The Effect of Retinoids in Vascular Smooth Muscle Cells: From Phenotyping Switching to Proliferation and Migration
Abstract
1. Introduction
2. Methods
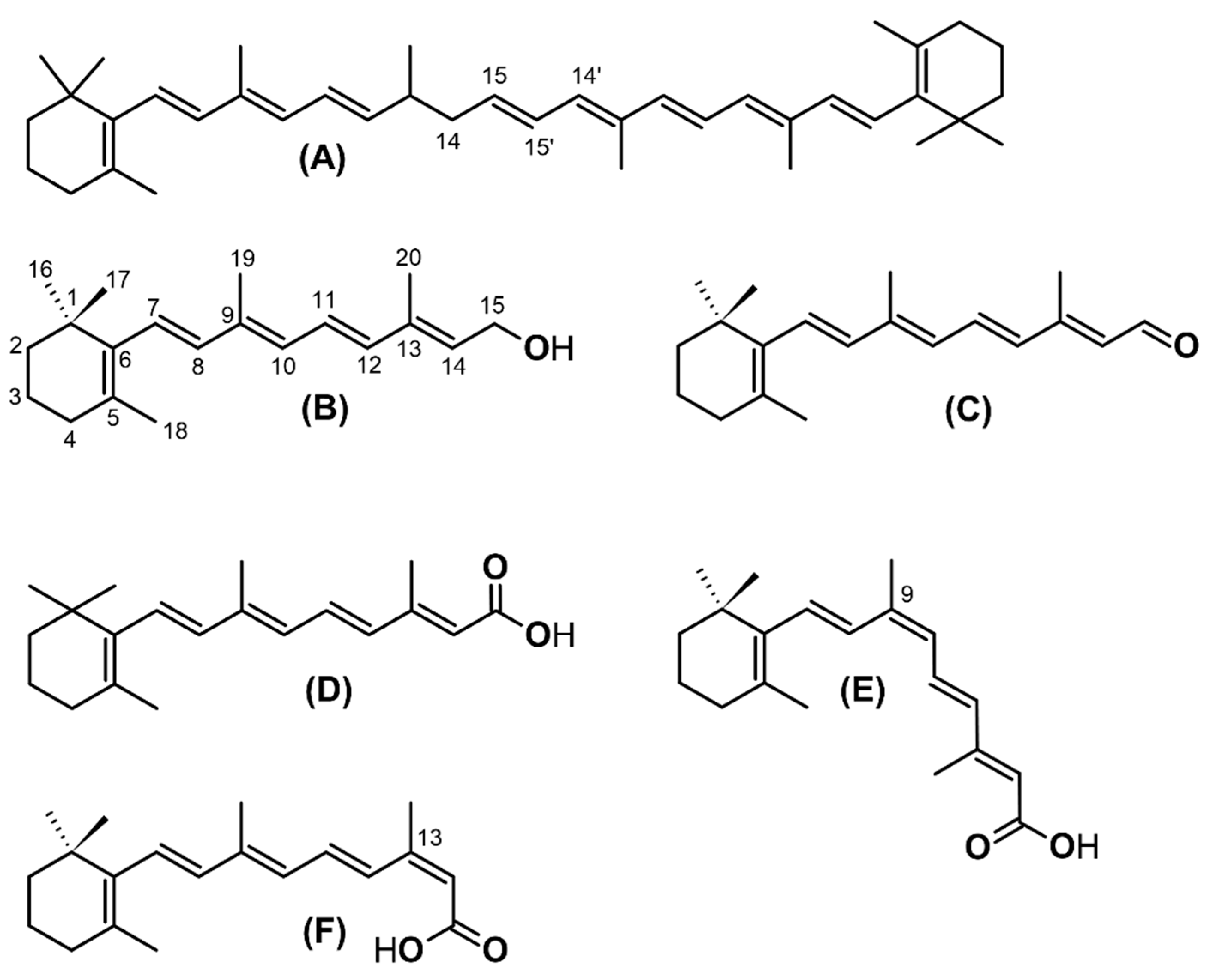
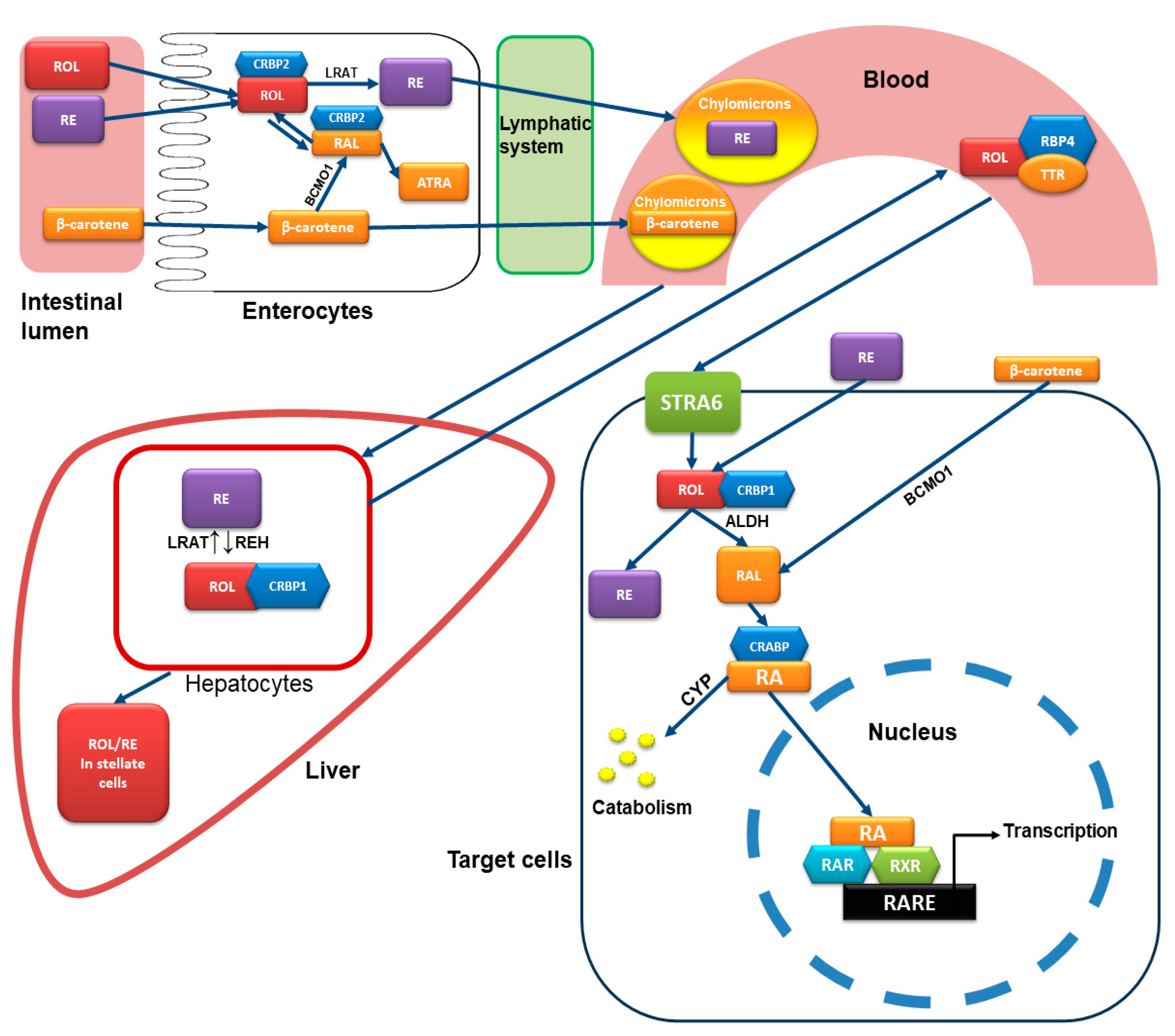
3. Chemistry and Metabolism of Retinoids
4. Mode of Action of Retinoids
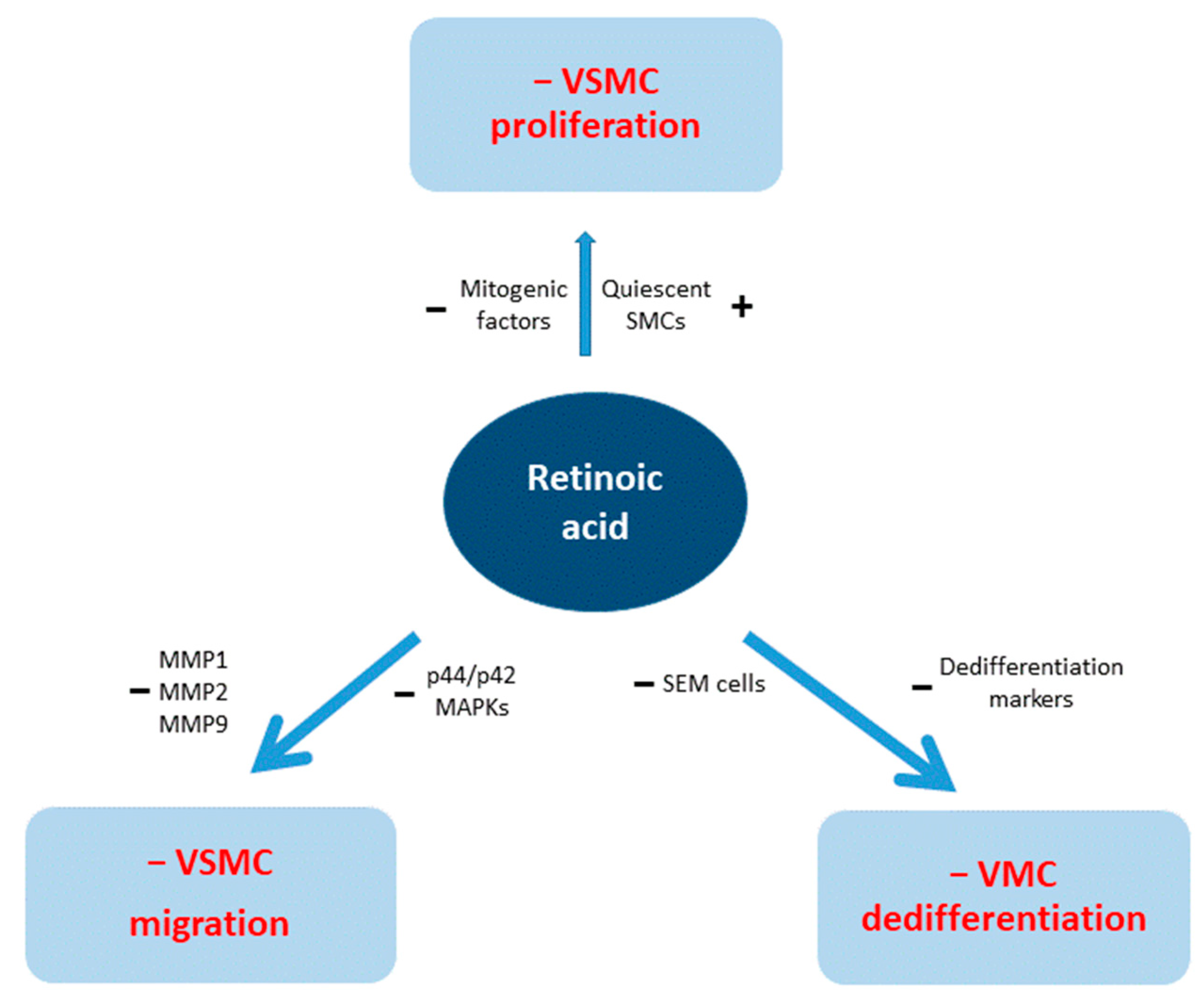
5. RA and VSMCs
5.1. RA and VSMCs Proliferation
5.2. RA and Migration
5.3. RA and Differentiation
6. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Geovanini, G.R.; Libby, P. Atherosclerosis and Inflammation: Overview and Updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. G. Ital. Cardiol. 2024, 25, e1–e112. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The Immune System in Atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Vascular Smooth Muscle Cells in Atherosclerosis: Time for a Re-Assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef]
- Chen, R.; McVey, D.G.; Shen, D.; Huang, X.; Ye, S. Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e031121. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam Cell Formation: A New Target for Fighting Atherosclerosis and Cardiovascular Disease. Vasc. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef]
- Nakatani, M.; Takeyama, Y.; Shibata, M.; Yorozuya, M.; Suzuki, H.; Koba, S.; Katagiri, T. Mechanisms of Restenosis after Coronary Intervention: Difference between Plain Old Balloon Angioplasty and Stenting. Cardiovasc. Pathol. 2003, 12, 40–48. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic Acid Signaling Pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef]
- Zawada, D.; Kornherr, J.; Meier, A.B.; Santamaria, G.; Dorn, T.; Nowak-Imialek, M.; Ortmann, D.; Zhang, F.; Lachmann, M.; Dreßen, M.; et al. Retinoic Acid Signaling Modulation Guides in Vitro Specification of Human Heart Field-Specific Progenitor Pools. Nat. Commun. 2023, 14, 1722. [Google Scholar] [CrossRef]
- Hunsu, V.O.; Facey, C.O.B.; Fields, J.Z.; Boman, B.M. Retinoids as Chemo-Preventive and Molecular-Targeted Anti-Cancer Therapies. Int. J. Mol. Sci. 2021, 22, 7731. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.d.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef] [PubMed]
- Cassim Bawa, F.N.; Gopoju, R.; Xu, Y.; Hu, S.; Zhu, Y.; Chen, S.; Jadhav, K.; Zhang, Y. Retinoic Acid Receptor Alpha (RARα) in Macrophages Protects from Diet-Induced Atherosclerosis in Mice. Cells 2022, 11, 3186. [Google Scholar] [CrossRef]
- Maitra, U.; Parks, J.S.; Li, L. An Innate Immunity Signaling Process Suppresses Macrophage ABCA1 Expression through IRAK-1-Mediated Downregulation of Retinoic Acid Receptor Alpha and NFATc2. Mol. Cell. Biol. 2009, 29, 5989–5997. [Google Scholar] [CrossRef]
- Deng, Q.; Chen, J. Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis. Biomolecules 2022, 12, 869. [Google Scholar] [CrossRef]
- Axel, D.I.; Frigge, A.; Dittmann, J.; Runge, H.; Spyridopoulos, I.; Riessen, R.; Viebahn, R.; Karsch, K.R. All-Trans Retinoic Acid Regulates Proliferation, Migration, Differentiation, and Extracellular Matrix Turnover of Human Arterial Smooth Muscle Cells. Cardiovasc. Res. 2001, 49, 851–862. [Google Scholar] [CrossRef]
- Samara, I.; Katsouras, C.S.; Semertzioglou, A.; Vratimos, A.; Moula, A.I.; Dimitriou, C.A.; Theofanis, M.; Papadimitropoulou, T.; Bouratzis, V.; Karanasiou, G.; et al. Histopathological Evaluation of a Retinoic Acid Eluting Stent in a Rabbit Iliac Artery Model. Sci. Rep. 2022, 12, 13305. [Google Scholar] [CrossRef] [PubMed]
