Abstract
Microorganisms colonize all barrier tissues and are present on the skin and all mucous membranes from birth. Bacteria have many ways of influencing the host organism, including activation of innate immunity receptors by pathogen-associated molecular patterns and synthesis of various chemical compounds, such as vitamins, short-chain fatty acids, bacteriocins, toxins. Bacteria, using extracellular vesicles, can also introduce high-molecular compounds, such as proteins and nucleic acids, into the cell, regulating the metabolic pathways of the host cells. Epithelial cells and immune cells recognize bacterial bioregulators and, depending on the microenvironment and context, determine the direction and intensity of the immune response. A large number of factors influence the maintenance of symbiotic microflora, the diversity of which protects hosts against pathogen colonization. Reduced bacterial diversity is associated with pathogen dominance and allergic diseases of the skin, gastrointestinal tract, and upper and lower respiratory tract, as seen in atopic dermatitis, allergic rhinitis, chronic rhinosinusitis, food allergies, and asthma. Understanding the multifactorial influence of microflora on maintaining health and disease determines the effectiveness of therapy and disease prevention and changes our food preferences and lifestyle to maintain health and active longevity.
1. Introduction
Microorganisms colonize skin and mucous membranes from birth and promote the formation of an adequate immune response to pathogenic microorganisms and harmless antigens [1]. Disruption of symbiotic and mutualistic relationships between the microflora and the host organism can lead to various pathological conditions, including allergic diseases [2,3]. It was found that the use of antibiotics during the first 2 years of life was a risk factor for the development of asthma, atopic dermatitis, and allergic rhinitis at the age of five [4,5,6]. This is how the hygiene hypothesis arose, referring to the T-helper type 1/2 (Th1/Th2) model [7,8]. According to the hygiene hypothesis, allergic diseases are associated with insufficient activation of innate immune receptors in early childhood during the development of the immune system. This hypothesis was also confirmed by the discovery that children from urban areas are predominantly affected by allergic diseases compared to children from rural areas [9]. It should be noted that along with the “hygiene hypothesis”, a “counter-regulatory” hypothesis emerged, according to which the epidemic of allergic diseases observed in recent years is a small price to pay for a significant reduction in child mortality, achieved through measures such as improved sanitation, access to drinking water, and vaccination [10]. Microbial colonization of mucosal tissues during infancy may have long-term consequences, such as promoting tolerance to environmental insults or contributing to the development of diseases in later life, including inflammatory bowel disease, allergy, and asthma [11,12]. With increasing understanding of the complexity of the regulation of allergic reactions, other theories have emerged, such as the “biodiversity” hypothesis, which shows a correlation between reduced biodiversity of the skin and mucosal microbiota and the incidence of allergic diseases [13]. In addition, among siblings, younger siblings were found to be less likely to develop allergic diseases [14,15]. With the discovery of bacteria in the meconium of newborns, it became obvious that acquaintance with microorganisms occurs in the womb and the problem of the impact of microflora should be analyzed from periods earlier than birth [16,17,18,19,20].
Modern technologies have revealed associations of genetic polymorphisms in numerous genes involved in the implementation of innate immunity and the formation of tight junctions between epithelial cells with allergic and inflammatory diseases [21,22,23]. However, the discovered polymorphisms could not fully explain the “non-heritability of asthma”, which necessitated determining the influence of environmental factors on epigenetic changes leading to allergic diseases [24]. Among these factors are air pollutants, allergens, and infectious agents in the prenatal and postnatal periods. A modified strategy that includes gene polymorphisms in combination with environmental factors has shown a correlation with allergic diseases [24]. The recently introduced “Epithelial Barrier Theory” includes even more parameters to explain the occurrence of allergic inflammation [25,26]. The Epithelial Barrier Theory combines all previous hypotheses and suggests that toxic substances in hygiene products, as well as microplastics and air pollution, damage the epithelium of our skin, lungs, and gastrointestinal tract [27,28,29,30,31]. In particular, serine proteases and metalloproteinases contained in pollen disrupt the integrity of the lung epithelial barrier by degrading the transmembrane adhesion proteins E-cadherin, claudin-1, and occludin, as well as the cytosolic complex zonula occludens-1 (ZO-1), which leads to an increase in transepithelial permeability [32]. Disruption of tight junctions and an increase in transepithelial permeability facilitate the action of allergens on the epithelial sublayers, promoting sensitization to a wide range of allergens [33].
The increase in allergic diseases since 1960 is also associated with the emergence of 350,000 new toxic compounds that, against the background of genetic susceptibility, contribute to disruption of the epithelial barrier and cause local and systemic inflammatory diseases [31]. Evolutionarily formed relationships between commensal microorganisms and their hosts are under pressure from new, previously unknown chemicals introduced to the immune system, and can significantly modulate immune reactivity along with resident microorganisms. Thus, there is a need to summarize the possible ways in which microorganisms influence the immune system to determine their contribution to the prevention or aggravation of allergic inflammation. The aim of this review was to analyze the influence of bacteria inhabiting the gastrointestinal tract, skin, upper and lower respiratory tract, as well as bioregulators of bacterial origin on allergic diseases.
2. Bacteria Modes of Action on Host Cells
Human microflora includes bacteria, archaea, fungi, protozoa, and viruses that inhabit the skin and all mucous membranes [34,35]. Bacterial communities are the most studied and most numerous in the gastrointestinal tract, where their number can reach 1013–1014 [36]. At the same time, it was found that resident microflora not only help to ferment food [37,38], supply various nutrients and vitamins [17,39,40,41,42], and prevent the colonization of pathogenic microflora [43,44], but also to strengthen the epithelial barrier [45,46]. Such a diverse effect of commensal bacteria on the human body is achieved due to the existence of many ways of activating host cells (Figure 1). First of all, bacteria affect specific receptors of innate immunity located on the surface and in the cytosol of host cells through pathogen-associated molecular patterns (PAMPs). These include peptidoglycans, lipopolysaccharides, flagellin, CpG nucleotides, muramyl peptides, and lectins that activate TLR2, 4, 5, 9, NLR and CLR, respectively [47,48,49,50,51,52]. In this case, lipopolysaccharides and muramyl peptides, which are agonists of TLR and NLR, respectively, may have structural differences characteristic of different strains of microorganisms [53,54,55,56,57]. Activation of PAMP receptors of innate immunity TLR and NLR triggers a cascade of reactions with activation of the transcription factor NFkB, synthesis of proinflammatory cytokines and chemokines, expression of cellular receptors, markers of differentiation, and maturation of immunocompetent cells, which normally leads to elimination of the pathogen [58,59,60]. In this case, cross-interaction occurs between different classes of innate immunity receptors [61,62,63]. In addition, innate immunity receptors cross-interact with other receptors on the cell surface. The microbial metabolite indole-3-propionic acid (IPA) has been shown to suppress enterocyte TNF-α via the pregnane X receptor (PXR). PXR-deficient mice (Nr1i2(-/-)) exhibit a distinctly leaky gut physiology coupled with upregulation of the Toll-like receptor (TLR) signaling pathway. These epithelial barrier defects were corrected in Nr1i2(-/-)Tlr4(-/-) mice [64]. Thus, the bacterial metabolite indole-3-propionic acid, via PXR, normally regulates TLR4 activity. Subsequently, PXR was shown to regulate the intestinal epithelial barrier during inflammation by reducing cytokine-induced myosin light chain kinase expression and c-Jun N-terminal kinase 1/2 activation [65]. The resulting cross-talk and positive and negative feedback loops, depending on the intensity of the stimuli that cause them, ultimately determine the nature of the immune response [52,66].
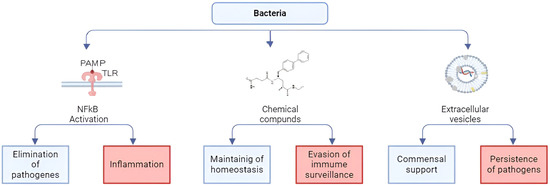
Figure 1.
Bacteria modes of action on host cells. Bacteria activate host cells via different ways. First of all, bacteria affect specific receptors of the innate immunity located on the surface and in the cytosol of host cells through pathogen-associated molecular patterns (PAMPs). Activation of PAMPs receptors of innate immunity TLR and NLR triggers a cascade of reactions with activation of the transcription factor NFkB, which normally leads to pathogen elimination. When the NFkB pathway is aberrantly active, inflammation occurs. The second way of action is a non-specific penetration through the host cells of amino acids, vitamins, hormones, short-chain fatty acids, bile acid intermediates, bacteriocins, and other chemical compounds produced by bacteria. Bacterial secretion systems can deliver proteins and nucleic acids directly into the host cell cytosol, directly influencing cellular metabolic processes including facilitating evasion of immune surveillance. The third way bacteria influence host cells is through extracellular vesicles. Extracellular vesicles from commensal bacteria help maintain trained immunity, whereas extracellular vesicles from pathogenic bacteria can alter intracellular pathways and colonize eukaryotic cells.
In addition to specific activity, bacteria have a non-specific effect on host cells by producing amino acids, vitamins, hormones, short-chain fatty acids, bile acid intermediates, bacteriocins, and other chemical compounds [17,39,40,41,42,67,68]. Gut bacterial metabolites exert their biological activity through specific recognition by G protein-coupled receptors (GPRs) [69,70]. GPRs are differentially expressed in different cell types, and the response to the same metabolite can vary depending on the specific roles of the cells [71]. Therefore, the response to gut microbiota-derived metabolites exhibits a huge combinatorial diversity, which poses significant challenges in understanding their effects [72]. Bacteria do not only secrete small molecular compounds; bacterial secretion systems, in particular type III and IV, can deliver proteins and nucleic acids directly into the host cell cytosol, directly influencing cellular metabolic processes including facilitating evasion of immune surveillance [73,74,75].
Another way bacteria influence host cells is through extracellular vesicles, when bacteria can introduce enzymes, RNA, toxins, components of the bacterial membrane, including LPS, into the host cell [76,77,78,79]. In this case, pathogenic bacteria can change intracellular pathways, which allow bacteria to evade the immune response and colonize eukaryotic cells [80,81,82]. Extracellular vesicles of pathogenic bacteria have been studied in more detail than those of commensal bacteria; however, the formation of extracellular vesicles in commensals has been established, with the ability to modulate interaction with the host and immune training [56,82,83,84]. Moreover, it has been established that extracellular vesicles of Lactobacillus plantarum Q7 have an anti-inflammatory effect [85], and bacterial tryptophan catabolites enclosed in extracellular vesicles can enhance the barrier functions of the intestinal epithelium and regulatory immune responses [86,87].
