Exogenous Application of dsRNA—Inducing Silencing of the Fusarium oxysporum Tup1 Gene and Reducing Its Virulence
Abstract
1. Introduction
2. Results
2.1. Identification of Fusarium oxysporum Tup1 Gene
2.2. Synthesis of Tup1-dsRNA
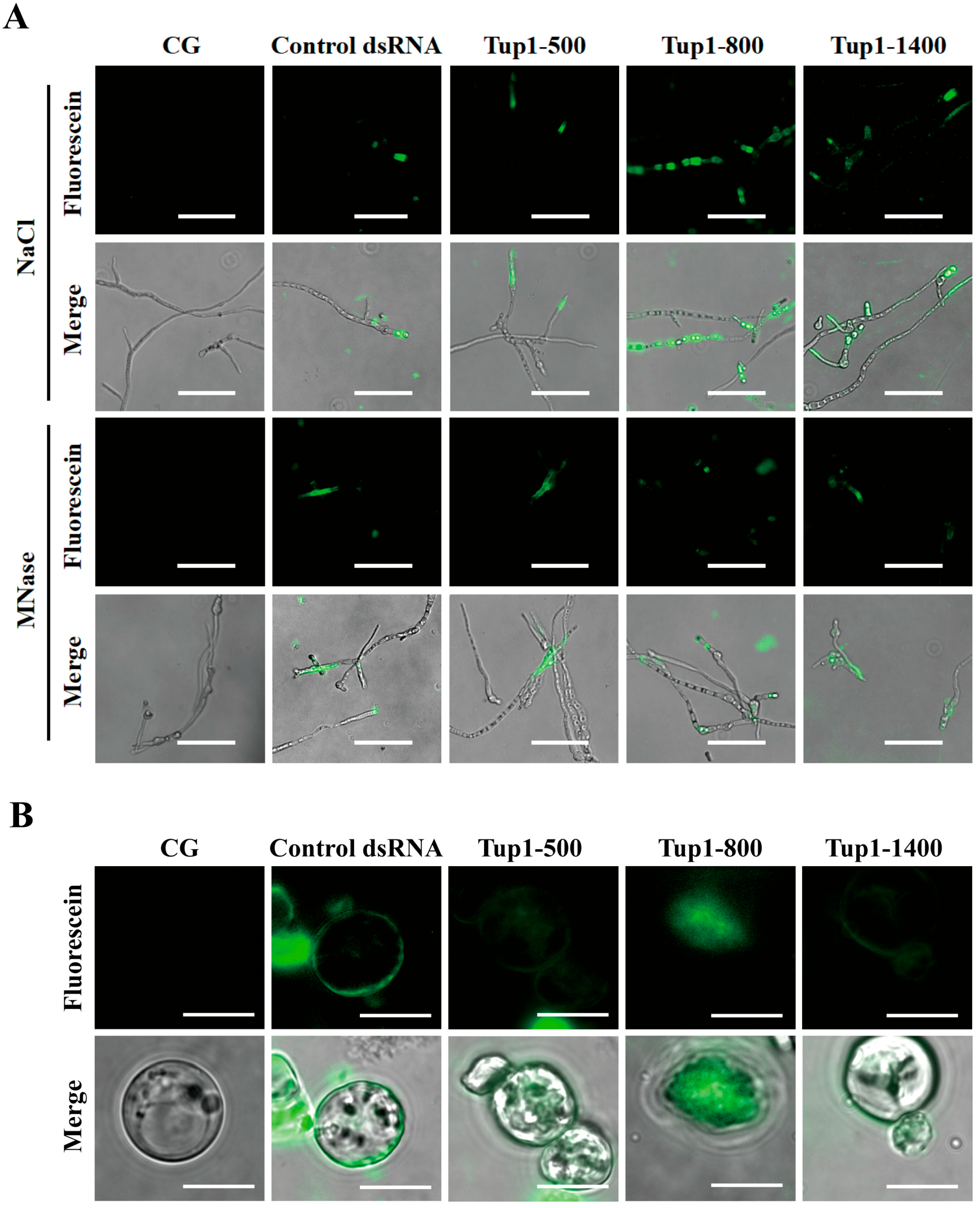
2.3. Tup1-dsRNA Was Effectively Taken Up and Absorbed by Fusarium oxysporum
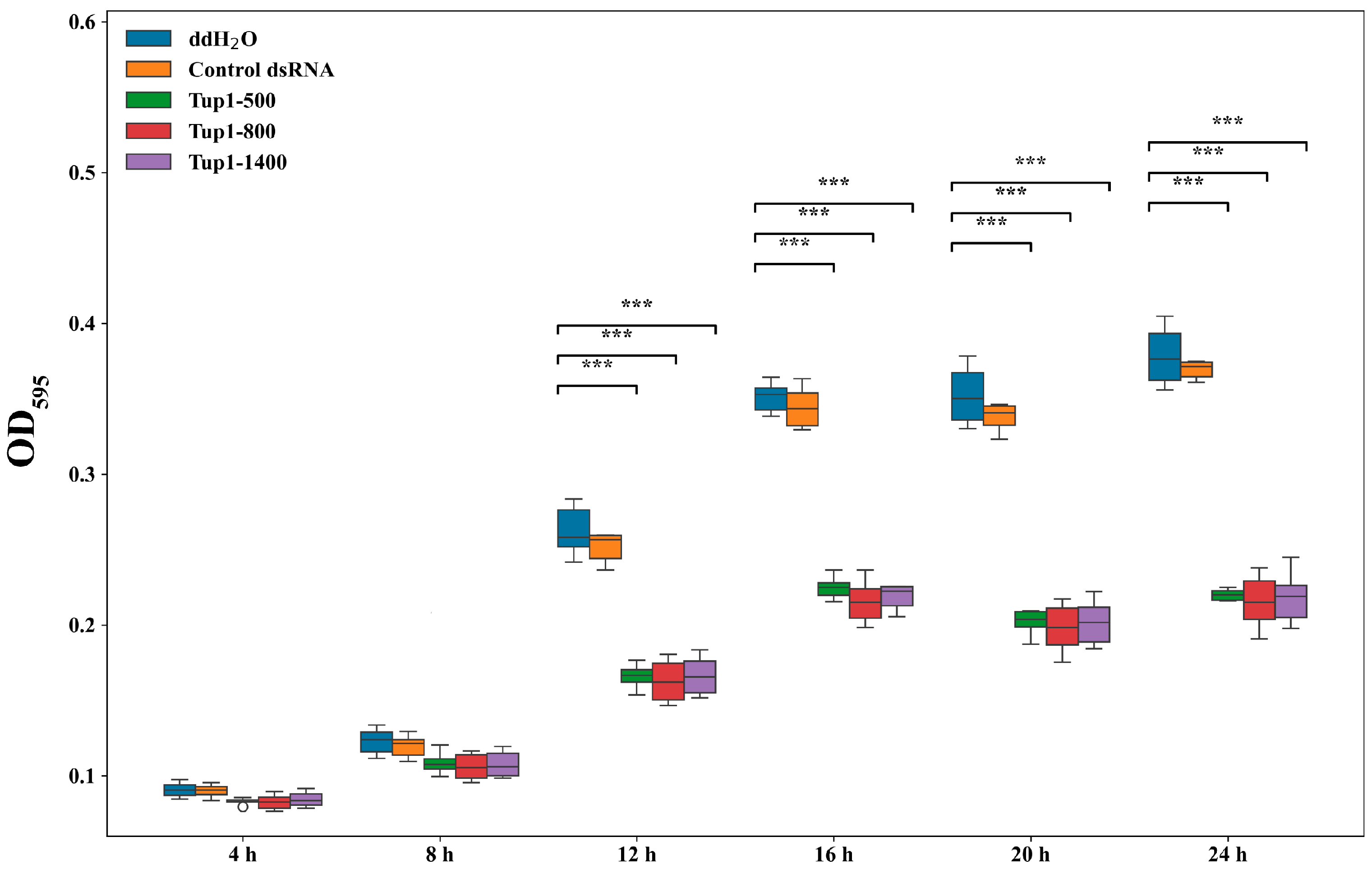
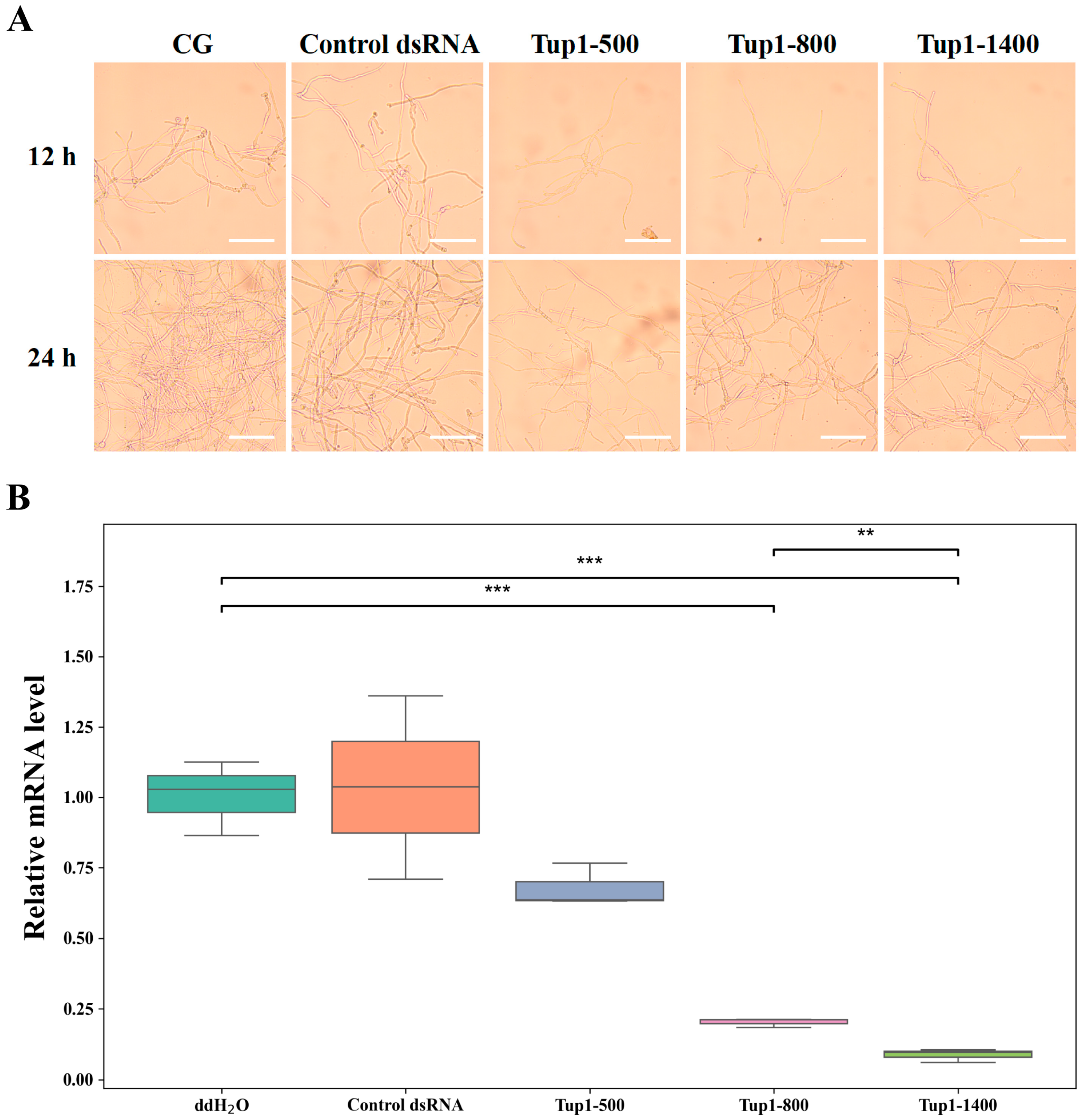
2.4. Effect of Exogenous Application of Tup1-dsRNA on the Growth and Germination of Fusarium oxysporum Spores and Fungal Transcript Analysis
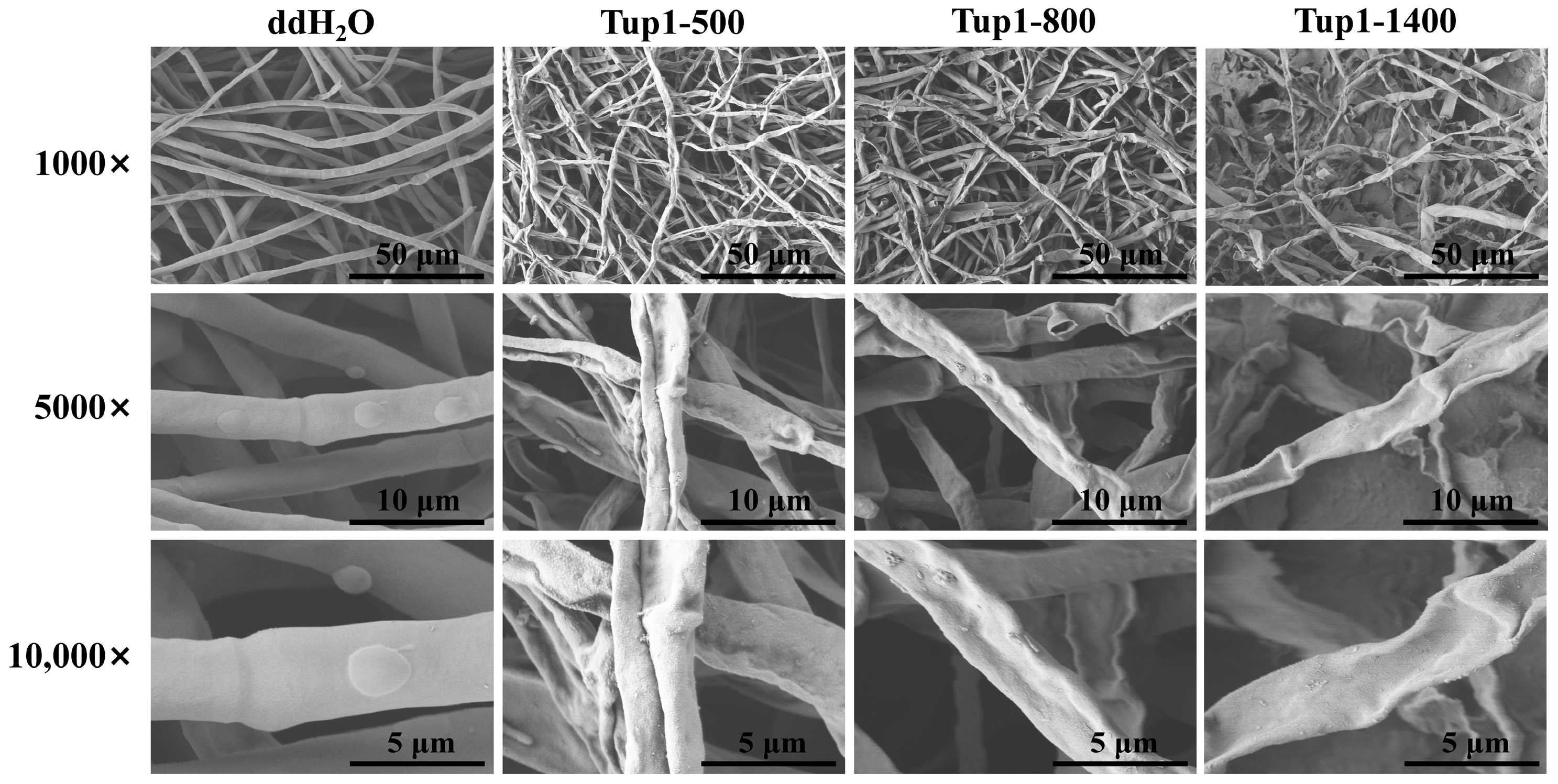
2.5. Effect of Exogenous Application of Tup1-dsRNA on the Growth and Morphology of Fusarium oxysporum Hyphae
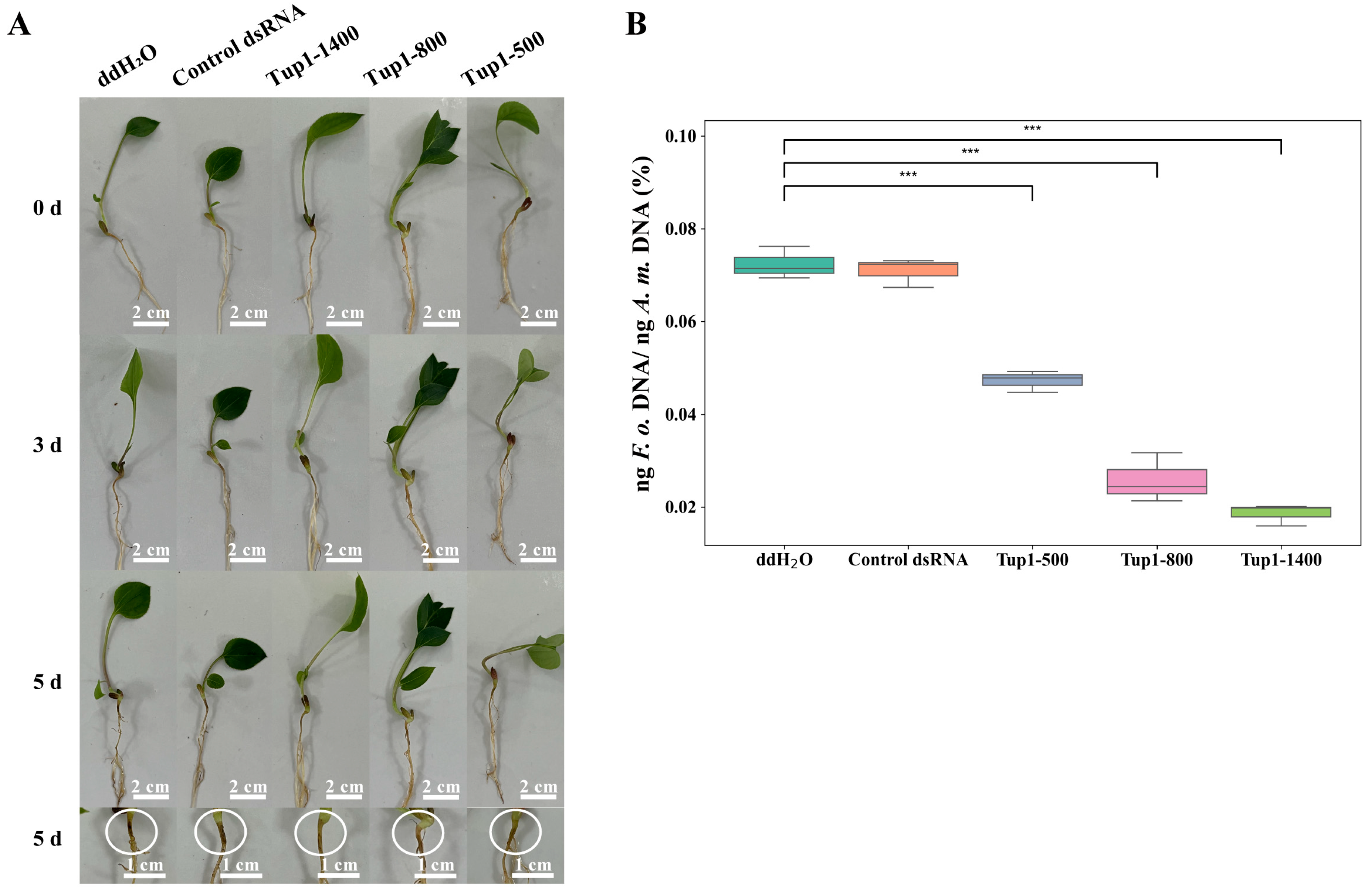
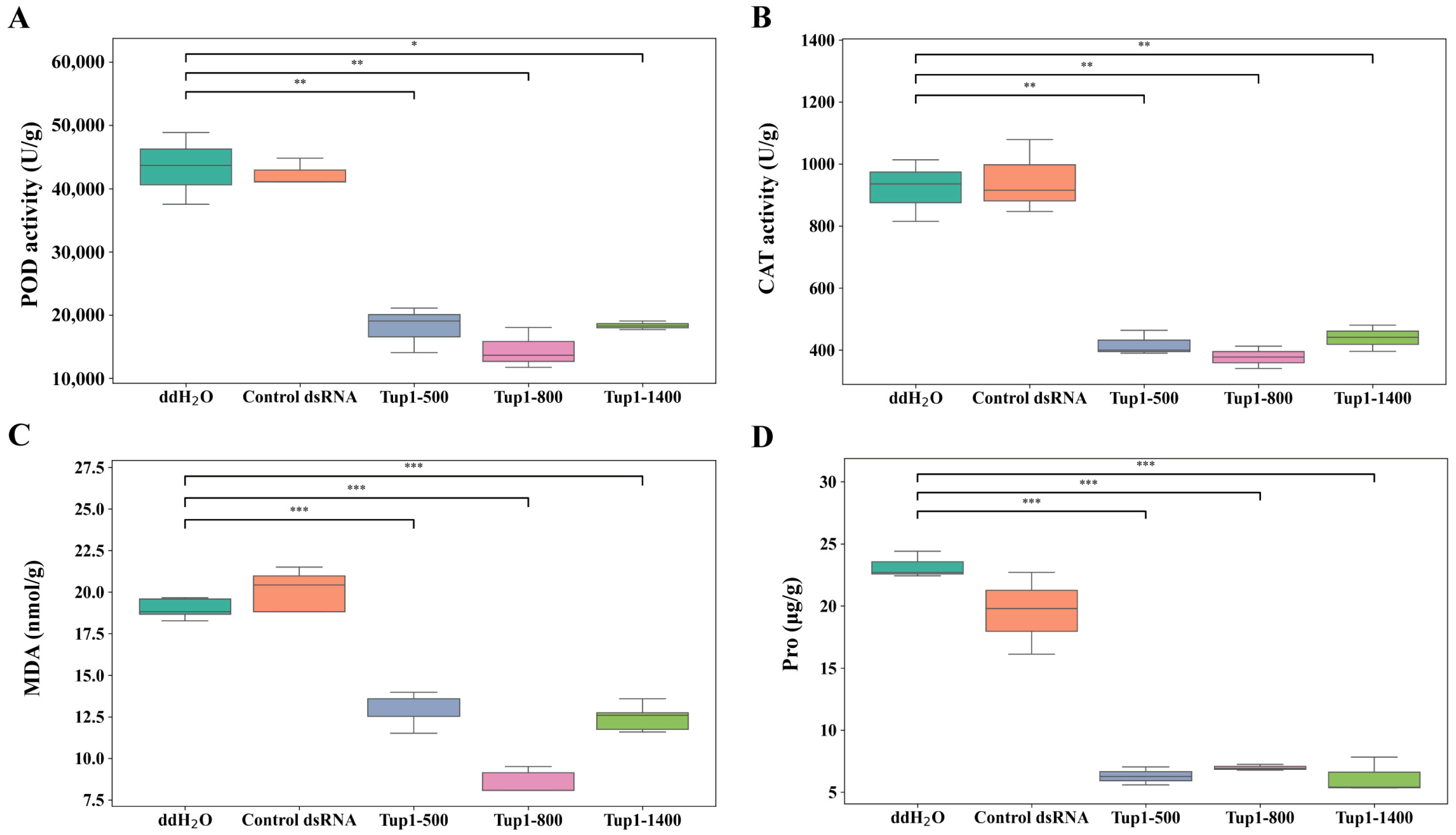
2.6. Exogenous Application of Tup1-dsRNA Alleviates the Occurrence of Atractylodes macrocephala Root Rot
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Plant Materials
4.3. Preparation of Fusarium oxysporum Protoplasm
4.4. DNA Extraction and Tup1 Gene Amplification
4.5. Total RNA Extraction and cDNA Synthesis
4.6. dsRNA Synthesis
4.7. Assessment of dsRNA Uptake by Fusarium oxysporum
4.8. Effects of Tup1-dsRNA on the Growth of Fusarium oxysporum Conidia In Vitro
4.9. Effects of Tup1-dsRNA on Fusarium oxysporum Mycelial Morphology and Growth In Vitro
4.10. Application of Tup1-dsRNA on the Roots of Atractylodes macrocephala
4.11. Biochemical Indicators and Enzyme Activity Assays
4.12. Gene Expression Analysis by Reverse Transcription Quantitative Polymerase Chain Reaction
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F. oxysporum | Fusarium oxysporum |