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of Retinoids: Recommendations 1981. Eur. J. Biochem. 1982, 129, 1–5. [Google Scholar]
- Harrison, E.H.; Curley, R.W. Carotenoids and Retinoids: Nomenclature, Chemistry, and Analysis. In The Biochemistry of Retinoid Signaling II; Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2016; Volume 81, pp. 1–19. [Google Scholar] [CrossRef]
- Gudas, L.J. Retinoid Metabolism: New Insights. J. Mol. Endocrinol. 2022, 69, T37–T49. [Google Scholar] [CrossRef]
- Brun, P.-J.; Yang, K.J.Z.; Lee, S.-A.; Yuen, J.J.; Blaner, W.S. Retinoids: Potent Regulators of Metabolism. BioFactors 2013, 39, 151–163. [Google Scholar] [CrossRef]
- Lerner, U.H. Vitamin A—Discovery, Metabolism, Receptor Signaling and Effects on Bone Mass and Fracture Susceptibility. Front. Endocrinol. 2024, 15, 1298851. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L.; Yoo, H.S. Retinoid Metabolism and Functions Mediated by Retinoid Binding-Proteins. Methods Enzymol. 2020, 637, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and Retinoid Signaling: Genomic and Nongenomic Effects. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- le Maire, A.; Alvarez, S.; Shankaranarayanan, P.; de Lera, A.R.; Bourguet, W.; Gronemeyer, H. Retinoid Receptors and Therapeutic Applications of RAR/RXR Modulators. Curr. Top. Med. Chem. 2012, 12, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J. Synthetic Retinoids Beyond Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Samara, I.; Moulas, A.N.; Karanasiou, G.; Papadimitropoulou, T.; Fotiadis, D.; Michalis, L.K.; Katsouras, C.S. Is It Time for a Retinoic Acid-Eluting Stent or Retinoic Acid-Coated Balloon? Insights from Experimental Studies of Systemic and Local Delivery of Retinoids. Hell. J. Cardiol. 2024, 76, 75–87. [Google Scholar] [CrossRef]
- Peclo, M.M.; Printseva, O.Y. Retinoic Acid Enhances the Proliferation of Smooth Muscle Cells. Experientia 1987, 43, 196–198. [Google Scholar] [CrossRef]
- Neuville, P.; Yan, Z.; Gidlöf, A.; Pepper, M.S.; Hansson, G.K.; Gabbiani, G.; Sirsjö, A. Retinoic Acid Regulates Arterial Smooth Muscle Cell Proliferation and Phenotypic Features in Vivo and in Vitro through an RARalpha-Dependent Signaling Pathway. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1430–1436. [Google Scholar] [CrossRef][Green Version]
- Tran-Lundmark, K.; Tannenberg, P.; Rauch, B.H.; Ekstrand, J.; Tran, P.-K.; Hedin, U.; Kinsella, M.G. Perlecan Heparan Sulfate Is Required for the Inhibition of Smooth Muscle Cell Proliferation by All-Trans-Retinoic Acid. J. Cell. Physiol. 2015, 230, 482–487. [Google Scholar] [CrossRef]
- Streb, J.W.; Long, X.; Lee, T.-H.; Sun, Q.; Kitchen, C.M.; Georger, M.A.; Slivano, O.J.; Blaner, W.S.; Carr, D.W.; Gelman, I.H.; et al. Retinoid-Induced Expression and Activity of an Immediate Early Tumor Suppressor Gene in Vascular Smooth Muscle Cells. PLoS ONE 2011, 6, e18538. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, B.; Jiang, X.; Cai, M.; Liu, N.; Zhang, S.; Tan, Y.; Huang, G.; Jin, W.; Liu, B.; et al. All-Trans-Retinoic Acid Suppresses Neointimal Hyperplasia and Inhibits Vascular Smooth Muscle Cell Proliferation and Migration via Activation of AMPK Signaling Pathway. Front. Pharmacol. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Choi, Y.-K.; Han, Y.-H.; Kim, H.-J.; Lee, I.-K.; Lee, M.-O. RORα Suppresses Proliferation of Vascular Smooth Muscle Cells through Activation of AMP-Activated Protein Kinase. Int. J. Cardiol. 2014, 175, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Bilbija, D.; Elmabsout, A.A.; Sagave, J.; Haugen, F.; Bastani, N.; Dahl, C.P.; Gullestad, L.; Sirsjö, A.; Blomhoff, R.; Valen, G. Expression of Retinoic Acid Target Genes in Coronary Artery Disease. Int. J. Mol. Med. 2014, 33, 677–686. [Google Scholar] [CrossRef]