Interestingly, some biological processes used by cells to combat microorganisms, such as neutrophil extracellular traps (NETs) formation, can be induced by a variety of stimuli, including PAMP activation, as well as independently of PAMPs [88]. NETs formation is a key mechanism of microbe–host cells conversation and is involved in pathogenesis of allergic diseases, especially in asthma exacerbation [89].
Thus, bacteria inhabiting skin and mucous membranes affect all systems and organs in numerous ways, normally contribute to the maintenance of immune homeostasis, and can affect diseases [90,91,92,93].
3. Modulation of Allergic Reactions by Bacterial Regulators
Allergic reactions occur in tissues that are adjacent to the external environment and populated by various microorganisms, which, along with heredity and environmental factors, contribute to inflammatory reactions. There are many phenotypes and endotypes of allergic diseases, differing in the causes of occurrence, clinical signs, and immune cells involved in pathological processes [94]. Despite the differences in the manifestation of allergic reactions on the skin, mucous membranes of the gastrointestinal tract, upper and lower respiratory tract, genitals, etc., there are common signs of ensuring the homeostatic state of barrier tissues [95,96,97]. Commensal bacteria play a significant role in maintaining the homeostatic state of barrier tissues, which normally keep innate immune cells active to quickly repel pathogen attacks and ensure tolerance to resident bacteria [1,98,99]. The balance between excessive immune reactivity to harmless antigens and insufficient immune response to dangerous microorganisms can be disrupted by the negative impact of external factors. As a result, the integrity of epithelial barriers can be disrupted, the composition of microflora can be changed and, as a consequence, inflammatory, allergic, and autoimmune reactions can occur [100,101,102].
Depending on the localization, the epithelium of barrier tissues uses different methods of protection from external influences: antimicrobial peptides, mucins, IgA, or various enzymes. However, when recognizing allergens and bacterial metabolites, common response mechanisms can be traced (Figure 2). In the case of recognizing allergens by dendritic antigen-presenting cells (APCs), they have been processed to form complexes of class II major histocompatibility complex (MHC II) molecules and the antigen, migrate to the lymph nodes, and promote the differentiation of naive T cells into Th2 cells [103,104]. At the same time, bacterial bioregulators interacting with APCs through TLRs and NLRs trigger the activation of Th1 and Th17 types by producing cytokines TNF, IL-1β, IL-6, IL-12, IL-23, and IFN γ [105,106,107,108]. When stimulated, naïve T cells secrete IL-2, interferon (IFN)-γ, lymphotoxin, and tumor necrosis factor (TNF)-α, as well as low levels of IL-4, IL-13, and IL-10 [109]. The preferential activation of Th1, Th2, Th17, or Treg depends on the cytokine milieu of the microenvironment [110]. The balance between these endogenously produced cytokines has been determined by the phenotype of the lymphokine-producing primed cells. This balance depends on the genetic background, the nature and strength of the signal, and the activation state of DCs h2 which migrate to the site of inflammation and produce proinflammatory cytokines (IL-4, IL-5, IL-13) to activate eosinophils and stimulate IgE synthesis by B cells and subsequent degranulation of mast cells [111]. Allergen-stimulated epithelial cells secrete IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) to activate innate lymphoid cells type 2 (ILC2), which secrete IL-5, act on eosinophils, and stimulate inflammatory responses [112,113,114]. ILC2 are critical drivers of type 2 (T2) inflammatory responses associated with allergic inflammatory conditions and can secrete IL-4, IL-5, IL-9, and IL-13 [115]. Interestingly, depending on the microenvironment, namely, under the influence of retinoic acid, IL-2, IL-4, IL-10, and IL-33, a subpopulation of KLRG1 + ILC2 may appear which has the ability to produce the anti-inflammatory cytokine IL-10 and exert an anti-inflammatory effect [115]. Cross-interaction of immunocompetent cells, their mutual influence, and sensitivity to the microenvironment normally ensures homeostasis and maintains the integrity of the epithelium [116].
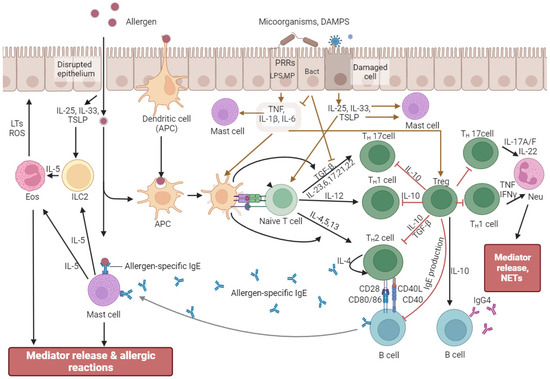
Figure 2.
Mechanism of formation and attenuation of allergic reactions. Allergens are recognized and processed by dendritic antigen-presenting cells (APCs) to form complexes of major histocompatibility complex class II (MHC II) molecules and antigens, which promote differentiation of naive T cells into Th2 cells. Th2 cells produce proinflammatory cytokines (IL-4, IL-5, IL-13) to activate eosinophils and stimulate IgE synthesis by B cells and subsequent degranulation of mast cells. When microorganisms and/or their metabolic products (brown arrows) affect epithelial cells, the latter produce cytokines (in particular, IL-1, Il-6, TNF), which activate APCs and T regulatory cells (Treg). Tregs can modulate the responses of other T cell populations, changing the direction of the immune response. Allergen-stimulated epithelial cells secrete IL-25, IL-33, and thymic stromal lymphopoietin to activate ILC2, which secretes IL-5, which affects eosinophils, stimulating inflammatory responses. When the epithelial barrier is compromised, allergens directly activate APCs and mast cells, enhancing inflammatory responses. Abbreviations: APCs—antigen-presenting cells; DAMPs—danger-associated molecular patterns; Neu—neutrophils; Eos—eosinophils; ILC2—group 2 innate lymphoid cells; NETs—neutrophil extracellular traps; LTs—leukotrienes; ROS—reactive oxygen species; TNF—tumor necrosis factor; TSLP—thymic stromal lymphopoietin.
In the case of a compromised epithelial barrier, allergens directly activate APCs and mast cells, enhancing inflammatory responses [117]. In addition, damaged epithelium increases immune cell infiltration, further enhancing dysfunction [117]. When microorganisms and/or their metabolic products affect epithelial cells, the latter produce cytokines (in particular, IL-1, Il-6, and TNF), which activate APCs and T-regulatory cells [118,119,120,121]. Tregs increase immune tolerance during inflammation, highlighting the role of the microbiota in maintaining homeostasis [98]. In addition, Tregs can modulate the responses of other T-cell populations, changing the direction of the immune response [122,123,124,125]. Commensal bacteria are known to induce the production of type I IFN (IFNα and IFNβ) and IL-27 by dendritic cells to influence Tregs [98]. Moreover, IL-27 plays a crucial role in the expression of IL-10 by T-regulatory cells [126,127,128,129]. The importance of IL10 is due to its ability to exert an anti-inflammatory effect, controlling the intensity of immune responses, and maintaining immune homeostasis [115,130,131]. Interleukin 10 is produced by almost all populations of immunocompetent cells—T-helpers, cytotoxic and regulatory T-cells, B-lymphocytes, macrophages, NK-cells, monocytes, dendritic cells, neutrophils, eosinophils and mast cells [132,133,134,135]. At the same time, B-cells secreting IL-10 were isolated into a separate subpopulation, namely regulatory B-cells (Breg), which have anti-inflammatory functions in allergic diseases [136]. The key role of IL-10 in ensuring tolerance is its effect on dendritic cells, which, after interaction with Tregs, promotes allergen-specific and cross-reactive tolerance [137,138]. IL10 is also produced by keratinocytes, intestinal mucosal epithelial cells, and tumor cells [139,140]. The ability of IL10 to suppress the immune response is used by some pathogens to evade immune surveillance [139,140]. In particular, Helicobacter pylori stimulate IL10 to survive in the stomach, Mycobacterium tuberculosis induces B cells, and Streptococcus pneumoniae induces neutrophils to produce IL10, which allows the bacteria to colonize their niches [139,141,142,143]. Some viruses, such as HIV, hepatitis C, and hepatitis B, also stimulate IL10 production to suppress immune response and promote viral persistence [140,144]. Importantly, bacteria and their cell wall fragments stimulate the production of both proinflammatory and anti-inflammatory cytokines [11,12,145]. The ability of bacteria to activate anti-inflammatory responses, including IL10 production, limits tissue damage and is important for mediating an effective immune response necessary for host survival [143,146,147].
It should be noted that activation of innate immune receptors in experimental models protects animals from lethal infection with a wide range of pathogens and has therapeutic activity in allergic and oncological diseases [49,148,149]. At the same time, stimulation with commensal bacteria through the induction of type 1 interferons triggers not only antibacterial and antiviral activity, but also the activation of dendritic cells and Treg cells, providing tolerance to commensals [98].
Various combinations of PAMPs can enhance or inhibit the synthesis of cytokines induced by the activation of innate immune receptors, as well as cause activation or suppression of various populations of immunocompetent cells [63]. In particular, PBMCs, monocytes, and dendritic cells secreted high levels of IL-10 when TLR5 was stimulated with flagellin, whereas TLR9 stimulation with CpG oligodeoxynucleotides did not induce IL-10 secretion in any of the three cell types but synergized with flagellin in this induction [63]. Moreover, TLR5 stimulation completely abolished the NK cell cytotoxicity induced by TLR9 stimulation [63].
Hundreds of genes are involved in the regulation of inflammatory processes, including ubiquitinases and deubiquitinases, the activity of which can be modulated by bacterial effectors [76,150]. Bacterial ligases, such as the E3 SopA, NleL, and IpaH families, can act on the principle of molecular mimicry and modulate ubiquitination processes in eukaryotic cells in the direction necessary for bacteria to manipulate the host signaling to facilitate infection [151,152,153,154,155].