| A. macrocephala | Atractylodes macrocephala |
| dsRNA | Double-stranded RNA |
| siRNA | Small interfering RNA |
| OD | Optical density |
| TCA | Tricarboxylic acid (cycle) |
| PDB | Potato dextrose broth |
| PDA | Potato dextrose agar |
| YPD | Yeast peptone dextrose |
| SIGS | Spray-induced gene silencing |
| SEM | Scanning electron microscope |
| NTP | Nucleoside triphosphate |
| STC | Sucrose–tris–calcium buffer |
| POD | Peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| Pro | proline |
References
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, H.; Hou, A.; Man, W.; Wang, S.; Zhang, J.; Wang, X.; Zheng, S.; Jiang, H.; Kuang, H. A Review of the Ethnopharmacology, Phytochemistry, Pharmacology, Application, Quality Control, Processing, Toxicology, and Pharmacokinetics of the Dried Rhizome of Atractylodes macrocephala. Front. Pharmacol. 2021, 12, 727154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Atractylodes macrocephala Koidz. volatile oil relieves acute ulcerative colitis via regulating gut microbiota and gut microbiota metabolism. Front. Immunol. 2023, 14, 1127785. [Google Scholar] [CrossRef] [PubMed]
- Ruqiao, L.; Yueli, C.; Xuelan, Z.; Huifen, L.; Xin, Z.; Danjie, Z.; Le, S.; Yanxue, Z. Rhizoma Atractylodis macrocephalae: A review of photochemistry, pharmacokinetics and pharmacology. Pharmazie 2020, 75, 42–55. [Google Scholar] [CrossRef]
- Huang, X.G.; Li, M.Y.; Yan, X.N.; Yang, J.S.; Rao, M.C.; Yuan, X.F. The potential of Trichoderma brevicompactum for controlling root rot on Atractylodes macrocephala. Can. J. Plant Pathol. 2021, 43, 794–802. [Google Scholar] [CrossRef]
