The Effects of swnN Gene Function of Endophytic Fungus Alternaria oxytropis OW 7.8 on Its Swainsonine Biosynthesis
Abstract
1. Introduction
2. Results
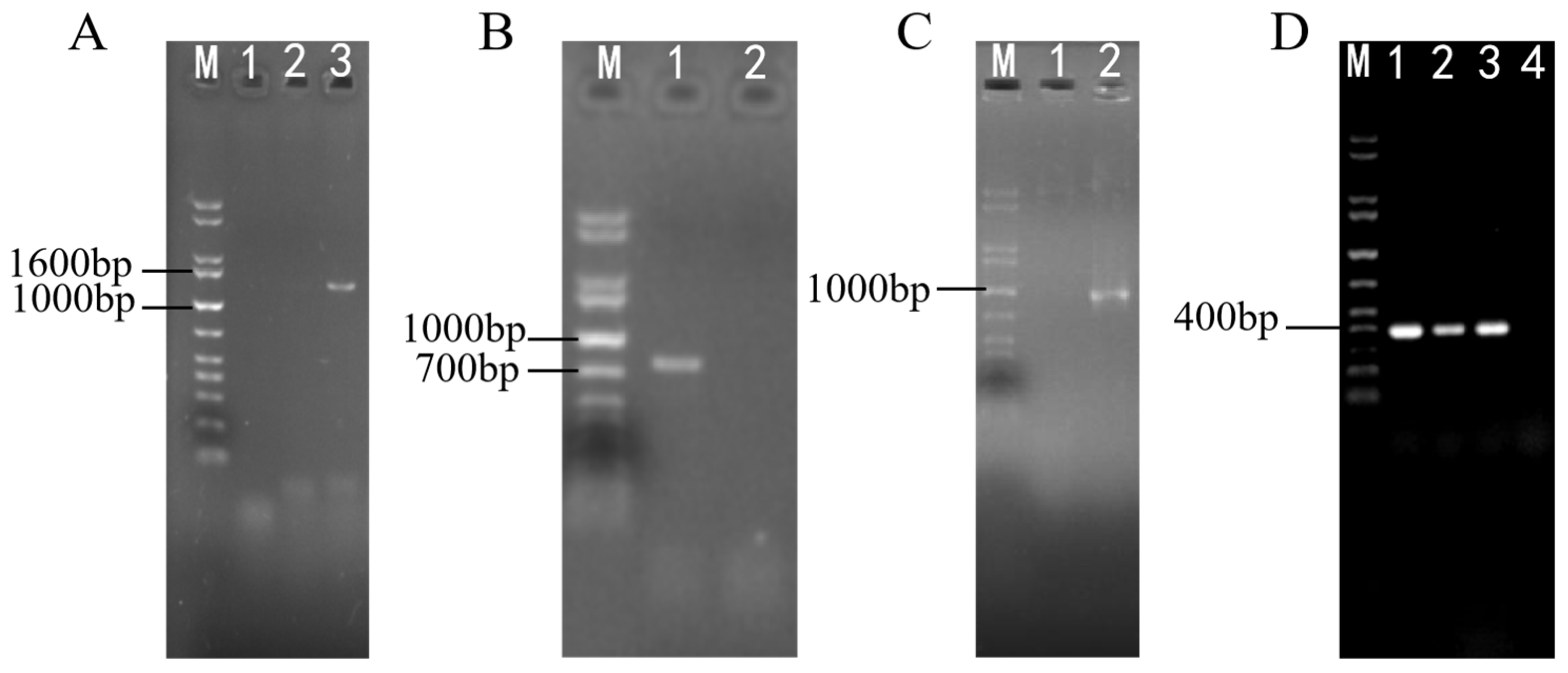
2.1. Cloning and Bioinformatics Analysis of the swnN Gene in A. oxytropis OW 7.8
2.2. Sensitivity Screening of A. oxytropis OW 7.8 to Hyg B
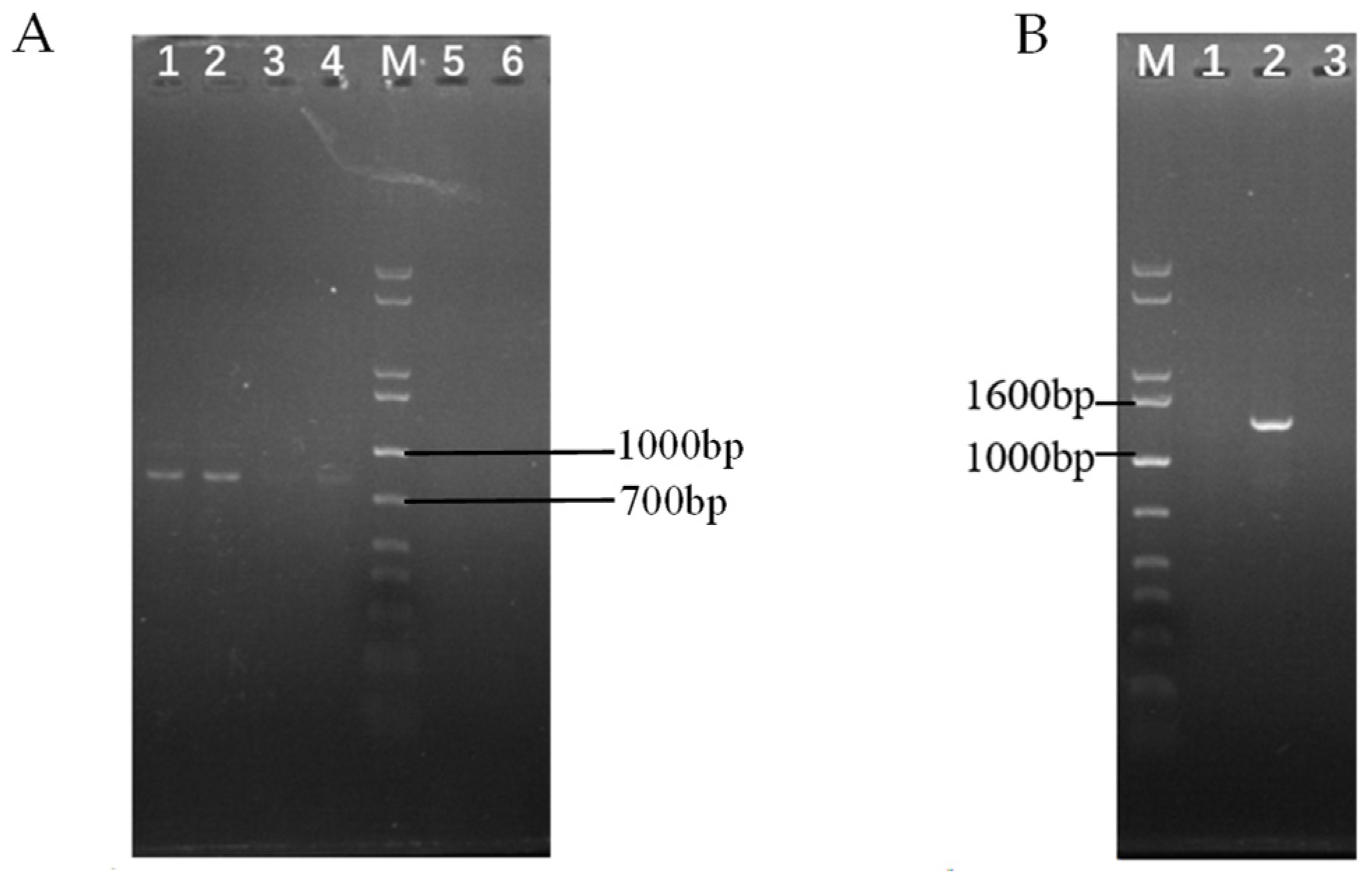
2.3. Identification for Transformants of ΔswnN Colonies
2.4. Morphology of Colonies and Mycelia
2.5. Screening of ΔswnN for Glufosinate Sensitivity
2.6. Screening and Identification of ΔswnN/swnN
2.7. SW Levels in A. oxytropis OW 7.8, ΔswnN, and ΔswnN/swnN Mycelia
2.8. Transcriptome Sequencing Analysis of A. oxytropis OW 7.8 and ΔswnN
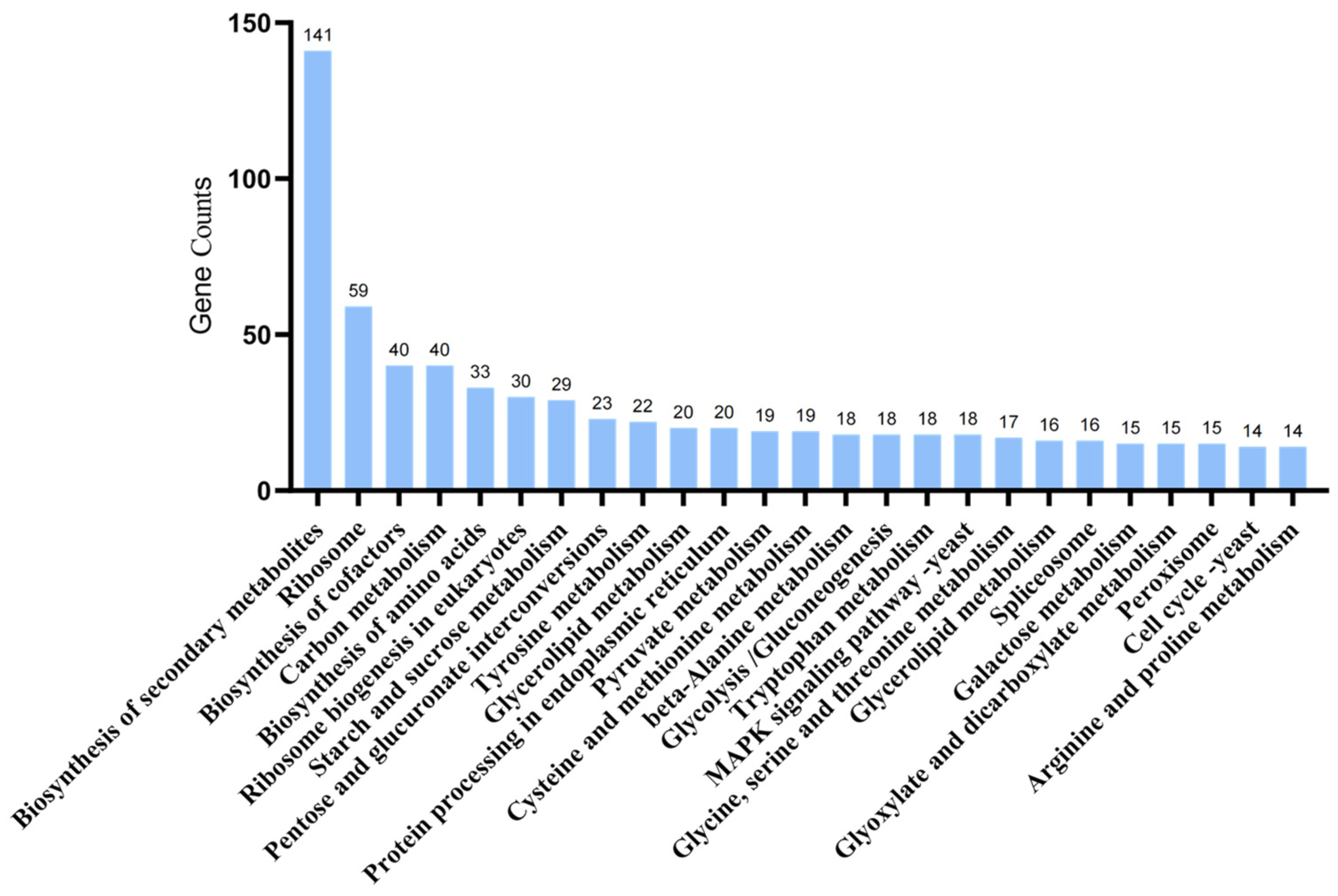
2.9. Metabolomic Analysis of A. oxytropis OW 7.8 and ΔswnN
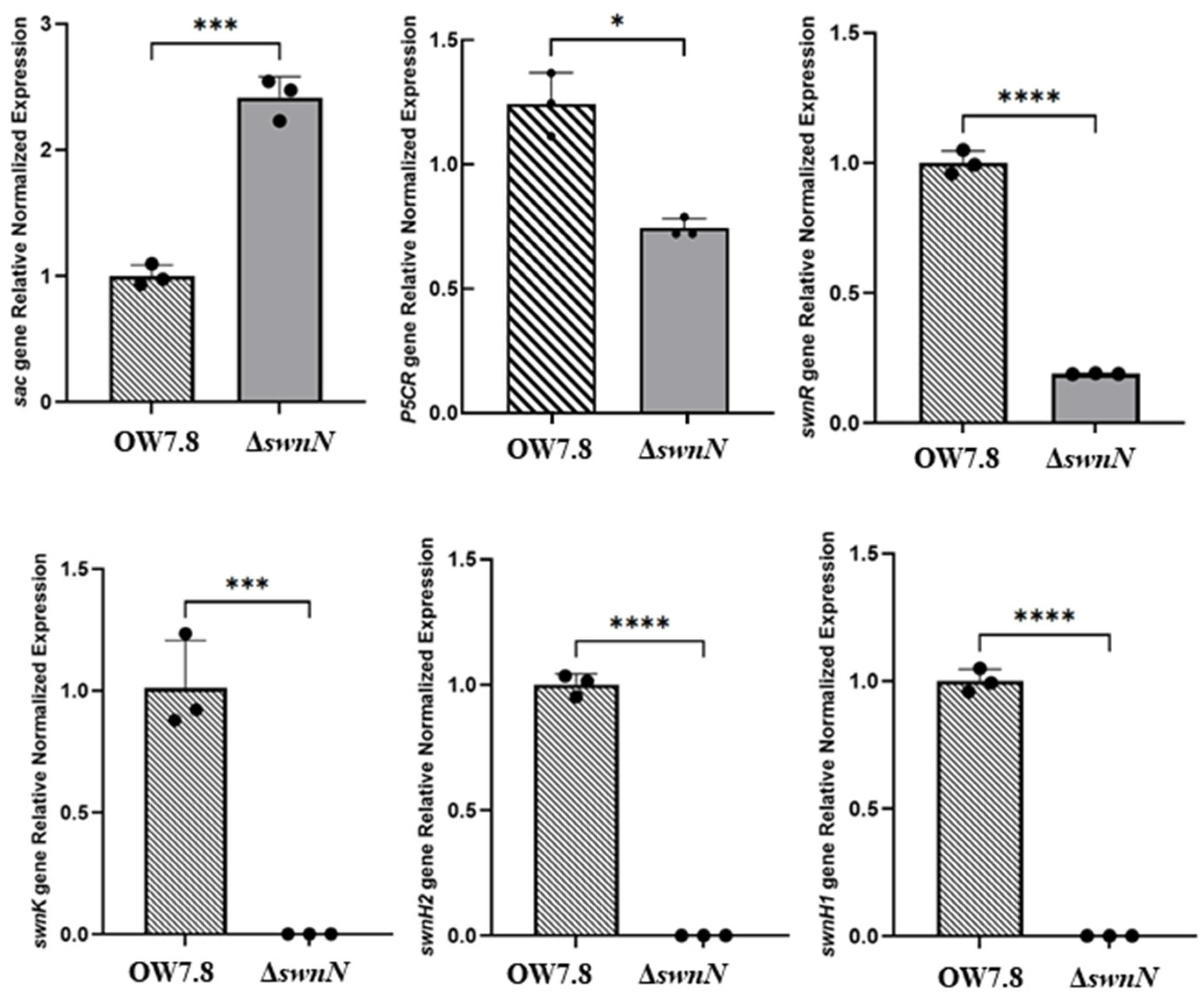
2.10. Expression of sac, P5CR, and SWN Cluster Genes in A. oxytropis OW 7.8 and ΔswnN
2.11. SW Synthesis Pathway in A. oxytropis OW 7.8
3. Discussion
4. Materials and Methods
4.1. Strain
4.2. Extraction of Genomic DNA and Identification of swnN Gene from A. oxytropis OW 7.8