4. The Impact of Bacteria on Allergic Diseases
Allergic diseases are a serious problem in both developed and developing countries. According to the World Health Organization, 30–40% of the population has one or more allergic diseases [156]. Worldwide, 400 million people suffer from allergic rhinitis, 300 million from asthma, and 250 million people suffer from food allergies [156,157]. The incidence of asthma has increased significantly in recent decades in both developed and developing countries, with more than 40 million new cases diagnosed each year [158]. By 2050, it is predicted that up to 4 billion people worldwide will suffer from asthma, allergic rhinitis, or atopic dermatitis [159]. Allergic diseases impair the quality of life of patients and are a significant burden on healthcare systems. In Europe alone, more than EUR 150 billion are spent annually on combating allergic diseases [160]. With such an increase in allergopathologies, the problem of their treatment and prevention is a pressing issue and requires comprehensive study. The study of bacterial communities in various diseases complements the understanding of the mechanisms of pathology development and is one of the ways to influence the course of the disease.
4.1. Gastrointestinal Tract
First contacts of bacteria with the microbiota occur during fetal development in the womb. Live bacteria have been found in amniotic fluid, fetal gut, skin, placenta, and fetal lungs during healthy pregnancy [161,162,163]. Staphylococcus and Lactobacillus present in fetal tissues induced the activation of memory T cells in fetal mesenteric lymph nodes [161]. Eighteen taxa were detected in fetal meconium, with Micrococcaceae and Lactobacillus being the most abundant [163]. Newborns with an asthmatic parent were found to have significantly different meconium bacteria compared to those without an asthmatic parent [164]. The first postnatal stool of neonates with an asthmatic parent was enriched in Enterobacteriaceae and Bacteroidaceae, depleted in several genera including Akkermansia, Faecalibacterium, and Rothia, was less diverse, and had a significant delay in bacterial richness gain [164]. However, oral administration of Lactobacillus rhamnosus GG reversed the deficit in anti-inflammatory lipids, but did not alleviate the deficit in bacterial species diversity [164]. Deficiencies in the bacterial genera Lachnospira, Veillonella, Faecalibacterium, and Rothia have been associated with asthma in later life (Table 1) [165]. It turned out that the presence of Bifidobacterium catenulatum in the gut microbiota was associated with a higher risk of developing eczema, while Bifidobacterium breve and Bifidobacterium lactis were identified in healthy children [166,167,168,169,170,171]. Bifidobacterium are known to produce short-chain fatty acids (SCFA), and insufficient SCFA content was observed in the intestines of asthma, atopic dermatitis, and allergic rhinitis [172,173,174]. Compensation for the deficiency of B. breve alleviated the course of allergic rhinitis and asthma [175]. Another bacterial bioregulator, polysaccharide A, suppresses the production of proinflammatory IL-17 and promotes the expression of IL-10 by CD4 + T cells [176]. Polysaccharide A (PSA) of Bacteroides fragilis acts via TLR2 directly on Foxp3(+) regulatory T cells, promoting immune tolerance. PSA-deficient B. fragilis is unable to inhibit T helper 17 cell responses and is defective in mucosal colonization [177]. Bacterial bioregulators such as SCFA and polysaccharide A have been implicated in the discovery and mechanism of the anti-inflammatory action of Bifidobacterium and Bacteroides fragilis. Microbiota-derived metabolites play a major role in the formation of tight junctions of intestinal epithelial cells, which are renewed every 3–5 days. These metabolites influence the expression and function of tight junction-associated proteins and promote a stronger intestinal barrier. Various gut microbiota-derived metabolites such as butyrate, quercetin, indole-3-propionic acid, bile acid intermediates, and L-homoserine can induce increased production of these junctional proteins [64,178,179].
Imbalances in microbiota and their metabolites may affect tight junction function, promoting microbial entry into the bloodstream, and inflammatory diseases. In experimental models, it was shown that, unlike other bifidobacteria, only B. breve significantly suppressed airway reactivity to methacholine, reduced acute allergic skin reactions to ovalbumin, and activated IFN-gamma and IL-10 secretion, affecting the maintenance of systemic Th1/Th2 balance [180,181]. In an experimental model, it was also shown that the presence of SCFA in the intestine protected against allergic inflammation in the lungs [182]. In another study, when examining changes in the number of Lachnospira (L) and Clostridium neonatale (C) bacteria, an increase in the L/C ratio was found in asthmatics [183]. Reduced microbiota diversity is characteristic of various allergic diseases: asthma, allergic rhinitis, and dermatitis [184]. Reduced microbiota diversity characterized by high abundance of the phylum Firmicutes and low abundance of Bacteroidetes, as well as the presence of the families Clostridiaceae, Ruminococcaceae, Lachnospiraceae or Erysipelotrichaceae is associated with hypersensitivity to milk, egg whites, and peanut [185,186]. Decreased biodiversity, consumption of industrially processed foods, food additives, preservatives, and hygiene products increase the allergen load and are considered key factors in the development of allergic diseases [100,187]. For example, cow’s milk can cause allergies, while raw milk has been shown to protect against allergies [187,188].
4.2. Upper and Lower Respiratory Tract
Prospective cohort studies have shown that six dominant genera make up the upper respiratory tract microbiota, including Moraxella, Streptococcus, Corynebacterium, Alloiococcus, Haemophilus, and Staphylococcus [162,189]. It has been noted that dysbiosis of the nasal microbiome is characterized by altered bacterial species composition and is associated with the occurrence of infections and subsequent allergic diseases [190]. Asthma patients have been shown to have low levels of Lactobacillus in the nasopharynx [191] and increased numbers of Bacteroidetes and Proteobacteria [192]. Early colonization of the nasopharynx by Streptococcus and Moraxella has been associated with asthma [189,191,192,193]. With regard to Moraxella species, specific species need to be considered when investigating associations with allergic diseases. For example, Moraxella catarrhalis has been reported as a potential risk factor for asthma in children and adults, with neutrophilic inflammation in the lungs observed in adults [194,195,196,197,198].
These findings were confirmed by other studies, which showed that Proteobacteria, especially Haemophilus spp. and Moraxella spp., were significantly increased in neutrophilic asthma, while Firmicutes, Actinobacteria, and Saccharibacteria were reduced in relative abundance compared to healthy controls [199,200]. Bacterial infections with Haemophilus, Moraxella, or Streptococcus spp. induce the secretion of IL-17, which in turn attracts neutrophils to the airways [201]. Neutrophilic asthma is characterized by an impaired response to corticosteroid treatment. The presence of bacteria in sputum has led to the development of antibiotic treatments for neutrophilic asthma, particularly azithromycin [201]. Eosinophilic asthma has been correlated with the presence of Tropheryma whipplei in sputum [200,202].
Klebsiella has been detected in bronchial lavage samples in asthmatics [203]. A study of bronchial lavage in children and adults revealed a predominance of Proteobacteria, particularly Haemophilus spp., in asthmatics, while Bacteroidetes, particularly Prevotella spp., were less common than in healthy controls [204].
Low levels of Bacteroidetes are present in the upper airways of patients with chronic rhinosinusitis [205]. Nasal lavage fluid samples from patients with chronic rhinosinusitis had lower bacterial diversity, and higher bacterial abundance of Staphylococcus aureus and its extracellular vesicles [205].
A study by Ruokolainen L. and colleagues examined the dependence of allergic rhinitis on genetic factors and lifestyle. It turned out that allergic manifestations and sensitization to common allergens were significantly influenced by the microbiota of the nasal epithelium and skin and determined by lifestyle. Acinetobacter, in particular, was associated with the absence of allergic rhinitis [206].
The study of bacterial communities in the upper and lower respiratory tract serves as a basis for the development of asthma therapy using probiotics [207]. However, it should be taken into account that the formation of the lung microbiome is associated with aerobic bacteria, and the microbiota of the gastrointestinal tract with anaerobic bacteria [208,209]. In addition, the effect of probiotics may depend on diet, and in some cases aggravate allergic inflammation. For example, the popular probiotic Akkermansia, containing the bacterium Akkermansia muciniphila, aggravates food allergies in the absence of fiber by activating the Th2 pathway [210]. The use of modern methods, including bioinformatics analysis and machine learning, can help in predicting possible reactions to bacteria and using bacteria as diagnostic markers of diseases [211]. Intensive research on bacterial metabolites has been conducted in the intestine. The study of bacterial bioregulators of microorganisms inhabiting the upper and lower respiratory tract is associated with the difficulty of excluding bacterial contamination from the oral cavity; however, their identification can reveal new molecules of therapeutic value.
Bioregulators of bacterial origin—PAMPs and bacterial metabolites—play a central role in regulating host homeostasis [45,46,47,72]. It is believed that bacterial metabolites and their derivatives can be effective treatments [212].
4.3. Skin
Microorganisms colonize the skin immediately after birth. The composition of the skin microbiota of a newborn during the first three months depends on the method of delivery. Infants born vaginally acquired bacterial communities similar to their mother’s vaginal microbiota, dominated by Lactobacillus, Prevotella, or Sneathia spp., whereas infants born by cesarean section harbored bacterial communities similar to those found on the skin surface, dominated by Staphylococcus, Corynebacterium, and Propionibacterium spp. [213]. The neonatal microbiota was relatively uniform across all body sites at delivery, with the exception of neonatal meconium. However, by 6 weeks, the infant microbiota had expanded significantly, become more diverse, and was not affected by delivery mode [214]. However, as early as 3 months, infants can be diagnosed with atopic dermatitis (AD) if their skin is colonized by Staphylococcus aureus [215,216,217]. In pediatric AD, the skin commensal S. epidermidis was significantly reduced in abundance, and Streptococcus, Propionibacterium, and Corynebacterium species were significantly reduced [216]. Skin commensals S. epidermidis and Staphylococcus hominis protect the host from pathogens, including Staphylococcus aureus, which stimulate the host to produce antimicrobial peptides such as cathelicidin and human beta-defensin (HBD), and directly produce PSM-γ and PSM-δ, which inhibit the growth of pathogenic bacteria [218]. It should be noted that local skin anatomy, lipid content, and pH also influence the occurrence of AD [219]. The skin is known to have an acidic pH, which promotes optimal barrier function. In humans, the skin surface pH is close to neutral at birth (pH 6.5), and it takes several weeks after birth for the pH to reach the normal range of 5.4–5.9 [220]. Staphylococcus aureus can exist in a wide pH range, tolerate acid loads, and form biofilms [221,222]. Staphylococcus aureus exerts its pathogenic effects through the production of virulence factors such as α-toxin, protein A (SpA), lipotechoic acid (LTA), phenol-soluble modulin (PSM)-α, and proteases that can damage keratinocytes [223,224,225].