- Dzinyela, R.; Alhassan, A.R.; Kiani-Pouya, A.; Rasouli, F.; Yang, L.; Movahedi, A. Role of small RNAs in plant stress response and their potential to improve crops. Crop Pasture Sci. 2023, 74, 1116–1127. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Qasim, M.; Wang, L. Plant Responses to Pathogen Attack: Small RNAs in Focus. Int. J. Mol. Sci. 2018, 19, 515. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs—Big Players in Plant-Microbe Interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, Z.; Liu, Z.; Xia, R. Small RNAs, emerging regulators critical for the development of horticultural traits. Hortic. Res. 2018, 5, 63. [Google Scholar] [CrossRef]
- Niu, D.; Hamby, R.; Sanchez, J.N.; Cai, Q.; Yan, Q.; Jin, H. RNAs—A new frontier in crop protection. Curr. Opin. Biotech. 2021, 70, 204–212. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Weiberg, A.; Buck, A.H.; Jin, H. Small RNAs and extracellular vesicles: New mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog. 2020, 15, e1008090. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, C.; Leonetti, P.; Macovei, A. Plant miRNA Cross-Kingdom Transfer Targeting Parasitic and Mutualistic Organisms as a Tool to Advance Modern Agriculture. Front. Plant Sci. 2020, 11, 930. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; Dos, S.E.; Smagghe, G.; Zotti, M.J. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.T.; Gaffar, F.Y.; Schuemann, J.; Biedenkopf, D.; Koch, A.M. RNA-Spray-Mediated Silencing of Fusarium graminearum AGO and DCL Genes Improve Barley Disease Resistance. Front. Plant Sci. 2020, 11, 476. [Google Scholar] [CrossRef]