4.3. Total RNA Extraction and swnN cDNA Cloning of A. oxytropis OW 7.8
4.4. Construction of Vectors
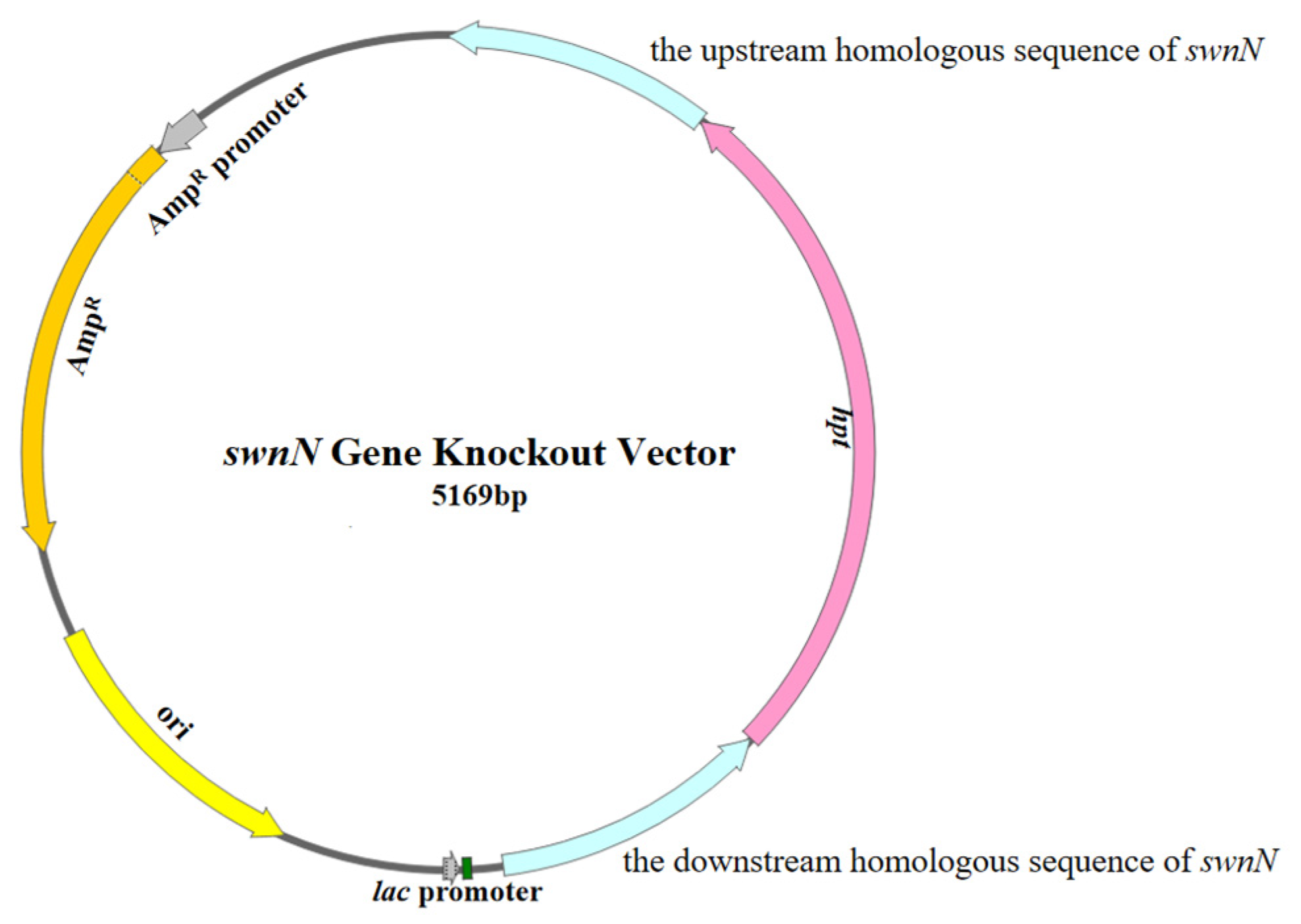
4.4.1. Construction of the swnN Gene Knockout Vector
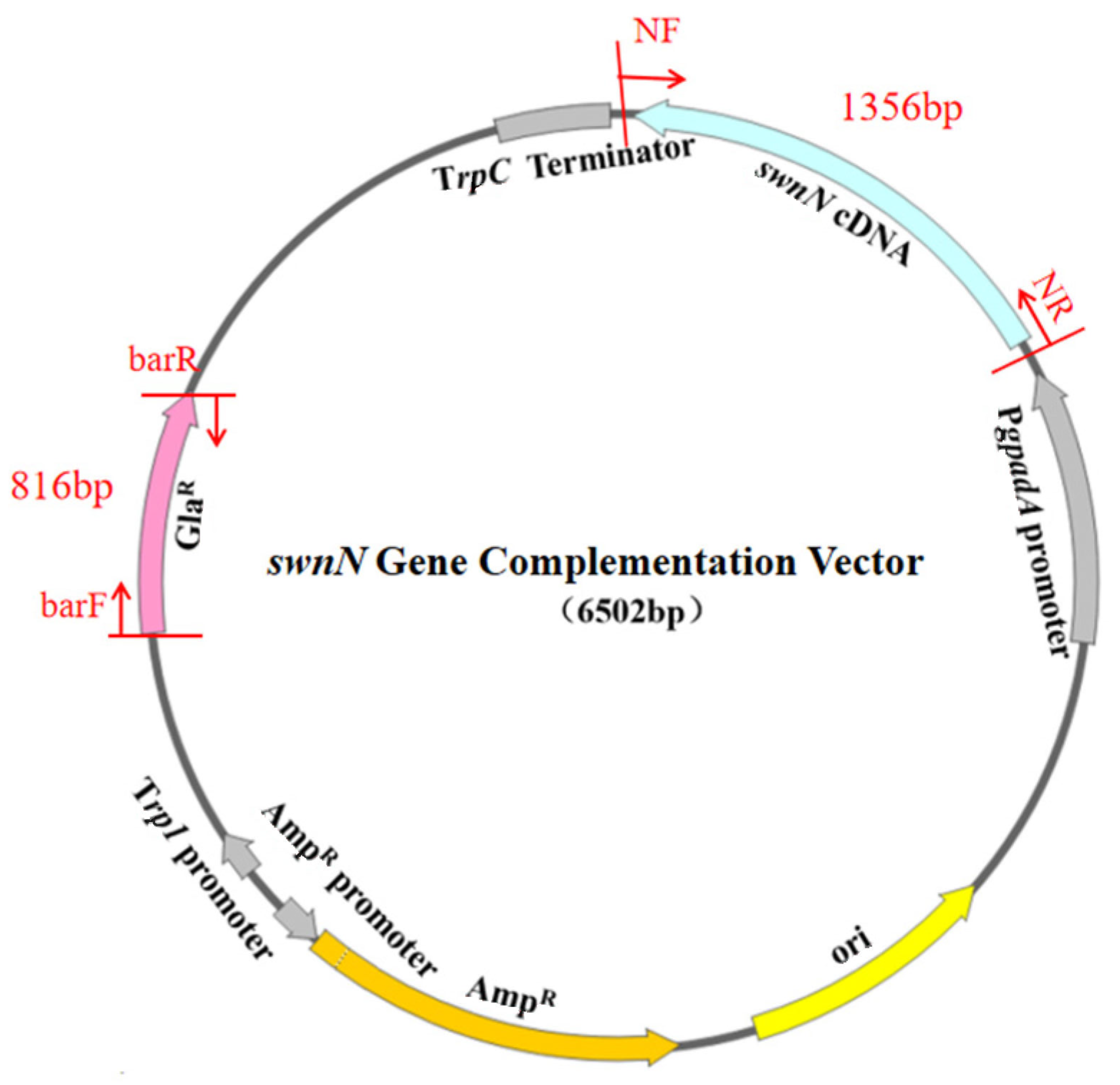
4.4.2. Construction of the swnN Gene Complementation Vector
4.5. Sensitivity Test of A. oxytropis OW 7.8 to Hygromycin B
4.6. Preparation and Transformation of A. oxytropis OW 7.8 Protoplasts
4.7. Screening and Identification of Gene Knockout Transformants
4.8. Sensitivity Test of ΔswnN to Glufosinate
4.9. Preparation and Transformation of ΔswnN Protoplasts
4.10. Screening and Identification of Gene Complementation Transformants
4.11. Colony and Mycelia Morphology
4.12. Extraction and Detection of SW in Mycelia of A. oxytropis OW 7.8, ΔswnN, and ΔswnN/swnN
4.13. Transcriptome Analysis of A. oxytropis OW 7.8 and ΔswnN
4.14. Metabolome Detection and Analysis of A. oxytropis OW 7.8 and ΔswnN
4.15. RT-qPCR Detection of Genes Closely Related to SW Synthesis in A. oxytropis OW 7.8 and ΔswnN
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, L.F.; Foote, W. Estrogenic Properties of Locoweed (Astragalus lentiginosus). Can. J. Comp. Med. 1972, 36, 360–365. [Google Scholar] [PubMed]