Skin colonization by S. aureus has been associated with food allergies. In a study of 718 children aged 0–18 years and 640 infants with severe eczema, an association was found between S. aureus colonization and egg or peanut allergy [226,227,228]. The increased risk of peanut and egg allergy in the first 5 years of life was independent of AD severity [227,228]. S. aureus enterotoxins are classified as superantigens (SAgs) due to their ability to activate B and T cells and induce immunoglobulin E production against SAgs in individuals with atopic dermatitis, allergic rhinitis, chronic sinusitis, and asthma [229,230,231,232,233]. Severe atopic dermatitis has been found to be characterized by an expansion of circulating Th2 and Th22 cells, but not Th17, in the skin-homing T cell population [233,234]. It should be noted that the virulence of S. aureus can be altered by the presence of other bacteria. In particular, the presence of Corynebacterium promotes the transition of S. aureus from a virulent to a commensal state, which explains the presence of S. aureus on the skin and in the nasal cavity in 25% of healthy individuals [235].
An association between Acinetobacter skin colonization and allergic rhinitis has been documented. Thus, when identifying the causes of allergic rhinitis in populations with a similar genotype but different lifestyle, genome-wide association analysis revealed that lifestyle and environment affect gene expression in blood mononuclear cells and depend on the microbiome of the skin and nasal epithelium [206]. Allergic rhinitis was found to be correlated with increased activity in 261 genes of the innate immune pathway, with statistically significant differences observed with decreased Acinetobacter representation on the skin and in the nasal cavity. In addition, a high number of expressed genes leads to a more balanced innate immunity and is associated with a low prevalence of allergies [206].

Table 1.
Bacteria in allergic diseases.
Table 1.
Bacteria in allergic diseases.
| Bacteria or Substances | Disease | Reference | |
|---|---|---|---|
| Gastrointestinal tract | Deficiency of Lachnospira, Veillonella, Rothia and Faecalibacterium | Asthma | [165] |
| An increase in the Lachnospira/Clostridium neonatale ratio | [183] | ||
| Deficiency of odd-chain fatty acids, 10-nonadecenoate and 10-hepatadecenoate Enriched for the aconitate | [164] | ||
| Deficiency of short-chain fatty acids | [172,182] | ||
| Presence of Bifidobacterium catenulatum | Atopic dermatitis | [166,167,168] | |
| Deficiency of Bifidobacterium breve | [169] | ||
| An increase of the species Firmicutes such as Clostridium and deficiency of Bacteroides | [170] | ||
| Deficiency of oligosaccharides and short-chain fatty acids | [172] | ||
| Deficiency of Bifidobacterium breve | Allergic rhinitis | [175] | |
| Deficiency of Bifidobacterium lactis | [171] | ||
| Deficiency of short-chain fatty acids | [173] | ||
| Presence of Enterobacter and deficiency of Bifidobacterium | Chronic rhinosinusitis | [174] | |
| Presence of Clostridiaceae, Ruminococcaceae, Lachnospiraceae and Erysipelotrichaceae | Food allergy | [185] | |
| Deficiency of Lactobacillales, Bacteroidales | [186] | ||
| Upper and lower respiratory tract | Streptococcus in nasopharynx | Asthma | [189] |
| Predominance of Moraxella in nasopharynx | [193,194,195] | ||
| Low abundance of Lactobacillus in nasopharynx | [191] | ||
| Low abundance of Firmicutes, Actinobacteria and Saccharibacteria in nasopharynx | [199] | ||
| Haemophilus influenza, Moraxella catarrhalis and Tropheryma whipplei in sputum | [200,202] | ||
| Moraxella catarrhalis, Haemophilus and Streptococcus in sputum | [198] | ||
| Enriched with taxa from Bacteroidetes and Proteobacteria in nasopharynx | [192] | ||
| Klebsiella in bronchial swab samples | [203] | ||
| Low abundance of Bacteroidetes and predominance of Staphylococcus aureus in nasal lavage | Chronic rhinosinusitis | [205] | |
| Low abundance of Acinetobacter | Allergic rhinitis | [206] | |
| Skin | Predominance of Staphylococcus aureus | Atopic dermatitis | [215,216,217] |
| Deficiency of Staphylococcus epidermidis, Streptococcus, Propionibacterium and Corynebacterium | [216] | ||
| Predominance of Staphylococcus aureus and deficiency of Staphylococcus epidermidis and Staphylococcus hominis | [218] | ||
| Predominance of Staphylococcus aureus | Food allergy | [226,227,228] | |
| Low abundance of Acinetobacter | Allergic rhinitis | [206] |
5. Concluding Remarks
New approaches to the diagnosis and therapy of asthma and associated diseases are being developed, including metagenomic studies, transcriptomics, systems biology, and cell therapy [150,236,237,238,239,240]. At the same time, it is necessary to take into account a large number of factors influencing the occurrence of allergic diseases, in particular, the influence of the mother’s microbiome, her diet, and the use of antibiotics during pregnancy on the formation of the allergic phenotype of her future child [162,241]. In addition, it is necessary to take into account the influence of viruses, fungi, and archaea that are part of the human microbiome and enter into complex mutualistic, symbiotic, and antagonistic interactions. It is important to determine not only the phyla and genera of bacteria associated with diseases, but also to study the species that can compete with each other for the habitat, as is shown by the example of S. epidermidis and S. aureus (phyla Firmicutes, genera Staphylococci).
The accumulated knowledge about the features of the impact of various microorganisms on humans makes it possible to prevent allergic diseases using both whole bacteria and their fragments and metabolites [162,242,243,244]. Currently, active research is underway to study the effect of microorganisms or their fragments on their hosts, while determining the effect of microorganism metabolites on humans requires more complex technologies. Modern methods developed for registering low-molecular compounds, as well as modern technologies, including machine learning, inspire hope for significant achievements in the near future. The ability of microbiota and bacterial metabolites to strengthen the integrity of the intestinal barrier, provide protection against inflammation, improve nutrient uptake pathways, and improve protection against age-related diseases has been associated with increased lifespan and health [245,246].
Understanding the mechanisms by which the immune response to various microorganisms and the substances they produce can help to discover how our immune system can detect commensal and pathogenic bacteria and develop new strategies to combat pathogens and allergic diseases. The numerous ways in which microorganisms affect their hosts require the consideration of many thousands of signaling pathways to identify associations with diseases. It is clear that maintaining health requires the participation of a large number of factors, provided by biodiversity. A thorough analysis of the influence of all possible bioregulators of bacterial origin and their cross-interaction, taking into account specific genetic characteristics and environmental factors, will allow us to accurately assess the events taking place and develop personalized therapies and strategies for the prevention of allergic diseases. Our knowledge of the influence of microflora on the functioning of all systems and organs leads to changes our food preferences and lifestyle.
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Obeso, D.; Villaseñor, A.; Barber, D.; Pérez-Gordo, M. Microbiome and Allergy: New Insights and Perspectives. J. Investig. Allergol. Clin. Immunol. 2022, 32, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Droste, J.H.; Wieringa, M.H.; Weyler, J.J.; Nelen, V.J.; Vermeire, P.A.; Van Bever, H.P. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy. 2000, 30, 1547–1553. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Yang, L.; Narita, M.; Saito, H.; Ohya, Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann. Allergy Asthma Immunol. 2017, 119, 54–58. [Google Scholar] [CrossRef]
- Metzler, S.; Frei, R.; Schmaußer-Hechfellner, E.; von Mutius, E.; Pekkanen, J.; Karvonen, A.M.; Kirjavainen, P.V.; Dalphin, J.C.; Divaret-Chauveau, A.; Riedler, J.; et al. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatr. Allergy Immunol. 2019, 30, 423–433. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, W.; Huang, C.; Sun, C.; Zhang, J. First-Year Antibiotics Exposure in Relation to Childhood Asthma, Allergies, and Airway Illnesses. Int. J. Environ. Res. Public Health 2020, 17, 5700. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Ronchetti, R. Are infections protecting from atopy? Curr. Opin. Allergy Clin. Immunol. 2001, 1, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. The airway epithelium is central to the pathogenesis of asthma. Allergol. Int. 2008, 57, 1–10. [Google Scholar] [CrossRef]
- Kilpeläinen, M.; Terho, E.O.; Helenius, H.; Koskenvuo, M. Farm environment in childhood prevents the development of allergies. Clin. Exp. Allergy 2000, 30, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Santeliz, J.; Karp, C.L. The germless theory of allergic disease: Revisiting the hygiene hypothesis. Nat. Rev. Immunol. 2001, 1, 69–75. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hoskinson, C.; Dai, D.L.Y.; Del Bel, K.L.; Becker, A.B.; Moraes, T.J.; Mandhane, P.J.; Finlay, B.B.; Simons, E.; Kozyrskyj, A.L.; Azad, M.B.; et al. Delayed gut microbiota maturation in the first year of life is a hallmark of pediatric allergic disease. Nat. Commun. 2023, 14, 4785. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Lisik, D.; Ermis, S.S.Ö.; Ioannidou, A.; Milani, G.P.; Nyassi, S.; Spolidoro, G.C.I.; Kankaanranta, H.; Goksör, E.; Wennergren, G.; Nwaru, B.I. Siblings and risk of allergic rhinitis: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2023, 34, e13991. [Google Scholar] [CrossRef]