- Ouyang, S.Q.; Ji, H.M.; Feng, T.; Luo, S.J.; Cheng, L.; Wang, N. Artificial trans-kingdom RNAi of FolRDR1 is a potential strategy to control tomato wilt disease. PLoS Pathog. 2023, 19, e1011463. [Google Scholar] [CrossRef]
- Sarkar, A.; Roy-Barman, S. Spray-Induced Silencing of Pathogenicity Gene MoDES1 via Exogenous Double-Stranded RNA Can Confer Partial Resistance Against Fungal Blast in Rice. Front. Plant Sci. 2021, 12, 733129. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Silencing of the Slt2-Type MAP Kinase Bmp3 in Botrytis cinerea by Application of Exogenous dsRNA Affects Fungal Growth and Virulence on Lactuca sativa. Int. J. Mol. Sci. 2021, 22, 5362. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Palpacelli, D.; Pecchia, S. Knockdown of Bmp1 and Pls1 Virulence Genes by Exogenous Application of RNAi-Inducing dsRNA in Botrytis cinerea. Int. J. Mol. Sci. 2023, 24, 4869. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Das, P.R.; Sherif, S.M. Application of Exogenous dsRNAs-induced RNAi in Agriculture: Challenges and Triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Papadopoulou, K.K. Epigenetic Modifications: An Unexplored Facet of Exogenous RNA Application in Plants. Plants 2020, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Asada, R.; Umeda, M.; Adachi, A.; Senmatsu, S.; Abe, T.; Iwasaki, H.; Ohta, K.; Hoffman, C.S.; Hirota, K. Recruitment and delivery of the fission yeast Rst2 transcription factor via a local genome structure counteracts repression by Tup1-family corepressors. Nucleic Acids Res. 2017, 45, 9361–9371. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.B.; Whitty, P.A.; Selker, E.U.; McKnight, J.N.; McKnight, L.E. Tup1 is critical for transcriptional repression in Quiescence in S. cerevisiae. PLoS Genet. 2022, 18, e1010559. [Google Scholar] [CrossRef] [PubMed]
