The Oldest of Old Male C57B/6J Mice Are Protected from Sarcopenic Obesity: The Possible Role of Skeletal Muscle Protein Kinase B Expression
Abstract
:1. Introduction
2. Results
2.1. Body Composition
2.2. GT Testing
2.3. IT Testing
2.4. AT Testing
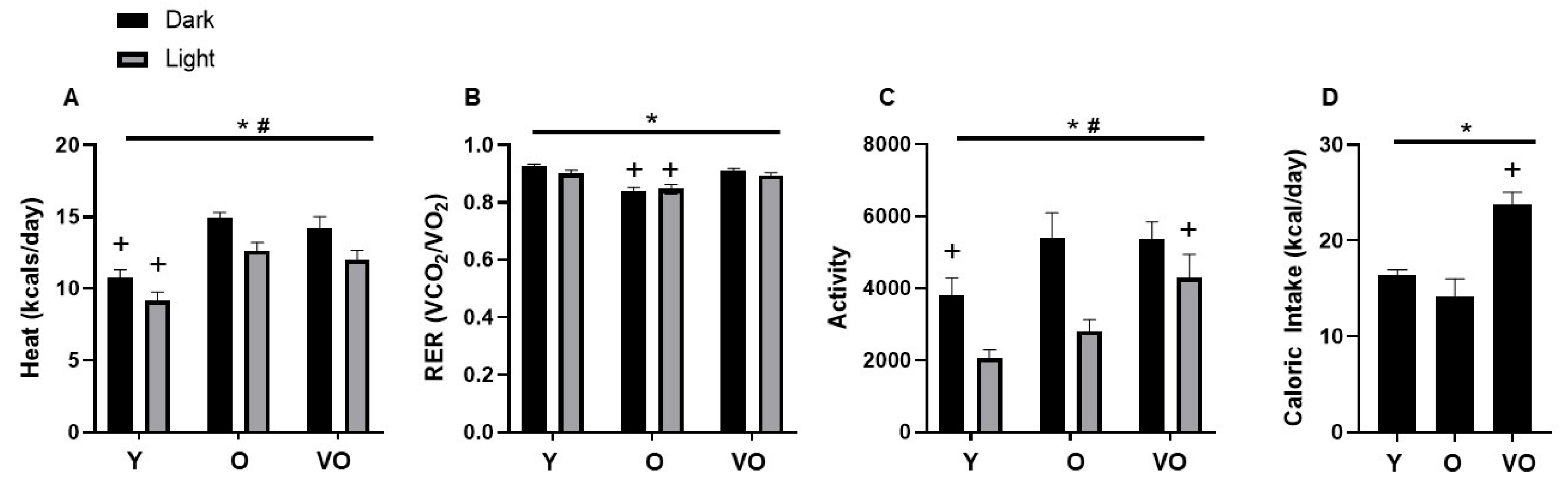
2.5. Energy Homeostasis
2.6. AKT Protein Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Body Composition
4.3. GT Testing
4.4. IT Testing
4.5. AT Testing
4.6. Energy Homeostasis
4.7. Preparation of Muscle Extracts
4.8. AKT Protein Expression
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J. Definitions, Classification, and Epidemiology of Obesity; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- NCD-RisC. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. Available online: https://search.ebscohost.com/login.aspx?direct=true&AuthType=sso&db=cmedm&AN=32487284&site=ehost-live&custid=s6847256 (accessed on 15 June 2024).
- Colby, S.L.; Ortman, J.M. Projections of the Size and Composition of the U.S. Population: 2014-60: Current Population Reports. Series P-25: Population Estimates and Projections; 2015 ASI 2546-3.194; Census P-25, No. 1143. 2015. Available online: https://statistical.proquest.com/statisticalinsight/result/pqpresultpage.previewtitle?docType=PQSI&titleUri=/content/2015/2546-3.194.xml (accessed on 15 June 2024).