- Aizawa, K.; Suzuki, T.; Kada, N.; Ishihara, A.; Kawai-Kowase, K.; Matsumura, T.; Sasaki, K.; Munemasa, Y.; Manabe, I.; Kurabayashi, M.; et al. Regulation of Platelet-Derived Growth Factor-A Chain by Krüppel-like Factor 5: New Pathway of Cooperative Activation with Nuclear Factor-kappaB. J. Biol. Chem. 2004, 279, 70–76. [Google Scholar] [CrossRef]
- Sakamoto, H.; Sakamaki, T.; Kanda, T.; Hoshino, Y.; Sawada, Y.; Sato, M.; Sato, H.; Oyama, Y.; Nakano, A.; Takase, S.; et al. Smooth Muscle Cell Outgrowth from Coronary Atherectomy Specimens in Vitro Is Associated with Less Time to Restenosis and Expression of a Key Transcription Factor KLF5/BTEB2. Cardiology 2003, 100, 80–85. [Google Scholar] [CrossRef]
- Shindo, T.; Manabe, I.; Fukushima, Y.; Tobe, K.; Aizawa, K.; Miyamoto, S.; Kawai-Kowase, K.; Moriyama, N.; Imai, Y.; Kawakami, H.; et al. Krüppel-like Zinc-Finger Transcription Factor KLF5/BTEB2 Is a Target for Angiotensin II Signaling and an Essential Regulator of Cardiovascular Remodeling. Nat. Med. 2002, 8, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, B.; Han, M.; Miao, S.; Wen, J. Synthetic Retinoid Am80 Inhibits Interaction of KLF5 with RAR Alpha through Inducing KLF5 Dephosphorylation Mediated by the PI3K/Akt Signaling in Vascular Smooth Muscle Cells. FEBS Lett. 2009, 583, 1231–1236. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Fei, X.; Song, Z.; Xie, F.; Yang, F.; Liu, X.; Xu, Z.; Wang, G. All-Trans Retinoic Acid Prevented Vein Grafts Stenosis by Inhibiting Rb-E2F Mediated Cell Cycle Progression and KLF5-RARα Interaction in Human Vein Smooth Muscle Cells. Cardiovasc. Drugs Ther. 2021, 35, 103–111. [Google Scholar] [CrossRef]
- Zheng, B.; Han, M.; Bernier, M.; Zhang, X.; Meng, F.; Miao, S.; He, M.; Zhao, X.; Wen, J. Krüppel-like Factor 4 Inhibits Proliferation by Platelet-Derived Growth Factor Receptor Beta-Mediated, Not by Retinoic Acid Receptor Alpha-Mediated, Phosphatidylinositol 3-Kinase and ERK Signaling in Vascular Smooth Muscle Cells. J. Biol. Chem. 2009, 284, 22773–22785. [Google Scholar] [CrossRef]
- Miano, J.M.; Topouzis, S.; Majesky, M.W.; Olson, E.N. Retinoid Receptor Expression and All-Trans Retinoic Acid-Mediated Growth Inhibition in Vascular Smooth Muscle Cells. Circulation 1996, 93, 1886–1895. [Google Scholar] [CrossRef]
- Hayashi, A.; Suzuki, T.; Tajima, S. Modulations of Elastin Expression and Cell Proliferation by Retinoids in Cultured Vascular Smooth Muscle Cells. J. Biochem. 1995, 117, 132–136. [Google Scholar] [CrossRef]
- Pakala, R.; Benedict, C.R. RAR Gamma Agonists Inhibit Proliferation of Vascular Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2000, 35, 302–308. [Google Scholar] [CrossRef]
- Takeda, K.; Ichiki, T.; Funakoshi, Y.; Ito, K.; Takeshita, A. Downregulation of Angiotensin II Type 1 Receptor by All-Trans Retinoic Acid in Vascular Smooth Muscle Cells. Hypertension 2000, 35, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gardner, D.G. Retinoic Acid Uses Divergent Mechanisms to Activate or Suppress Mitogenesis in Rat Aortic Smooth Muscle Cells. J. Clin. Investig. 1998, 102, 653–662. [Google Scholar] [CrossRef]