- Ruben, S.; Garbe, E.; Mogavero, S.; Albrecht-Eckardt, D.; Hellwig, D.; Häder, A.; Krüger, T.; Gerth, K.; Jacobsen, I.D.; Elshafee, O.; et al. Ahr1 and Tup1 Contribute to the Transcriptional Control of Virulence-Associated Genes in Candida albicans. mBio 2020, 11. [Google Scholar] [CrossRef]
- Huang, Z.; Lou, J.; Gao, Y.; Noman, M.; Li, D.; Song, F. FonTup1 functions in growth, conidiogenesis and pathogenicity of Fusarium oxysporum f. sp. niveum through modulating the expression of the tricarboxylic acid cycle genes. Microbiol. Res. 2023, 272, 127389. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Cao, P.; Yang, J.; Xia, L.; Zhang, Z.; Wu, Z.; Hao, Y.; Liu, P.; Wang, C.; Li, C.; Yang, J.; et al. Two gene clusters and their positive regulator SlMYB13 that have undergone domestication-associated negative selection control phenolamide accumulation and drought tolerance in tomato. Mol. Plant 2024, 17, 579–597. [Google Scholar] [CrossRef]
- Ingrisano, R.; Tosato, E.; Trost, P.; Gurrieri, L.; Sparla, F. Proline, Cysteine and Branched-Chain Amino Acids in Abiotic Stress Response of Land Plants and Microalgae. Plants 2023, 12, 3410. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-Based Control of Fusarium graminearum Infections through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-Stranded RNAs (dsRNAs) as a Sustainable Tool against Gray Mold (Botrytis cinerea) in Grapevine: Effectiveness of Different Application Methods in an Open-Air Environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, R.; Anastasaki, A.; Truong, N.P.; Cook, A.B.; Omedes-Pujol, M.; Rose, V.L.; Nguyen, T.A.H.; Burns, J.A.; Perrier, S.; Davis, T.P.; et al. Efficient Binding, Protection, and Self-Release of dsRNA in Soil by Linear and Star Cationic Polymers. ACS Macro Lett. 2018, 7, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, S.; Sun, M.; Yu, Q.; Li, C.; Graham, R.I.; Wang, X.; Wang, X.; Xu, P.; Ren, G. Delivery of Methoprene-Tolerant dsRNA to Improve RNAi Efficiency by Modified Liposomes for Pest Control. ACS Appl. Mater. Interfaces 2023, 15, 13576–13588. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant Roots Release Small Extracellular Vesicles with Antifungal Activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J.; Cook, D.E. Targeting microbial pathogens. Science 2018, 360, 1070–1071. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; Depamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef]
- Dunker, F.; Trutzenberg, A.; Rothenpieler, J.S.; Kuhn, S.; Pröls, R.; Schreiber, T.; Tissier, A.; Kemen, A.; Kemen, E.; Hückelhoven, R.; et al. Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. eLife 2020, 9, e56096. [Google Scholar] [CrossRef]
- Ji, H.; Mao, H.; Li, S.; Feng, T.; Zhang, Z.; Cheng, L.; Luo, S.; Borkovich, K.A.; Ouyang, S. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol. 2021, 232, 705–718. [Google Scholar] [CrossRef]