- Molyneux, R.J.; McKenzie, R.A.; O’Sullivan, B.M.; Elbein, A.D. Identification of the Glycosidase Inhibitors Swainsonine and Calystegine B2 in Weir Vine (Ipomoea sp. Q6 {AFF. CALOBRA}) and Correlation with Toxicity. J. Nat. Prod. 1995, 58, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, L.; Li, N.; Li, Q.; Li, P.; Fung, K.; Leung, P.; Gao, J. The Healing and Anti-Scar Effects of Astragaloside IV on the Wound Repair in Vitro and in Vivo. J. Ethnopharmacol. 2012, 139, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, W.; Liu, X.; Ma, F.; Cao, D.; Yang, X.; Wang, S.; Geng, P.; Lu, H.; Zhao, B. Pathogenesis and Preventive Treatment for Animal Disease Due to Locoweed Poisoning. Environ. Toxicol. Pharmacol. 2014, 37, 336–347. [Google Scholar]
- Cook, D.; Gardner, D.R.; Lee, S.T.; Pfister, J.A.; Stonecipher, C.A.; Welsh, S.L. A Swainsonine Survey of North American Astragalus and Oxytropis Taxa Implicated as Locoweeds. Toxicon 2016, 118, 104–111. [Google Scholar] [CrossRef]