- Kosaka, C.; Sasaguri, T.; Komiyama, Y.; Takahashi, H. All-Trans Retinoic Acid Inhibits Vascular Smooth Muscle Cell Proliferation Targeting Multiple Genes for Cyclins and Cyclin-Dependent Kinases. Hypertens. Res. 2001, 24, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Kintscher, U.; Kim, S.; Jackson, S.; Yin, F.; Nagpal, S.; Chandraratna, R.A.; Hsueh, W.A.; Law, R.E. Retinoids Inhibit Proliferation of Human Coronary Smooth Muscle Cells by Modulating Cell Cycle Regulators. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 746–751. [Google Scholar] [CrossRef]
- Kato, S.; Sasaguri, Y.; Morimatsu, M. Down-Regulation in the Production of Matrix Metalloproteinase 1 by Human Aortic Intimal Smooth Muscle Cells. Biochem. Mol. Biol. Int. 1993, 31, 239–248. [Google Scholar]
- Suganuma, E.; Sato, S.; Honda, S.; Nakazawa, A. All Trans Retinoic Acid Alleviates Coronary Stenosis by Regulating Smooth Muscle Cell Function in a Mouse Model of Kawasaki Disease. Sci. Rep. 2021, 11, 13856. [Google Scholar] [CrossRef] [PubMed]
- Johst, U.; Betsch, A.; Wiskirchen, J.; Schöber, W.; Vonthein, R.; Rinkert, N.; Kehlbach, R.; Claussen, C.D.; Duda, S.H. All-Trans and 9-Cis Retinoid Acids Inhibit Proliferation, Migration, and Synthesis of Extracellular Matrix of Human Vascular Smooth Muscle Cells by Inducing Differentiation in Vitro. J. Cardiovasc. Pharmacol. 2003, 41, 526–535. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, J.; Han, B.; Liu, J.; Xiang, X.; Zhang, M.; Xia, S.; Zhang, W.; Zhang, X. Novel Zinc Finger Transcription Factor ZFP580 Facilitates All-Trans Retinoic Acid -Induced Vascular Smooth Muscle Cells Differentiation by Rarα-Mediated PI3K/Akt and ERK Signaling. Cell. Physiol. Biochem. 2018, 50, 2390–2405. [Google Scholar] [CrossRef]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Shi, Y.; Niculescu, R.; Chung, E.H.; Martin, J.L.; Zalewski, A. Characteristics of Coronary Smooth Muscle Cells and Adventitial Fibroblasts. Circulation 2000, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, P.J.; Barry, W.L.; McPherson, J.A.; McNamara, C.A.; Gimple, L.W.; Sanders, J.M.; Bishop, G.G.; Powers, E.R.; Ragosta, M.; Owens, G.K.; et al. All-Trans-Retinoic Acid Limits Restenosis after Balloon Angioplasty in the Focally Atherosclerotic Rabbit: A Favorable Effect on Vessel Remodeling. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Herdeg, C.; Oberhoff, M.; Baumbach, A.; Schroeder, S.; Leitritz, M.; Blattner, A.; Siegel-Axel, D.I.; Meisner, C.; Karsch, K.R. Effects of Local All-Trans-Retinoic Acid Delivery on Experimental Atherosclerosis in the Rabbit Carotid Artery. Cardiovasc. Res. 2003, 57, 544–553. [Google Scholar] [CrossRef][Green Version]
- Colbert, M.C.; Kirby, M.L.; Robbins, J. Endogenous Retinoic Acid Signaling Colocalizes with Advanced Expression of the Adult Smooth Muscle Myosin Heavy Chain Isoform during Development of the Ductus Arteriosus. Circ. Res. 1996, 78, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Lindschau, C.; Quass, P.; Distler, A.; Luft, F.C. Differentiation of Vascular Smooth Muscle Cells and the Regulation of Protein Kinase C-Alpha. Circ. Res. 1995, 76, 21–29. [Google Scholar] [CrossRef]