- Caligiore-Gei, P.F.; Valdez, J.G. Adjustment of a rapid method for quantification of Fusarium spp. spore suspensions in plant pathology. Rev. Argent. Microbiol. 2015, 47, 152–154. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Govindaraj, L.; Dhanasekaran, M.; Vetrivel, S.; Kumar, K.K.; Ebenezar, E. Combination of driselase and lysing enzyme in one molar potassium chloride is effective for the production of protoplasts from germinated conidia of Fusarium verticillioides. J. Microbiol. Methods 2015, 111, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Weng, Z. Sequence Alignment and Homology Search with BLAST and ClustalW. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot093088. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zamore, P.D. Preparation of dsRNAs for RNAi by In Vitro Transcription. Cold Spring Harb. Protoc. 2019, 2019, pdb.prot097469. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, A.; Doody, O. The one-way ANOVA test explained. Nurse Res. 2023, 31, 8–14. [Google Scholar] [CrossRef]



| Gene | 5′-3′ Sequence | Annealing Temperature |
|---|---|---|
| Tup1 | ATGTCGATGTATCCGCATCGC | 63.1 °C |
| TGACTTCGACAGAACCGGAC | 58.0 °C |
| dsRNA | 5′-3′ Sequence | Annealing Temperature |
|---|---|---|
| Tup1-500 | TAATACGACTCACTATAGGGCTCAAGTCAGCCATCCGACTCC | 80.8 °C |
| TAATACGACTCACTATAGGGGCGAGTAAATATCTTGCTCGTGACC | 79.6 °C | |
| Tup1-800 | TAATACGACTCACTATAGGGCCTTACTCTCAGAACTATCCTCCGG | 78.8 °C |
| TAATACGACTCACTATAGGGGACGCCATCCTCTATTGTCAGAG | 79.0 °C | |
| Tup1-1400 | TAATACGACTCACTATAGGGTCTCGTTCCTCCTCAGCCAGG | 79.9 °C |
| TAATACGACTCACTATAGGGGAGCCAGTGGCAAGTATCTTCC | 79.0 °C |
| Primer | 5′-3′ Sequence | Annealing Temperature |
|---|---|---|
| Q-Matk (A. macrocephala) | TGCCCCAATGCGTTACAAAATTTCG | 70.7 °C |