- Cook, D.; Ralphs, M.H.; Welch, K.D.; Stegelmeier, B.L. Locoweed Poisoning in Livestock. Rangelands 2009, 31, 16–21. [Google Scholar] [CrossRef]
- Fu, J.; Guo, Y.; Huang, W.; Guo, R.; Luo, H.; Wu, C.; Zhao, B. The Distribution of Locoweed in Natural Grassland in the United States and the Current Status and Prospects of Research on Animal Poisoning. Acta Agrestia Sin. 2019, 27, 519–530. [Google Scholar]
- Guo, C.; Zhang, L.; Zhao, Q.; Beckmann, M.; Phillips, H.; Meng, H.; Mo, C.; Mur, L.A.J.; He, W. Host-Species Variation and Environment Influence Endophyte Symbiosis and Mycotoxin Levels in Chinese Oxytropis Species. Toxins 2022, 14, 181. [Google Scholar] [CrossRef]
- Tulsiani, D.R.P.; Broquist, H.P.; James, L.F.; Touster, O. The Similar Effects of Swainsonine and Locoweed on Tissue Glycosidases and Oligosaccharides of the Pig Indicate That the Alkaloid Is the Principal Toxin Responsible for the Induction of Locoism. Arch. Biochem. Biophys. 1984, 232, 76–85. [Google Scholar] [CrossRef]
- Wang, S.; Tan, P.; Wang, H.; Wang, J.; Zhang, C.; Lu, H.; Zhao, B. Swainsonine Inhibits Autophagic Degradation and Causes Cytotoxicity by Reducing CTSD O-GlcNAcylation. Chem. Biol. Interact. 2023, 382, 110629. [Google Scholar] [CrossRef]