- Gollasch, M.; Haase, H.; Ried, C.; Lindschau, C.; Morano, I.; Luft, F.C.; Haller, H. L-Type Calcium Channel Expression Depends on the Differentiated State of Vascular Smooth Muscle Cells. FASEB J. 1998, 12, 593–601. [Google Scholar] [CrossRef]
- Rogers, M.A.; Chen, J.; Nallamshetty, S.; Pham, T.; Goto, S.; Muehlschlegel, J.D.; Libby, P.; Aikawa, M.; Aikawa, E.; Plutzky, J. Retinoids Repress Human Cardiovascular Cell Calcification With Evidence for Distinct Selective Retinoid Modulator Effects. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, M.; Zhao, X.-M.; Wen, J.-K. Kruppel-like Factor 4 Is Required for the Expression of Vascular Smooth Muscle Cell Differentiation Marker Genes Induced by All-Trans Retinoic Acid. J. Biochem. 2008, 144, 313–321. [Google Scholar] [CrossRef]
- Meng, F.; Han, M.; Zheng, B.; Wang, C.; Zhang, R.; Zhang, X.; Wen, J. All-Trans Retinoic Acid Increases KLF4 Acetylation by Inducing HDAC2 Phosphorylation and Its Dissociation from KLF4 in Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2009, 387, 13–18. [Google Scholar] [CrossRef]
- Yu, K.; Zheng, B.; Han, M.; Wen, J. ATRA Activates and PDGF-BB Represses the SM22α Promoter through KLF4 Binding to, or Dissociating from, Its Cis-DNA Elements. Cardiovasc. Res. 2011, 90, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, B.; Chen, S.; Ma, G.; Wen, J. Retinoic Acid Receptor α Mediates All-Trans-Retinoic Acid-Induced Klf4 Gene Expression by Regulating Klf4 Promoter Activity in Vascular Smooth Muscle Cells. J. Biol. Chem. 2012, 287, 10799–10811. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M.; O’Brien, E.R.; DeBlois, D.; Giachelli, C.M. Relevance of Smooth Muscle Replication and Development to Vascular Disease. In The Vascular Smooth Muscle Cell; Elsevier: Amsterdam, The Netherlands, 1995; pp. 81–139. ISBN 978-0-12-632310-8. [Google Scholar]
- Muller, D.W.M.; Ellis, S.G.; Topol, E.J. Experimental Models of Coronary Artery Restenosis. J. Am. Coll. Cardiol. 1992, 19, 418–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Christen, T.; Bochaton-Piallat, M.-L.; Neuville, P.; Rensen, S.; Redard, M.; Van Eys, G.; Gabbiani, G. Cultured Porcine Coronary Artery Smooth Muscle Cells: A New Model With Advanced Differentiation. Circ. Res. 1999, 85, 99–107. [Google Scholar] [CrossRef]
- Patatanian, E.; Thompson, D.F. Retinoic Acid Syndrome: A Review. J. Clin. Pharm. Ther. 2008, 33, 331–338. [Google Scholar] [CrossRef]
- Gregory, E.K.; Webb, A.R.; Vercammen, J.M.; Flynn, M.E.; Ameer, G.A.; Kibbe, M.R. Periadventitial atRA Citrate-Based Polyester Membranes Reduce Neointimal Hyperplasia and Restenosis after Carotid Injury in Rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1419–H1429. [Google Scholar] [CrossRef]
- Gregory, E.K.; Webb, A.; Vercammen, J.M.; Kelly, M.E.; Akar, B.; Van Lith, R.; Bahnson, E.M.; Jiang, W.; Ameer, G.A.; Kibbe, M.R. Inhibiting Intimal Hyperplasia in Prosthetic Vascular Grafts via Immobilized All-Trans Retinoic Acid. J. Control. Release 2018, 274, 69–80. [Google Scholar] [CrossRef]
| First Author (Year) | Experimental Model | Agent, Dose of Administration | Main Results | Other Results |
|---|---|---|---|---|
| Peclo M.M (1987) [28] | Cultured rat aortic SMC | RA 10−5–10−7 M | • Enhancement of proliferation of SMC • Delay of the exit of cells from the proliferative cycle • Higher saturation density of the culture | • Increased expression of L1 |