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Hong, S.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Dou, Q.; Liu, C.; Zhang, W.; Yang, Y.; Deng, R.; Cheng, A.S.K. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: A updated meta-analysis. BMC Geriatr. 2019, 19, 183. [Google Scholar] [CrossRef]
- Brown, L.A.; Perry Richard, A., Jr.; Haynie, W.S.; Lee, D.E.; Rosa-Caldwell, M.; Brown, J.L.; Greene, N.P.; Wolchok, J.C.; Washington, T.A. Moderators of skeletal muscle maintenance are compromised in sarcopenic obese mice. Mech. Ageing Dev. 2021, 194, 111404. [Google Scholar] [CrossRef]
- Kob, R.; Bollheimer, L.C.; Bertsch, T.; Fellner, C.; Djukic, M.; Sieber, C.C.; Fischer, B.E. Sarcopenic obesity: Molecular clues to a better understanding of its pathogenesis? Biogerontology 2015, 16, 15–29. [Google Scholar] [CrossRef]
- Bollheimer, L.C.; Buettner, R.; Pongratz, G.; Brunner-Ploss, R.; Hechtl, C.; Banas, M.; Singler, K.; Hamer, O.W.; Stroszczynski, C.; Sieber, C.C.; et al. Sarcopenia in the aging high-fat fed rat: A pilot study for modeling sarcopenic obesity in rodents. Biogerontology 2012, 13, 609–620. [Google Scholar] [CrossRef]
- Gonzalez, E.; McGraw, T.E. The Akt kinases: Isoform specificity in metabolism and cancer. Cell Cycle 2009, 8, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.W.; Elliott, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Edward, F. Adolph Distinguished Lecture. Skeletal muscle atrophy: Multiple pathways leading to a common outcome. J. Appl. Physiol. (1985) 2020, 129, 272–282. [Google Scholar] [CrossRef]
- Guillet, C.; Prod’homme, M.; Balage, M.; Gachon, P.; Giraudet, C.; Morin, L.; Grizard, J.; Boirie, Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004, 18, 1586–1587. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, M.; Brown, A.; Merrell, E.; DiSanto-Rose, M.; Rathmacher, J.A.; Reynolds Thomas, H., 4th. PKB signaling and atrogene expression in skeletal muscle of aged mice. J. Appl. Physiol. 2011, 111, 192–199. [Google Scholar] [CrossRef]
- Joseph, G.A.; Wang, S.X.; Jacobs, C.E.; Zhou, W.; Kimble, G.C.; Tse, H.W.; Eash, J.K.; Shavlakadze, T.; Glass, D.J. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol. Cell. Biol. 2019, 39, e00141-19. [Google Scholar] [CrossRef] [PubMed]
- Hwee, D.T.; Bodine, S.C. Age-related deficit in load-induced skeletal muscle growth. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 618–628. [Google Scholar] [CrossRef]
- Halldorsdottir, S.; Carmody, J.; Boozer, C.N.; Leduc, C.A.; Leibel, R.L. Reproducibility and accuracy of body composition assessments in mice by dual energy x-ray absorptiometry and time domain nuclear magnetic resonance. Int. J. Body Compos. Res. 2009, 7, 147–154. [Google Scholar]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- White, Z.; Terrill, J.; White, R.B.; McMahon, C.; Sheard, P.; Grounds, M.D.; Shavlakadze, T. Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skelet. Muscle 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Van Long, N.; Chien, P.N.; Tung, T.X.; Van Anh, L.T.; Giang, N.N.; Nga, P.T.; Linh, L.T.T.; Nam, S.; Heo, C. Complementary combination of biomarkers for diagnosis of sarcopenia in C57BL/6J mice. Life Sci. 2023, 312, 121213. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, V.L.; Ayers, R.A.; Bateman, T.A.; Simske, S.J. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.H.; Dalton, A.; Calzini, L.; Tuluca, A.; Hoyte, D.; Ives, S.J. The impact of age and sex on body composition and glucose sensitivity in C57BL/6J mice. Physiol. Rep. 2019, 7, e13995. [Google Scholar] [CrossRef]