- Panter, K.E.; James, L.F.; Stegelmeier, B.L.; Ralphs, M.H.; Pfister, J.A. Locoweeds: Effects on Reproduction in Livestock. J. Nat. Toxins 1999, 8, 53–62. [Google Scholar] [PubMed]
- Dennis, J.W. Effects of Swainsonine and Polyinosinic:Polycytidylic Acid on Murine Tumor Cell Growth and Metastasis. Cancer Res. 1986, 46, 5131–5136. [Google Scholar] [PubMed]
- Stegelmeier, B.L.; James, L.F.; Panter, K.E.; Ralphs, M.H.; Gardner, D.R.; Molyneux, R.J.; Pfister, J.A. The Pathogenesis and Toxicokinetics of Locoweed (Astragalus and Oxytropis spp.) Poisoning in Livestock. J. Nat. Toxins 1999, 8, 35–45. [Google Scholar]
- Molyneux, R.J.; James, L.F. Loco Intoxication: Indolizidine Alkaloids of Spotted Locoweed (Astragalus lentiginosus). Science 1982, 216, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Inamura, N.; Nakahara, K.; Kiyoto, S.; Goto, T.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. Studies of an Immunomodulator, Swainsonine. II. Effect of Swainsonine on Mouse Immunodeficient System and Experimental Murine Tumor. J. Antibiot. 