| Neuville P. (1999) [29] | Cultured aortic media and intimal thickening rat SMC, angioplasty of rat carotid artery and thoracic aorta | 10−6 final concentration, 0.5 mg/kg intraperitoneally/day for 14 days of RA | • Medial and intimal thickening inhibition • Proliferation inhibition by the presence of CRBP-1 • Increased migration and tissue-type plasminogen activator activity • Decreased α-smooth muscle actin in SMC cultured from the IT | • Reduced the intimal hyperplasia in the carotid artery in vivo |
| Tran-Lundmark K. (2015) [30] | Murine SMC cultures | 2 μg/mL ATRA | • Regulation of SMC growth through upregulation of perlecan expression | • Activation and phosphorylation of PTEN and Akt in wild-type and mutant SMCs |
| Streb JW. (2011) [31] | Rat pulmonary artery SMC, rat aortic SMC, human coronary artery SMC, adult male mice model | In vitro: 2 × 10−6 of RA, 13-cis RA In vivo: 10 mg/kg ATRA | • Attenuation of SMC growth through inducible expression of AKAP12β via PKA-mediated signaling | |
| Zhang J. (2019) [32] | Common carotid artery ligation mouse model | 10 mg/kg and 20 mg/kg of RA for 21 days | • Inhibition of neointima hyperplasia • Suppression of SMC proliferation and migration through direct activation of AMPK and inhibition of mTOR signaling | • Alteration of expression of proliferation-related in proteins (Cyclin D1, Cyclin D3, Cyclin A2, CDK4, CDK6) |
| Kim EJ. (2014) [33] | Human aortic SMC rat A7r5 Balloon injury rats | RORa, cholesterol sulfate | • Activation of AMPK-mTOR-S6K1 signaling pathway • Suppression of neointima formation after ballon injury | • Modulate the expression of cell-cycle-regulating factors and induces G1 arrest |
| Bilbija D. (2014) [34] | Human SMC | 1 μM ATRA | • Inhibition of proliferation through expression of RA-target genes (RBP1, STRA6, CYP26B1, CRABP1 and RARβ) | |
| Zhang X. (2009) [38] | thoracic aorta VSMC rat model Ballon injury rats | 2 μmol/LAm80 1 mg/kg/d | • Suppression of interaction between KLF5-RARa through dephosphorylation in VSMCs induced by the activation of PI3/Akt and p38 MAPK signaling pathways | • Induction of RARa expression • Inhibition of KLF5 |
| Yu Y. (2021) [39] | rabbit autogenous vein graft model | 10 mg/kg/day ATRA | • Decrease in intima-media thickening, • Reduced expression of Ki67 inhibition of the growth activity of HUVSMCs mediated by the Rb-E2F pathway | • Reduction in KLF5-RARa interaction • Inhibition of the iNO expression |
| Zheng B. (2009) [40] | Rat aortic VSMC | 10 μΜ Am80, Am50 | • Suppression of RARa expression and inhibition of PI3K/ERK signaling | • Klf4 inhibits the proliferation by PDGFRβ induced by PDGF-BB |
| Miano JM. (1996) [41] | Rat aortic SMC | 2 × 10−6 mol/L | • inhibition of SMC growth by the growth-inhibitory action of PDGF-BB | • Expression of retinoid receptors in rat aortic SMC |
| Hayashi A. (1995) [42] | Chick embryos aortas SMC | 10−6 RA | • Inhibition of cell proliferation through an increase in elastin mRNA levels | |
| Pakala R. (2000) [43] | Canine aortic SCM | RARγ-specific agonist | • Inhibition of serotonin- and serum-induced VSMC proliferation | • 1–10 nM ATRA inhibited serum- and 5-HT-induced 3[H]thymidine incorporation and cell number |
| Takeda K. (2000) [44] | Rat thoracic aorta SMC | 1 mmol/L ATRA | • Downregulation of AT1-R mRNA through de novo protein synthesis-dependent on the RAR/RXR heterodimer | • Reduced AT1-R gene expression |