- Strachan, D.P.; Aït-Khaled, N.; Foliaki, S.; Mallol, J.; Odhiambo, J.; Pearce, N.; Williams, H.C.; ISAAC Phase Three Study Group. Siblings, asthma, rhinoconjunctivitis and eczema: A worldwide perspective from the International Study of Asthma and Allergies in Childhood. Clin. Exp. Allergy 2015, 45, 126–136. [Google Scholar] [CrossRef]
- Perez-Munoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Jimenez, E.; Fernandez, L.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Olivares, M.; Xaus, J. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Moles, L.; Gómez, M.; Heilig, H.; Bustos, G.; Fuentes, S.; de Vos, W.; Fernández, L.; Rodríguez, J.M.; Jiménez, E. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS ONE 2013, 8, e66986. [Google Scholar] [CrossRef]
- Senn, V.; Bassler, D.; Choudhury, R.; Scholkmann, F.; Righini-Grunder, F.; Vuille-Dit-Bile, R.N.; Restin, T. Microbial Colonization From the Fetus to Early Childhood-A Comprehensive Review. Front. Cell. Infect. Microbiol. 2020, 10, 573735. [Google Scholar] [CrossRef]
- Machado, M.E.; Porto, L.C.; Alves Galvão, M.G.; Sant’Anna, C.C.; Lapa E Silva, J.R. SNPs, adipokynes and adiposity in children with asthma. J. Asthma 2023, 60, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Ishida-Yamamoto, A.; Kishibe, M.; Honma, M. Desmosomes and corneodesmosomes and their relevance to genetic skin diseases. G. Ital. Dermatol. Venereol. 2017, 152, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Falcon, R.M.G.; Caoili, S.E.C. Immunologic, genetic, and ecological interplay of factors involved in allergic diseases. Front. Allergy 2023, 4, 1215616. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, J.D.; Park, C.S. Gene-Environment Interactions in Asthma: Genetic and Epigenetic Effects. Yonsei Med. J. 2015, 56, 877–886. [Google Scholar] [CrossRef]
- Losol, P.; Sokolowska, M.; Hwang, Y.K.; Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Radzikowska, U.; Ardicli, S.; Yoon, J.E.; et al. Epithelial Barrier Theory: The Role of Exposome, Microbiome, and Barrier Function in Allergic Diseases. Allergy Asthma Immunol. Res. 2023, 15, 705–724. [Google Scholar] [CrossRef]
- Ozdemir, C.; Kucuksezer, U.C.; Ogulur, I.; Pat, Y.; Yazici, D.; Ardicli, S.; Akdis, M.; Nadeau, K.; Akdis, C.A. Lifestyle Changes and Industrialization in the Development of Allergic Diseases. Curr. Allergy Asthma Rep. 2024, 24, 331–345. [Google Scholar] [CrossRef]
- Singh, N.; Diebold, Y.; Sahu, S.K.; Leonardi, A. Epithelial barrier dysfunction in ocular allergy. Allergy 2022, 77, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Zhou, Y.C.; Yang, L.T.; Zhou, Q.; Wang, X.J.; Qiu, S.Q.; Cheng, B.H.; Zeng, X.H. Involvement and repair of epithelial barrier dysfunction in allergic diseases. Front. Immunol. 2024, 15, 1348272. [Google Scholar] [CrossRef]
- Loxham, M.; Davies, D.E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 1736–1751. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Bohle, B.; Ovchinnikova, T.V. How Do Pollen Allergens Sensitize? Front. Mol. Biosci. 2022, 9, 900533. [Google Scholar] [CrossRef]
- Yazici, D.; Ogulur, I.; Pat, Y.; Babayev, H.; Barletta, E.; Ardicli, S.; Bel Imam, M.; Huang, M.; Koch, J.; Li, M.; et al. The epithelial barrier: The gateway to allergic, autoimmune, and metabolic diseases and chronic neuropsychiatric conditions. Semin. Immunol. 2023, 70, 101846. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; de Matos, M.R.; Cortes, L.; Nunes-Correia, I.; Todo-Bom, A.; Pires, E.; Veríssimo, P. Pollen Proteases Play Multiple Roles in Allergic Disorders. Int. J. Mol. Sci. 2020, 21, 3578. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Berdyshev, E.; Leung, D.Y. Epithelial barrier repair and prevention of allergy. J. Clin. Investig. 2019, 129, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Virgin, H.W. The virome in mammalian physiology and disease. Cell 2014, 157, 142–150. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.D. Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc. Soc. Exp. Biol. Med. 1967, 126, 301–304. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Høverstad, T.; Midtvedt, T. Short-chain fatty acids in germfree mice and rats. J. Nutr. 1986, 116, 1772–1776. [Google Scholar] [CrossRef]
- Gustafsson, B.E.; Daft, F.S.; Mcdaniel, E.G.; Smith, J.C.; Fitzgerald, R.J. Effects of vitamin K-active compounds and intestinal microorganisms in vitamin K-deficient germfree rats. J. Nutr. 1962, 78, 461–468. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Freter, R. The fatal enteric cholera infection in the guinea pig, achieved by inhibition of normal enteric flora. J. Infect. Dis. 1955, 97, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; Pamer, E.G. Commensal bacteria mediated defenses against pathogens. Curr. Opin. Immunol. 2014, 29, 16–22. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Hayes, C.L.; Dong, J.; Galipeau, H.J.; Jury, J.; McCarville, J.; Huang, X.; Wang, X.Y.; Naidoo, A.; Anbazhagan, A.N.; Libertucci, J.; et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci. Rep. 2018, 8, 14184. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Philpott, D.J. Peptidoglycan: A critical activator of the mammalian immune system during infection and homeostasis. Immunol. Rev. 2011, 243, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Roles of Toll-like receptors in innate immune responses. Genes Cells 2001, 6, 733–742. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Inohara, N.; Nuñez, G. NODs: Intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 2003, 3, 371–382. [Google Scholar] [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. When signaling pathways collide: Positive and negative regulation of toll-like receptor signal transduction. Immunity 2008, 29, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, R.P.; Isakov, V.V.; Nazarenko, E.L.; Ovodov, Y.S.; Guryanova, S.V.; Dmitriev, B.A. Structure of the O-specific polysaccharide of the lipopolysaccharide from Yersinia kristensenii O:25.35. Carbohydr. Res. 1993, 241, 201–208. [Google Scholar] [CrossRef]
- L’vov, V.L.; Gur’ianova, S.V.; Rodionov, A.V.; Dmitriev, B.A.; Shashkov, A.S.; Ignatenko, A.V.; Gorshkova, R.P.; Ovodov, I.S. The structure of a repetitive unit of the glycerolphosphate- containing O-specific polysaccharide chain from Yersinia kristensenii strain 103 (0:12,26) lipopolysaccharide. Bioorg. Khim. 1990, 16, 379–389. [Google Scholar]
- L’vov, V.L.; Gur’yanova, S.V.; Rodionov, A.V.; Gorshkova, R.P. Structure of the repeating unit of the O-specific polysaccharide of the lipopolysaccharide of Yersinia kristensenii strain 490 (O:12,25). Carbohydr. Res. 1992, 228, 415–422. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-Positive Bacterial Cell Wall. Microbiol Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Meshcheriakova, E.A.; Gur’ianova, S.V.; Makarov, E.A.; Andronova, T.M.; Ivanov, V.T. Structure-functional study of glycosaminylmuramoyl peptides. The effect of chemical modification of N-acetylglucosaminyl-N-acetylmuramoyldipeptide on its immunomodulating properties in vivo and in vitro. Bioorg. Chem. 1991, 17, 1157–1165. [Google Scholar]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef]
- Kufer, T.A.; Banks, D.J.; Philpott, D.J. Innate immune sensing of microbes by Nod proteins. Ann. N. Y. Acad. Sci. 2006, 1072, 19–27. [Google Scholar] [CrossRef]
- Guryanova, S.V. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms 2022, 10, 1526. [Google Scholar] [CrossRef]
- Tan, R.S.; Ho, B.; Leung, B.P.; Ding, J.L. TLR cross-talk confers specificity to innate immunity. Int. Rev. Immunol. 2014, 33, 443–453. [Google Scholar] [CrossRef]
- Becker, C.E.; O’Neill, L.A. Inflammasomes in inflammatory disorders: The role of TLRs and their interactions with NLRs. Semin. Immunopathol. 2007, 29, 239–248. [Google Scholar] [CrossRef]
- Merlo, A.; Calcaterra, C.; Mènard, S.; Balsari, A. Cross-talk between toll-like receptors 5 and 9 on activation of human immune responses. J. Leukoc. Biol. 2007, 82, 509–518. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Garg, A.; Zhao, A.; Erickson, S.L.; Mukherjee, S.; Lau, A.J.; Alston, L.; Chang, T.K.; Mani, S.; Hirota, S.A. Pregnane X Receptor Activation Attenuates Inflammation-Associated Intestinal Epithelial Barrier Dysfunction by Inhibiting Cytokine-Induced Myosin Light-Chain Kinase Expression and c-Jun N-Terminal Kinase 1/2 Activation. J. Pharmacol. Exp. Ther. 2016, 359, 91–101. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Zhu, W. Crosstalk between the Immune Receptors and Gut Microbiota. Curr. Protein. Pept. Sci. 2015, 16, 622–631. [Google Scholar] [CrossRef]
- Guryanova, S.V. Immunomodulation, Bioavailability and Safety of Bacteriocins. Life 2023, 13, 1521. [Google Scholar] [CrossRef]
- Gillor, O.; Ghazaryan, L. Recent advances in bacteriocin application as antimicrobials. Recent Pat. Antiinfect. Drug Discov. 2007, 2, 115–122. [Google Scholar] [CrossRef]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Y.; Song, M. Lactobacillus alleviates intestinal epithelial barrier function through GPR43-mediated M2 macrophage polarization. Animal Diseases 2024, 4, 20. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.U.; Kweon, M.N. Gut microbiota-derived metabolites tune host homeostasis fate. Semin. Immunopathol. 2024, 11, 2. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Rivera-Chávez, F.; Tsolis, R.M.; Bäumler, A.J. How bacterial pathogens use type III and type IV secretion systems to facilitate their transmission. Curr. Opin. Microbiol. 2017, 35, 1–7. [Google Scholar] [CrossRef]