1985, 38, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Romero, J.; Liddell, C.; Creamer, R. Production of Swainsonine by Fungal Endophytes of Locoweed. Mycol. Res. 2003, 107, 980–988. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria Redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Wang, Q.I.; Nagao, H.; Yu-Ling, L.I.; Wang, H.S.; Kakishima, M. Embellisia oxytropis, a New Species Isolated from Oxytropis kansuensis in China. Mycotaxon 2006, 95, 255–260. [Google Scholar]
- Pryor, B.M.; Creamer, R.; Shoemaker, R.A.; McLain-Romero, J.; Hambleton, S. Undifilum, a New Genus for Endophytic Embellisia oxytropis and Parasitic Helminthosporium bornmuelleri on Legumes. Botany 2009, 87, 178–194. [Google Scholar] [CrossRef]
- Wang, W.; Qian, Y.; Lu, P.; He, S.; Du, L.; Li, Y.; Gao, F. The Relationship between Swainsonine and Endophytic Fungi in Different Populations of Oxytropis glabra from Inner Mongolia Chin. J. Anim. Vet. Sci. 2022, 53, 304–314. [Google Scholar]
- Lu, P.; Child, D.; Zhao, M.; Gardener, D.R.; Lv, G.; Han, G. Culture and Identification of Endophytic Fungi from Oxytropis glabra DC. Acta Ecol. Sin. 2009, 29, 53–58. [Google Scholar]