- Izumiya, Y.; Hopkins, T.; Morris, C.; Sato, K.; Zeng, L.; Viereck, J.; Hamilton, J.A.; Ouchi, N.; LeBrasseur, N.K.; Walsh, K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008, 7, 159–172. [Google Scholar] [CrossRef]
- Norrby, M.; Evertsson, K.; Fjällström, A.; Svensson, A.; Tågerud, S. Akt (protein kinase B) isoform phosphorylation and signaling downstream of mTOR (mammalian target of rapamycin) in denervated atrophic and hypertrophic mouse skeletal muscle. J. Mol. Signal. 2012, 7, 7. [Google Scholar] [CrossRef]
- Kline, W.O.; Panaro, F.J.; Yang, H.; Bodine, S.C. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J. Appl. Physiol. (1985) 2007, 102, 740–747. [Google Scholar] [CrossRef]
- Stern, J.H.; Kim, K.; Ramsey, J.J. The influence of shc proteins and aging on whole body energy expenditure and substrate utilization in mice. PLoS ONE 2012, 7, e48790. [Google Scholar] [CrossRef]
- Marmentini, C.; Soares, G.M.; Bronczek, G.A.; Piovan, S.; Mareze-Costa, C.; Carneiro, E.M.; Boschero, A.C.; Kurauti, M.A. Aging Reduces Insulin Clearance in Mice. Front. Endocrinol. 2021, 12, 679492. [Google Scholar] [CrossRef]
- Gamage, S.; Peddibhotla, S.; Reddy, P.H.; Dhurandhar, N.V.; Hegde, V. Improvement in Glycemic Control in Mice of Different Age Groups. Exp. Clin. Endocrinol. Diabetes 2021, 129, 519–527. [Google Scholar] [CrossRef]
- Reynolds, T.H.; Ives, S.J. Life without Proteinase Activated Receptor 2 (PAR2) Alters Body Composition and Glucose Tolerance in Mice. Nutrients 2022, 14, 4096. [Google Scholar] [CrossRef] [PubMed]
- Leiter, E.H.; Premdas, F.; Harrison, D.E.; Lipson, L.G. Aging and glucose homeostasis in C57BL/6J male mice. FASEB J. 1988, 2, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kerr, H.L.; Krumm, K.; Anderson, B.; Christiani, A.; Strait, L.; Li, T.; Irwin, B.; Jiang, S.; Rybachok, A.; Chen, A.; et al. Mouse sarcopenia model reveals sex- and age-specific differences in phenotypic and molecular characteristics. J. Clin. Investig. 2024, 134. [Google Scholar] [CrossRef]
- Reznick, R.M.; Zong, H.; Li, J.; Morino, K.; Moore, I.K.; Yu, H.J.; Liu, Z.; Dong, J.; Mustard, K.J.; Hawley, S.A.; et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007, 5, 151–156. [Google Scholar] [CrossRef]



| Young (n = 7) | Old (n = 10) | Very Old (n = 9) | ANOVA p Value | |
|---|---|---|---|---|
| Body Mass (g) | 30.5 ± 1.28 * | 47.8 ± 2.09 * | 40.0 ± 1.82 * | 0.0001 |
| Fat Mass (g) | 6.03 ± 1.16 | 23.8 ± 1.82 * | 11.0 ± 2.09 | 0.0001 |
| Lean mass (g) | 20.1 ± 0.27 | 19.0 ± 0.63 | 23.1 ± 0.55 * | 0.0001 |
| % Body Fat | 19.1 ± 2.90 | 49.2 ± 2.09 * | 26.3 ± 3.75 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, T.H., IV; Mills, N.; Hoyte, D.; Ehnstrom, K.; Arata, A. The Oldest of Old Male C57B/6J Mice Are Protected from Sarcopenic Obesity: The Possible Role of Skeletal Muscle Protein Kinase B Expression. Int. J. Mol. Sci. 2024, 25, 10278. https://doi.org/10.3390/ijms251910278
Reynolds TH IV, Mills N, Hoyte D, Ehnstrom K, Arata A. The Oldest of Old Male C57B/6J Mice Are Protected from Sarcopenic Obesity: The Possible Role of Skeletal Muscle Protein Kinase B Expression. International Journal of Molecular Sciences. 2024; 25(19):10278. https://doi.org/10.3390/ijms251910278
Chicago/Turabian StyleReynolds, Thomas H., IV, Noa Mills, Dakembay Hoyte, Katy Ehnstrom, and Alex Arata. 2024. "The Oldest of Old Male C57B/6J Mice Are Protected from Sarcopenic Obesity: The Possible Role of Skeletal Muscle Protein Kinase B Expression" International Journal of Molecular Sciences 25, no. 19: 10278. https://doi.org/10.3390/ijms251910278