- Galán, J.E.; Waksman, G. Protein-Injection Machines in Bacteria. Cell 2018, 172, 1306–1318. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef]
- Charpentier, L.A.; Dolben, E.F.; Hendricks, M.R.; Hogan, D.A.; Bomberger, J.M.; Stanton, B.A. Bacterial Outer Membrane Vesicles and Immune Modulation of the Host. Membranes 2023, 13, 752. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Coelho, C.; Casadevall, A. Answers to naysayers regarding microbial extracellular vesicles. Biochem. Soc. Trans. 2019, 47, 1005–1012. [Google Scholar] [CrossRef]
- Zhao, G.; Jones, M.K. Role of Bacterial Extracellular Vesicles in Manipulating Infection. Infect. Immun. 2023, 91, e0043922. [Google Scholar] [CrossRef]
- Peregrino, E.S.; Castañeda-Casimiro, J.; Vázquez-Flores, L.; Estrada-Parra, S.; Wong-Baeza, C.; Serafín-López, J.; Wong-Baeza, I. The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 6210. [Google Scholar] [CrossRef] [PubMed]
- Verbunt, J.; Jocken, J.; Blaak, E.; Savelkoul, P.; Stassen, F. Gut-bacteria derived membrane vesicles and host metabolic health: A narrative review. Gut Microbes 2024, 16, 2359515. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles. 2021, 10, e12161. [Google Scholar] [CrossRef]
- Schorey, J.S.; Cheng, Y.; McManus, W.R. Bacteria- and host-derived extracellular vesicles—Two sides of the same coin? J. Cell Sci. 2021, 134, jcs256628. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chan, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Keir, H.R.; Shoemark, A.; Dicker, A.J.; Perea, L.; Pollock, J.; Giam, Y.H.; Suarez-Cuartin, G.; Crichton, M.L.; Lonergan, M.; Oriano, M.; et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: An international, observational, multicohort study. Lancet Respir. Med. 2021, 9, 873–884. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, N.V.; Kozlov, I.G.; Guryanova, S.V.; Kokov, E.A.; Andronova, T.M. Clinical and immunological efficiency of muramyl dipeptide in the treatment of atopic diseases. Med. Immunol. 2016, 1, 15–20. [Google Scholar] [CrossRef]
- Guryanova, S.; Khaitov, R. Glucosaminylmuramyldipeptide—GMDP: Effect on mucosal immunity (on the issue of immunotherapy and immunoprophylaxis). Immunologiya 2020, 41, 174–183. (In Russian) [Google Scholar] [CrossRef]
- Gholami, H.; Chmiel, J.A.; Burton, J.P.; Maleki Vareki, S. The Role of Microbiota-Derived Vitamins in Immune Homeostasis and Enhancing Cancer Immunotherapy. Cancers 2023, 15, 1300. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Diamant, Z.; Eiwegger, T.; Fokkens, W.J.; Traidl-Hoffmann, C.; Nadeau, K.; O’Hehir, R.E.; O’Mahony, L.; Pfaar, O.; Torres, M.J.; et al. Future research trends in understanding the mechanisms underlying allergic diseases for improved patient care. Allergy 2019, 74, 2293–2311. [Google Scholar] [CrossRef]
- Capucilli, P.; Hill, D.A. Allergic Comorbidity in Eosinophilic Esophagitis: Mechanistic Relevance and Clinical Implications. Clin. Rev. Allergy Immunol. 2019, 57, 111–127. [Google Scholar] [CrossRef]
- Letner, D.; Farris, A.; Khalili, H.; Garber, J. Pollen-food allergy syndrome is a common allergic comorbidity in adults with eosinophilic esophagitis. Dis. Esophagus. 2018, 31, 2. [Google Scholar] [CrossRef]
- Zysk, W.; Mesjasz, A.; Trzeciak, M.; Horvath, A.; Plata-Nazar, K. Gastrointestinal Comorbidities Associated with Atopic Dermatitis-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1194. [Google Scholar] [CrossRef]
- Vasquez Ayala, A.; Hsu, C.Y.; Oles, R.E.; Matsuo, K.; Loomis, L.R.; Buzun, E.; Carrillo Terrazas, M.; Gerner, R.R.; Lu, H.H.; Kim, S.; et al. Commensal bacteria promote type I interferon signaling to maintain immune tolerance in mice. J. Exp. Med. 2024, 221, e20230063. [Google Scholar] [CrossRef]
- Molloy, J.; Allen, K.; Collier, F.; Tang, M.L.; Ward, A.C.; Vuillermin, P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int. J. Environ. Res. Public Health 2013, 10, 7235–7256. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Komlósi, Z.I.; van de Veen, W.; Kovács, N.; Szűcs, G.; Sokolowska, M.; O’Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and molecular mechanisms of allergic asthma. Mol. Asp. Med. 2022, 85, 100995. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef]
- Feili-Hariri, M.; Falkner, D.H.; Morel, P.A. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: Implications for immunotherapy. J. Leukoc Biol. 2005, 78, 656–664. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 7, 491. [Google Scholar] [CrossRef]
- Creagh, E.M.; O’Neill, L.A. TLRs, NLRs and RLRs: A trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006, 27, 352–357. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Kozlov, I.G.; Meshcheryakova, E.A.; Alekseeva, L.G.; Andronova, T.M. Glucosaminylmuramyl Dipeptide Normalizes Th1/Th2 Balance in Atopic Bronchial Asthma. Immunology 2009, 5, 305–308. (In Russian) [Google Scholar]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Delespesse, G.; Ohshima, Y.; Shu, U.; Yang, L.; Demeure, C.; Wu, C.; Byun, D.; Sarfati, M. Differentiation of naive human CD4 T cells into TH2/TH1 effectors. Allergol. Int. 1997, 46, 63–72. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Kohanski, M.A.; Maina, I.W.; Workman, A.D.; Herbert, D.R.; Cohen, N.A. Sentinels at the wall: Epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int. Forum. Allergy Rhinol. 2019, 9, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Montaini, G.; Mazzoni, A.; Rossettini, B.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Liotta, F.; Rossi, O.; Gallo, O.; et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 2017, 139, 964–976.e4. [Google Scholar] [CrossRef]
- Emami Fard, N.; Xiao, M.; Sehmi, R. Regulatory ILC2—Role of IL-10 Producing ILC2 in Asthma. Cells 2023, 12, 2556. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, E.; Rodriguez-Coira, J.; Delgado-Dolset, M.I.; Gomez-Casado, C.; Barber, D.; Escribese, M.M. Epithelial Barrier: Protector and Trigger of Allergic Disorders. J. Investig. Allergol. Clin. Immunol. 2022, 32, 81–96. [Google Scholar] [CrossRef]
- Stadnyk, A.W. Cytokine production by epithelial cells. FASEB J. 1994, 8, 1041–1047. [Google Scholar] [CrossRef]
- Koch, S.; Nusrat, A. The life and death of epithelia during inflammation: Lessons learned from the gut. Annu. Rev. Pathol. 2012, 7, 35–60. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Colgan, S.P. Cytokine responses and epithelial function in the intestinal mucosa. Cell. Mol. Life Sci. 2016, 73, 4203–4212. [Google Scholar] [CrossRef]
- Svanborg, C.; Agace, W.; Hedges, S.; Linder, H.; Svensson, M. Bacterial adherence and epithelial cell cytokine production. Zentralbl. Bakteriol. 1993, 278, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Tsang, J.S.; Park, K. Systems immunology of regulatory T cells: Can one circuit explain it all? Trends Immunol. 2023, 44, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Gu, J.; Zhou, J.; Wang, Q.; Li, X.; Deng, Z.; Lu, L. Tissue Tregs and Maintenance of Tissue Homeostasis. Front. Cell Dev. Biol. 2021, 9, 717903. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Carrier, Y.; Peron, J.P.; Bettelli, E.; Kamanaka, M.; Flavell, R.A.; Kuchroo, V.K.; Oukka, M.; Weiner, H.L. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007, 8, 1380–1389. [Google Scholar] [CrossRef]
- Pot, C.; Jin, H.; Awasthi, A.; Liu, S.M.; Lai, C.Y.; Madan, R.; Sharpe, A.H.; Karp, C.L.; Miaw, S.C.; Ho, I.C.; et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009, 183, 797–801. [Google Scholar] [CrossRef]
- Kim, D.; Le, H.T.; Nguyen, Q.T.; Kim, S.; Lee, J.; Min, B. Cutting Edge: IL-27 Attenuates Autoimmune Neuroinflammation via Regulatory T Cell/Lag3-Dependent but IL-10-Independent Mechanisms In Vivo. J. Immunol. 2019, 202, 1680–1685. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Jang, E.; Le, H.T.; Kim, S.; Kim, D.; Dvorina, N.; Aronica, M.A.; Baldwin, W.M., 3rd; Asosingh, K.; Comhair, S.; et al. IL-27 targets Foxp3+ Tregs to mediate antiinflammatory functions during experimental allergic airway inflammation. JCI Insight 2019, 4, e123216. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L. Contrasting roles for all-trans retinoic acid in TGF-[beta]-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med. 2009, 206, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001, 2, 816–822. [Google Scholar] [CrossRef]
- Jung, M.; Sabat, R.; Krätzschmar, J.; Seidel, H.; Wolk, K.; Schönbein, C.; Schutt, S.; Friedrich, M.; Docke, W.D.; Asadullah, K.; et al. Expression profiling of IL-10-regulated genes in human monocytes and peripheral blood mononuclear cells from psoriatic patients during IL-10 therapy. Eur. J. Immunol. 2004, 34, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kliem, C.V.; Schaub, B. The role of regulatory B cells in immune regulation and childhood allergic asthma. Mol. Cell. Pediatr. 2024, 11, 1. [Google Scholar] [CrossRef]
- Heinl, P.V.; Graulich, E.; Weigmann, B.; Wangorsch, A.; Ose, R.; Bellinghausen, I.; Khatri, R.; Raker, V.K.; Scheurer, S.; Vieths, S.; et al. IL-10-modulated dendritic cells from birch pollen- and hazelnut-allergic patients facilitate Treg-mediated allergen-specific and cross-reactive tolerance. Allergy 2024, 28. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of immune tolerance to allergens: Role of IL-10 and Tregs. J. Clin. Investig. 2014, 124, 4678–4680. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Wilson, E.B.; Brooks, D.G. The role of IL-10 in regulating immunity to persistent viral infections. Curr. Top. Microbiol. Immunol. 2011, 350, 39–65. [Google Scholar]