- Cook, D.; Gardner, D.R.; Pfister, J.A. Swainsonine-Containing Plants and Their Relationship to Endophytic Fungi. J. Agric. Food Chem. 2014, 62, 7326–7334. [Google Scholar] [CrossRef] [PubMed]
- Dorling, P.R.; Huxtable, C.R.; Colegate, S.M. Inhibition of Lysosomal Alpha-Mannosidase by Swainsonine, an Indolizidine Alkaloid Isolated from Swainsona canescens. Biochem. J. 1980, 191, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Colegate, S.M.; Dorling, P.R.; Huxtable, C.R. A Spectroscopic Investigation of Swainsonine: An a-Mannosidase Inhibitor Isolated from Swainsona canescens. Aust. J. Chem. 1979, 32, 2257–2264. [Google Scholar] [CrossRef]
- Davis, D.; Schwarz, P.; Hernandez, T.; Mitchell, M.; Warnock, B.; Elbein, A.D. Isolation and Characterization of Swainsonine from Texas Locoweed (Astragalus emoryanus). Plant Physiol. 1984, 76, 972–975. [Google Scholar] [CrossRef]
- Gardner, D.R.; Molyneux, R.J.; Ralphs, M.H. Analysis of Swainsonine: Extraction Methods, Detection, and Measurement in Populations of Locoweeds (Oxytropis spp.). J. Agric. Food Chem. 2001, 49, 4573–4580. [Google Scholar] [CrossRef]