| AAAGCCTTCAATGGTCCGCAGTC | 66.6 °C | |
| Q-Prot (F. oxysporum) | CGAGGTGTGGATTTAGGAGATG | 58.9 °C |
| GTGCTGATTCGTTGTACGTTATTC | 58.8 °C |
| Primer | 5′-3′ Sequence | Annealing Temperature |
|---|---|---|
| Q-FoTup1 | GCCTCAAGCAGACGTATGAAGATG | 63.1 °C |
| TTGCCAGGAGCGATAGAAGGAG | 63.5 °C | |
| Q-Actin | GAGGGACCGCTCTCGTCGT | 62.1 °C |
| GGAGATCCAGACTGCCGCTGAG | 65.8 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Zhou, Y.; Zhu, N.; Meng, Q.; Zhao, Y.; Xu, J.; Tang, Y.; Dai, S.; Yuan, X. Exogenous Application of dsRNA—Inducing Silencing of the Fusarium oxysporum Tup1 Gene and Reducing Its Virulence. Int. J. Mol. Sci. 2024, 25, 10286. https://doi.org/10.3390/ijms251910286
Fan S, Zhou Y, Zhu N, Meng Q, Zhao Y, Xu J, Tang Y, Dai S, Yuan X. Exogenous Application of dsRNA—Inducing Silencing of the Fusarium oxysporum Tup1 Gene and Reducing Its Virulence. International Journal of Molecular Sciences. 2024; 25(19):10286. https://doi.org/10.3390/ijms251910286
Chicago/Turabian StyleFan, Sen, Yanguang Zhou, Na Zhu, Qingling Meng, Yujin Zhao, Jingyan Xu, Yunjia Tang, Shijie Dai, and Xiaofeng Yuan. 2024. "Exogenous Application of dsRNA—Inducing Silencing of the Fusarium oxysporum Tup1 Gene and Reducing Its Virulence" International Journal of Molecular Sciences 25, no. 19: 10286. https://doi.org/10.3390/ijms251910286
APA StyleFan, S., Zhou, Y., Zhu, N., Meng, Q., Zhao, Y., Xu, J., Tang, Y., Dai, S., & Yuan, X. (2024). Exogenous Application of dsRNA—Inducing Silencing of the Fusarium oxysporum Tup1 Gene and Reducing Its Virulence. International Journal of Molecular Sciences, 25(19), 10286. https://doi.org/10.3390/ijms251910286