- Müller, A.; Oertli, M.; Arnold, I.C.H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun. Signal. 2011, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Qu, Z.-L.; Tang, X.-L.; Liu, Q.; Luo, W.; Huang, C.; Pan, Q.; Zhang, X.-L. Mycobacterium tuberculosis Mannose-Capped Lipoarabinomannan Induces IL-10-Producing B Cells and Hinders CD4+Th1 Immunity. iScience 2019, 11, 13–30. [Google Scholar] [CrossRef] [PubMed]
- González, L.A.; Melo-González, F.; Sebastián, V.P.; Vallejos, O.P.; Noguera, L.P.; Suazo, I.D.; Schultz, B.M.; Manosalva, A.H.; Peñaloza, H.F.; Soto, J.A.; et al. Characterization of the Anti-Inflammatory Capacity of IL-10-Producing Neutrophils in Response to Streptococcus pneumoniae Infection. Front. Immunol. 2021, 12, 638917. [Google Scholar] [CrossRef] [PubMed]
- Rechkina, E.A.; Denisova, G.F.; Masalova, O.V.; Lideman, L.F.; Denisov, D.A.; Lesnova, E.I.; Ataullakhanov, R.I.; Gur’ianova, S.V.; Kushch, A. Epitope mapping of antigenic determinants of hepatitis C virus proteins by phage display. Mol. Biologiia 2006, 40, 357–368. (In Russian) [Google Scholar]
- Guryanova, S.V.; Sigmatulin, I.; Gigani, O.O.; Lipkina, S.A. Mechanisms of regulation allergic and autoimmune reactions by bacterial origin bioregulators. RUDN J. Med. 2023, 27, 470–482. [Google Scholar] [CrossRef]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef]
- Cebula, A.; Seweryn, M.; Rempala, G.A.; Pabla, S.S.; McIndoe, R.A.; Denning, T.L.; Bry, L.; Kraj, P.; Kisielow, P.; Ignatowicz, L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 2013, 497, 258–262. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192. [Google Scholar] [CrossRef]
- Prescott, D.; Maisonneuve, C.; Yadav, J.; Rubino, S.J.; Girardin, S.E.; Philpott, D.J. NOD2 modulates immune tolerance via the GM-CSF–dependent generation of CD103+ dendritic cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10946–10957. [Google Scholar] [CrossRef]
- Pablo-Torres, C.; Garcia-Escribano, C.; Romeo, M.; Gomez-Casado, C.; Arroyo Solera, R.; Bueno-Cabrera, J.L.; Del Mar Reaño Martos, M.; Iglesias-Cadarso, A.; Tarín, C.; Agache, I.; et al. Transcriptomics reveals a distinct metabolic profile in T cells from severe allergic asthmatic patients. Front. Allergy 2023, 4, 1129248. [Google Scholar] [CrossRef]
- Zhang, Y.; Higashide, W.M.; McCormick, B.A.; Chen, J.; Zhou, D. The nflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol. Microbiol. 2006, 62, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Piscatelli, H.; Kotkar, S.A.; McBee, M.E.; Muthupalani, S.; Schauer, D.B.; Mandrell, R.E.; Leong, J.M.; Zhou, D. The EHEC type III effector NleL is an E3 ubiquitin ligase that modulates pedestal formation. PLoS ONE 2011, 6, e19331. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Toyotome, T.; Kataoka, N.; Ohno, M.; Abe, H.; Shimura, Y.; Seyedarabi, A.; Pickersgill, R.; Sasakawa, C. Shigella effector IpaH9.8 binds to a splicing factor U2AF(35) to modulate host immune responses. Biochem. Biophys. Res. Commun. 2005, 333, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Ashida, H.; Kim, M.; Schmidt-Supprian, M.; Ma, A.; Ogawa, M.; Sasakawa, C. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat. Cell Biol. 2010, 12, 66–73. [Google Scholar] [CrossRef]
- Ashida, H.; Nakano, H.; Sasakawa, C. Shigella IpaH0722 E3 ubiquitin ligase effector targets TRAF2 to inhibit PKC-NF-κB activity in invaded epithelial cells. PLoS Pathog. 2013, 9, e1003409. [Google Scholar] [CrossRef]
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. (Eds.) WAO White Book on Allergy; World Allergy Organization: Milwaukee, WI, USA, 2011; 20p. [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Worldwide asthma epidemiology: Insights from the Global Health Data Exchange database. Int. Forum. Allergy Rhinol. 2020, 10, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Agache, I.; Bavbek, S.; Bilo, B.M.; Braido, F.; Cardona, V.; Custovic, A.; Demonchy, J.; Demoly, P.; Eigenmann, P.; et al. Research needs in allergy: An EAACI position paper, in collaboration with EFA. Clin. Transl. Allergy 2012, 2, 21. [Google Scholar] [CrossRef]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A. Microbial exposure during early human development primes fetal immune cells. Cell 2021, 184, 3394–3409.e20. [Google Scholar] [CrossRef]
- Kloepfer, K.M.; McCauley, K.E.; Kirjavainen, P.V. The Microbiome as a Gateway to Prevention of Allergic Disease Development. J. Allergy Clin. Immunol. Pract. 2022, 10, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Rackaityte, E.; Halkias, J.; Fukui, E.M.; Mendoza, V.F.; Hayzelden, C.; Crawford, E.D.; Fujimura, K.E.; Burt, T.D.; Lynch, S.V. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020, 26, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Kimes, N.E.; Lin, D.L.; Rauch, M.; McKean, M.; McCauley, K.; Panzer, A.R.; Mar, J.S.; Cabana, M.D.; Lynch, S.V. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat. Commun. 2018, 16, 707. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Ismail, I.H.; Boyle, R.J.; Licciardi, P.V.; Oppedisano, F.; Lahtinen, S.; Robins-Browne, R.M.; Tang, M.L. Early gut colonization by Bifidobacterium breve and B. catenulatum differentially modulates eczema risk in children at high risk of developing allergic disease. Pediatr. Allergy Immunol. 2016, 27, 838–846. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Fieten, K.B.; Totté, J.E.E.; Levin, E.; Reyman, M.; Meijer, Y.; Knulst, A.; Schuren, F.; Pasmans, S.G.M.A. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int. Arch. Allergy Immunol. 2018, 175, 77–84. [Google Scholar] [CrossRef]
- Hattori, K.; Yamamoto, A.; Sasai, M.; Taniuchi, S.; Kojima, T.; Kobayashi, Y.; Iwamoto, H.; Namba, K.; Yaeshima, T. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi 2003, 52, 20–30. [Google Scholar] [PubMed]
- Nylund, L.; Satokari, R.; Nikkila, J.; Rajilic-Stojanovic, M.; Kalliomaki, M.; Isolauri, E.; Salminen, S.; de Vos, W.M. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013, 13, 12. [Google Scholar] [CrossRef]
- Singh, A.; Hacini-Rachinel, F.; Gosoniu, M.L.; Bourdeau, T.; Holvoet, S.; Doucet-Ladeveze, R.; Beaumont, M.; Mercenier, A.; Nutten, S. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: An exploratory, randomized, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2013, 67, 161–167. [Google Scholar] [CrossRef]
- Pessôa, R.; Clissa, P.B.; Sanabani, S.S. The Interaction between the Host Genome, Epigenome, and the Gut–Skin Axis Microbiome in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 14322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Du, M.; Yang, H.; Shi, R.; Shi, Y.; Zhang, S.; Zhao, Y.; Lan, J. Short-chain fatty acid—A critical interfering factor for allergic diseases. Chem. Biol. Interact. 2023, 385, 110739. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.E.; Li, C.; Xie, A.; Lu, S.; Tang, L.; Liu, Y.H.; Elfadil, A.; Wen, S. Distinct Clinical Pathology and Microbiota in Chronic Rhinosinusitis with Nasal Polyps Endotypes. Laryngoscope 2021, 131, E34–E44. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef]
- Grosheva, I.; Zheng, D.; Levy, M.; Polansky, O.; Lichtenstein, A. High-throughput screen identifies host and microbiota regulators of intestinal barrier function. Gastroenterology 2020, 159, 1807–1823. [Google Scholar] [CrossRef]
- Wong, C.B.; Iwabuchi, N.; Xiao, J.Z. Exploring the Science behind Bifidobacterium breve M-16V in infant health. Nutrients 2019, 11, 1724. [Google Scholar] [CrossRef]
- Inoue, Y.; Iwabuchi, N.; Xiao, J.Z.; Yaeshima, T.; Iwatsuki, K. Suppressive effects of Bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol. Pharm. Bull. 2009, 32, 760–763. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Arrieta, M.C.; Dimitriu, P.A.; Cheng, J.; Thorson, L.; Lefebvre, D.L.; Azad, M.B.; Subbarao, P.; Mandhane, P.; Becker, A.; et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin. Sci. 2016, 130, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Kaczynska, A.; Klosinska, M.; Chmiel, P.; Janeczek, K.; Emeryk, A. The Crosstalk between the Gut Microbiota Composition and the Clinical Course of Allergic Rhinitis: The Use of Probiotics, Prebiotics and Bacterial Lysates in the Treatment of Allergic Rhinitis. Nutrients 2022, 14, 4328. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Huang, J.L.; Chen, K.J.; Kong, M.S.; Hua, M.C.; Yeh, Y.M.; Chang, H.J. Comparison of 16S rRNA gene sequencing microbiota among children with serological IgE-mediated food hypersensitivity. Pediatr. Res. 2024, 95, 241–250. [Google Scholar] [CrossRef]
- Joseph, C.L.; Sitarik, A.R.; Kim, H.; Huffnagle, G.; Fujimura, K.; Yong, G.J.M.; Levin, A.M.; Zoratti, E.; Lynch, S.; Ownby, D.R.; et al. Infant gut bacterial community composition and food-related manifestation of atopy in early childhood. Pediatr. Allergy Immunol. 2022, 33, e13704. [Google Scholar] [CrossRef]
- Agache, I.; Laculiceanu, A.; Spanu, D.; Grigorescu, D. The Concept of One Health for Allergic Diseases and Asthma. Allergy Asthma Immunol. Res. 2023, 15, 290–302. [Google Scholar] [CrossRef]
- Brick, T.; Hettinga, K.; Kirchner, B.; Pfaffl, M.W.; Ege, M.J. The Beneficial Effect of Farm Milk Consumption on Asthma, Allergies, and Infections: From Meta-Analysis of Evidence to Clinical Trial. J. Allergy Clin. Immunol. Pract. 2020, 8, 878–889.e3. [Google Scholar] [CrossRef]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, J.Q. Nasal Microbiome and Its Interaction with the Host in Childhood Asthma. Cells 2022, 11, 3155. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Shilts, M.H.; Tovchigrechko, A.; Schobel, S.; Chappell, J.D.; Larkin, E.K.; Gebretsadik, T.; Halpin, R.A.; Nelson, K.E.; Moore, M.L.; et al. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J. Allergy Clin. Immunol. 2018, 142, 1447–1456.e9. [Google Scholar] [CrossRef]
