---|---|---|---|
| MLD38_040423 | 12 | JAG | 5 | 0.22–0.30 | AT1G13400, AT1G68480 |
| MLD38_004029 | 2 | PNF | 1 | 0.22 | AT2G27990 |
| MLD38_023819 | 7 | FIL | 1 | 0.26 | AT2G45190 |
| MLD38_035107 | 10 | LAS | 2 | 0.24, 0.24 | AT1G55580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhong, Y.; Zou, P.; Ni, J.; Liu, Y.; Dai, S.; Zhou, R. Identification of Genomic Regions Associated with Differences in Flowering Time and Inflorescence Architecture between Melastoma candidum and M. normale. Int. J. Mol. Sci. 2024, 25, 10250. https://doi.org/10.3390/ijms251910250
Chen J, Zhong Y, Zou P, Ni J, Liu Y, Dai S, Zhou R. Identification of Genomic Regions Associated with Differences in Flowering Time and Inflorescence Architecture between Melastoma candidum and M. normale. International Journal of Molecular Sciences. 2024; 25(19):10250. https://doi.org/10.3390/ijms251910250
Chicago/Turabian StyleChen, Jingfang, Yan Zhong, Peishan Zou, Jianzhong Ni, Ying Liu, Seping Dai, and Renchao Zhou. 2024. "Identification of Genomic Regions Associated with Differences in Flowering Time and Inflorescence Architecture between Melastoma candidum and M. normale" International Journal of Molecular Sciences 25, no. 19: 10250. https://doi.org/10.3390/ijms251910250