| Chen S. (1998) [45] | Rat aortic SMC | ARTA, RXR-, RAR-selective agonist | • Suppress mitogenesis and ERK activity mediated by endothelin stimulation | • Stimulates mitogenesis without changing ERK activity • Coincident activation of p21 expression |
| Kosaka C. (2001) [46] | Rat aortic SMC | 1 mmol/l ATRA | • Inhibition of DNA synthesis mediated by basic fibroblast growth factor | • Suppression of the pRb kinase activities of Cdks |
| Wakino S. (2001) [47] | Human coronary SMC | TTNBP, ATRA, AGN4204, 9cRA | • Antiproliferative activity by regulation of G1/S cell cycle via inhibition of Rb phosphorylation | • Inhibition of DNA synthesis by stimulation of platelet-derived growth factor and insulin |
| First Author (Year) | Experimental Model | Agent, Dose of Administration | Main Results | Other Results |
|---|---|---|---|---|
| Kato S. (1993) [48] | human aortic SMCs | • Downregulation of the production of proMMP-1 after treatment with PDGF | ||
| Axel D. (2000) [16] | mono- and transfilter co-cultures of human arteries SMC | 0.01 nM–10.0 mM atRA | • Migration and proliferation inhibition through reduction in MMP-2 and MMP-9 expression | • Decrease in mRNA expression of the glycoproteins thrombospondin-1, fibronectin |
| Suganuma E. (2021) [49] | Male mouse model of Kawasaki disease | 30 mg/kg ATRA, oral administration | • Reduction in elastin break score of external and internal elastin lumina • Suppression of MMP-9 protein | • Lower coronary stenosis and inflammatory scores • Augmentation of their area coverage by migration cells after stimulation by platelet-derived PDGF-BB |
| Johst U. (2003) [50] | human aortic SMC | 10−6 M, 10−7 M, and 10−8 M ATRA, 9cis RA | • Decrease in migrated cells associated with reduced production of tenascin and downregulation of p44/p42 MAPKs | • Increased G1-phase • Stronger expression of α-actin |
| Neuville P. (1999) [29] | Cultured aortic media and intimal thickening rat SMC, angioplasty of rat carotid artery and thoracic aorta | 10−6 final concentration, 0.5 mg/kg intraperitoneally/day for 14 days of RA | • Increased migration and tissue-type plasminogen activator activity | • Reduced the intimal hyperplasia in the carotid artery in vivo |
| First Author (Year) | Experimental Model | Agent, Dose of Administration | Main Results | Other Results |
|---|---|---|---|---|
| Pan H. (2020) [52] | ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice | 10 μM ATRA for 72 h | • Attenuation of SEM cells transition • Suppression of SMC dedifferentiation • Reduction in atherosclerotic burden, • Promoted fibrous cap stability. | |
| Axel D. (2001) [16] | mono- and transfilter co-cultures of human arteries SMC | 0.01 nM–10.0 mM ATRA | • Enhanced a-smooth muscle actin and heavy chain myosin expression | • Decrease in mRNA expression of the glycoproteins thrombospondin-1, fibronectin |
| Neuville P. (1999) [29] | Cultured aortic media and intimal thickening rat SMC, angioplasty of rat carotid artery and thoracic aorta | 10−6 final concentration, 0.5 mg/kg intraperitoneally/day for 14 days of RA | • Decreased α-smooth muscle actin in SMC cultured from the IT | • Reduced the intimal hyperplasia in the carotid artery in vivo |
| Wiegman P.J. (2000) [54] | Balloon angioplasty on rabbits with focal femoral atherosclerosis | 25 mg ATRA for the 3 days before and for 28 days after balloon injury | • More a-actin and desmin immunostaining | • Larger lumen area, internal elastic lamina, and external elastic lamina areas |