- Fazlollahi, M.; Lee, T.D.; Andrade, J.; Oguntuyo, K.; Chun, Y.; Grishina, G.; Grishin, A.; Bunyavanich, S. The nasal microbiome in asthma. J. Allergy Clin. Immunol. 2018, 142, 834–843.e2. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.H.F.; Lang, A.; Teo, S.M.; Judd, L.M.; Gangnon, R.; Evans, M.D.; Lee, K.E.; Vrtis, R.; Holt, P.G.; Lemanske, R.F., Jr.; et al. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J. Allergy Clin. Immunol. 2021, 147, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Karppinen, S.; Schuez-Havupalo, L.; Waris, M.; He, Q.; Hoffman, K.L.; Petrosino, J.F.; Dumas, O.; Camargo, C.A., Jr.; Hasegawa, K.; et al. Longitudinal Changes in Early Nasal Microbiota and the Risk of Childhood Asthma. Pediatrics 2020, 146, e20200421. [Google Scholar] [CrossRef]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A. Distinct Nasal Airway Bacterial Microbiota Differentially Relate to Exacerbation in Pediatric Asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef]
- Depner, M.; Ege, M.J.; Cox, M.J.; Dwyer, S.; Walker, A.W.; Birzele, L.T. Bacterial microbiota of the upper respiratory tract and childhood asthma. J. Allergy Clin. Immunol. 2017, 139, 826–834.e13. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Hermansen, M.N.; Buchvald, F.; Loland, L.; Halkjaer, L.B.; Bønnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Thorsen, S.V.; et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, L.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, e100645. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Ma, Q.; Zhang, Q.; Wang, C. Neutrophilic Asthma Is Associated with Increased Airway Bacterial Burden and Disordered Community Composition. BioMed Res. Int. 2018, 2018, 9230234. [Google Scholar] [CrossRef] [PubMed]
- Versi, A.; Ivan, F.X.; Abdel-Aziz, M.I.; Bates, S.; Riley, J.; Baribaud, F.; Kermani, N.Z.; Montuschi, P.; Dahlen, S.E.; Djukanovic, R.; et al. Haemophilus influenzae and Moraxella catarrhalis in sputum of severe asthma with inflammasome and neutrophil activation. Allergy 2023, 78, 2906–2920. [Google Scholar] [CrossRef]
- Fraga-Silva, T.F.C.; Boko, M.M.M.; Martins, N.S.; Cetlin, A.A.; Russo, M.; Vianna, E.O.; Bonato, V.L.D. Asthma-associated bacterial infections: Are they protective or deleterious? J. Allergy Clin. Immunol. Glob. 2022, 2, 14–22. [Google Scholar] [CrossRef]
- Simpson, J.L.; Daly, J.; Baines, K.J.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Hugenholtz, P.; Willner, D.; et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur. Respir. J. 2016, 47, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- Choi, E.B.; Hong, S.W.; Kim, D.K.; Jeon, S.G.; Kim, K.R.; Cho, S.H.; Gho, Y.S.; Jee, Y.K.; Kim, Y.K. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy 2014, 69, 517–526. [Google Scholar] [CrossRef]
- Ruokolainen, L.; Fyhrquist, N.; Laatikainen, T.; Auvinen, P.; Fortino, V.; Scala, G.; Jousilahti, P.; Karisola, P.; Vendelin, J.; Karkman, A.; et al. Immune-microbiota interaction in Finnish and Russian Karelia young people with high and low allergy prevalence. Clin. Exp. Allergy 2020, 50, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Lee, S.W. Application of Microbiome-Based Therapies in Chronic Respiratory Diseases. J. Microbiol. 2024, 62, 201–216. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Sig. Transduct. Target Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar] [CrossRef]
- Taglialegna, A. Fibre-deprived Akkermansia worsens food allergy. Nat. Rev. Microbiol. 2023, 21, 769. [Google Scholar] [CrossRef]
- Tan, X.; Liu, H.; Qiu, W.; Li, Z.; Ge, S.; Luo, Y.; Zeng, N.; Chen, M.; Zhou, Q.; Cai, S.; et al. The nasal microbiota is a potential diagnostic biomarker for sepsis in critical care units. Microbiol. Spectr. 2024, 12, e0344123. [Google Scholar] [CrossRef]
- Dvořák, Z.; Li, H.; Mani, S. Microbial Metabolites as Ligands to Xenobiotic Receptors: Chemical Mimicry as Potential Drugs of the Future. Drug Metab. Dispos. 2023, 51, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Meylan, P.; Lang, C.; Mermoud, S.; Johannsen, A.; Norrenberg, S.; Hohl, D.; Vial, Y.; Prod’hom, G.; Greub, G.; Kypriotou, M.; et al. Skin Colonization by Staphylococcus aureus Precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J. Investig. Dermatol. 2017, 137, 2497–2504. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, aah4680. [Google Scholar] [CrossRef]
- Demessant-Flavigny, A.L.; Connétable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Nelson, D.; Wareham, J. The timing of skin acidification in very low birth weight infants. J. Perinatol. 1998, 18, 272–275. [Google Scholar]
- Zhou, C.; Fey, P.D. The acid response network of Staphylococcus aureus. Curr. Opin. Microbiol. 2020, 55, 67–73. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P. Environmental pH is a key modulator of Staphylococcus aureus biofilm development under predation by the virulent phage phiIPLA-RODI. ISME J. 2021, 15, 245–259. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Deng, L.; Costa, F.; Blake, K.J. S. aureus drives itch and scratch-induced skin damage through a V8 protease-PAR1 axis. Cell 2023, 186, 5375–5393.e5325. [Google Scholar] [CrossRef]
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; Macgowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef]
- Jones, A.L.; Curran-Everett, D.; Leung, D.Y.M. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1247–1248.e1243. [Google Scholar] [CrossRef]
- Tsilochristou, O.; du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Radulovic, S.; Basting, M.; Plaut, M.; et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J. Allergy Clin. Immunol. 2019, 144, 494–503. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 26, 803–813. [Google Scholar] [CrossRef]
- Muluk, N.B.; Altın, F.; Cingi, C. Role of Superantigens in Allergic Inflammation: Their Relationship to Allergic Rhinitis, Chronic Rhinosinusitis, Asthma, and Atopic Dermatitis. Am. J. Rhinol Allergy 2018, 32, 502–517. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef]
- Clowry, J.; Dempsey, D.J.; Claxton, T.J.; Towell, A.M.; Turley, M.B.; Sutton, M.; Geoghegan, J.A.; Kezic, S.; Jakasa, I.; White, A.; et al. Distinct T cell signatures are associated with Staphylococcus aureus skin infection in pediatric atopic dermatitis. JCI Insight 2024, 9, e178789. [Google Scholar] [CrossRef]
- Zollner, T.M.; Wichelhaus, T.A.; Hartung, A.; Von Mallinckrodt, C.; Wagner, T.O.; Brade, V.; Kaufmann, R. Colonization with superantigen-producing Staphylococcus aureus is associated with increased severity of atopic dermatitis. Clin. Exp. Allergy. 2000, 30, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Gonzalez, J.; Shemer, A.; Malajian, D.; Xu, H.; Zheng, X.; Khattri, S.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suárez-Fariñas, M.; et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J. Allergy Clin. Immunol. 2015, 136, 104–115.e7. [Google Scholar] [CrossRef] [PubMed]
- DeVore, S.B.; Gonzalez, T.; Sherenian, M.G.; Herr, A.B.; Khurana Hershey, G.K. On the surface: Skin microbial exposure contributes to allergic disease. Ann. Allergy Asthma Immunol. 2020, 125, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ménard, G.; Bonnaure-Mallet, M.; Donnio, P.Y. Adhesion of Staphylococcus aureus to epithelial cells: An in vitro approach to study interactions within the nasal microbiota. J. Med. Microbiol. 2020, 69, 1253–1261. [Google Scholar] [CrossRef]
- Guryanova, S.; Guryanova, A. sbv IMPROVER: Modern Approach to Systems Biology. Methods Mol Biol. 2017, 1613, 21–29. [Google Scholar] [CrossRef]
- Pirker, A.-L.; Vogl, T. Development of systemic and mucosal immune responses against gut microbiota in early life and implications for the onset of allergies. Front. Allergy 2024, 5, 1439303. [Google Scholar] [CrossRef]
- Namasivayam, A.A.; Morales, A.F.; Lacave, Á.M.; Tallam, A.; Simovic, B.; Alfaro, D.G.; Bobbili, D.R.; Martin, F.; Androsova, G.; Shvydchenko, I.; et al. Community-Reviewed Biological Network Models for Toxicology and Drug Discovery Applications. Gene Regul. Syst. Biol. 2016, 10, 51–66. [Google Scholar] [CrossRef]
- Cereta, A.D.; Oliveira, V.R.; Costa, I.P.; Afonso, J.P.R.; Fonseca, A.L.; de Souza, A.R.T.; Silva, G.A.M.; Mello, D.A.C.P.G.; de Oliveira, L.V.F.; da Palma, R.K. Emerging Cell-Based Therapies in Chronic Lung Diseases: What About Asthma? Front. Pharmacol. 2021, 12, 648506. [Google Scholar] [CrossRef]
- Tap, J.; Mondot, S.; Levenez, F.; Pelletier, E.; Caron, C.; Furet, J.P.; Ugarte, E.; Muñoz-Tamayo, R.; Paslier, D.L.; Nalin, R.; et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 2009, 11, 2574–2584. [Google Scholar] [CrossRef]
- Vuillermin, P.J.; Macia, L.; Nanan, R.; Tang, M.L.; Collier, F.; Brix, S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin. Immunopathol. 2017, 39, 669–675. [Google Scholar] [CrossRef]
- McCoy, K.D.; Köller, Y. New developments providing mechanistic insight into the impact of the microbiota on allergic disease. Clin. Immunol. 2015, 159, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Kou, F.; Men, X.; Liu, Y.; Tang, L.; Wen, S. The Changes in Bacterial Microbiome Associated with Immune Disorder in Allergic Respiratory Disease. Microorganisms 2022, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F.; Balas, I.; Robinson, M.J.; Bakdash, G. Border Control: The Role of the Microbiome in Regulating Epithelial Barrier Function. Cells 2024, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liang, S.; Jin, F. Gut microbiota and healthy longevity. Sci. China Life Sci. 2024, 2. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).