| Herdeg C. (2002) [55] | Right carotid artery of rabbits after induction of a fibromuscular plaque | 10 mL, 10 μM ATRA, local delivery with double-balloon catheter | • More intense a-actin staining pattern | • Reduced early neointimal proliferation and extent of stenosis |
| Colbert M. (1996) [56] | Transgenic mouse carrying lacZ transgene | • Inducing and maintaining smooth muscle differentiation in the developing ductus arteriosus • Promote the expression of the adult vascular phenotype | • Appearance of smooth muscle myosin heavy chain isoform | |
| Haller H. (1995) [57] | VSMC of rat aortas | 10−9 mol/L ATRA for 12 and 48 h | • Inhibition of differentiation through increase in PKC-a expression and PKC activity | • Elevated expression of PKC and PKC-a in differentiated cultured SMC |
| Gollasch M. (1998) [58] | rat aortic (A7r5) VSMC cultures | 10−8 M ATRA for 48 h | • Increase in the number of functional Ca2+ channel | • L-type Ca2+ channel is a novel marker for differentiation of VSMC • Correlation of L-type Ca2+ α1-subunits expression with alpha-SMA and SM-MHC expression |
| Rogers M. (2024) [59] | human coronary artery SMC | 1 μmol/L ATRA, 1 μmol/L 9-cis RA | • Inhibition of SMC differentiation into osteoblast-like phenotype | |
| Wang C. (2008) [60] | VSMC of thoracic aorta of male rats | 5, 10 or 20 mM of ATRA | • Inhibition of proliferation and migration of VSMCs through upregulation of KLF4, differentiation marker genes (SM22a, alpha-SMA) and KLF4 target genes p53 | • Downregulation of SMemb |
| Meng F. (2009) [61] | VSMC of thoracic aorta of male rats | 10 μM ATRA | • Induction of HDAC2 phosphorylation mediated by JNK signaling leading to the increase in KLF4 acetylation | • Inhibition of the interaction between KLF4 and HDAC2 |
| Yu K. (2011) [62] | VSMC of thoracic aorta of male rats | 5, 10, or 20 mM ATRA | • Activation of SM22α expression • Stimulation of KLF4 acetylation by induction of KLF4 phosphorylation | |
| Shi J. (2012) [63] | VSMC of thoracic aorta of male rats | 10 μM ATRA | • RARα mediated ATRA-KLF4 expression | |
| Wei S. (2018) [51] | VSMC of thoracic aorta of rats | 0, 5, 10, 20 μmol/L ATRA | • Suppression of dedifferentiation through RARα induced ZFP580 expression via the PI3K/Akt pathways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samara, I.; Moula, A.I.; Moulas, A.N.; Katsouras, C.S. The Effect of Retinoids in Vascular Smooth Muscle Cells: From Phenotyping Switching to Proliferation and Migration. Int. J. Mol. Sci. 2024, 25, 10303. https://doi.org/10.3390/ijms251910303
Samara I, Moula AI, Moulas AN, Katsouras CS. The Effect of Retinoids in Vascular Smooth Muscle Cells: From Phenotyping Switching to Proliferation and Migration. International Journal of Molecular Sciences. 2024; 25(19):10303. https://doi.org/10.3390/ijms251910303
Chicago/Turabian StyleSamara, Ioanna, Amalia I. Moula, Anargyros N. Moulas, and Christos S. Katsouras. 2024. "The Effect of Retinoids in Vascular Smooth Muscle Cells: From Phenotyping Switching to Proliferation and Migration" International Journal of Molecular Sciences 25, no. 19: 10303. https://doi.org/10.3390/ijms251910303
APA StyleSamara, I., Moula, A. I., Moulas, A. N., & Katsouras, C. S. (2024). The Effect of Retinoids in Vascular Smooth Muscle Cells: From Phenotyping Switching to Proliferation and Migration. International Journal of Molecular Sciences, 25(19), 10303. https://doi.org/10.3390/ijms251910303