- Colodel, E.M.; Gardner, D.R.; Zlotowski, P.; Driemeier, D. Identification of Swainsonine as a Glycoside Inhibitor Responsible for Sida Carpinifolia Poisoning. Vet. Hum. Toxicol. 2002, 44, 177–178. [Google Scholar]
- Haraguchi, M.; Gorniak, S.L.; Ikeda, K.; Minami, Y.; Kato, A.; Watson, A.A.; Nash, R.J.; Molyneux, R.J.; Asano, N. Alkaloidal Components in the Poisonous Plant, Ipomoea carnea (Convolvulaceae). J. Agric. Food Chem. 2003, 51, 4995–5000. [Google Scholar] [CrossRef]
- Lu, P.; Li, X.; Wang, S. Saccharopine Reductase Influences Production of Swainsonine in Alternaria oxytropis. Sydowia 2021, 73, 69–74. [Google Scholar]
- Wang, Y.; Lu, P.; Sarula; Xi, L.; Yang, B.; Du, L. Construction and Identification of sac Functional Complementary Strain in Alternaria oxytropis. Mol. Plant Breed. 2020, 18, 7777–7783. [Google Scholar]
- Cook, D.; Donzelli, B.G.G.; Creamer, R.; Baucom, D.L.; Gardner, D.R.; Pan, J.; Moore, N.; Krasnoff, S.B.; Jaromczyk, J.W.; Schardl, C.L. Swainsonine Biosynthesis Genes in Diverse Symbiotic and Pathogenic Fungi. G3 2017, 7, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Neyaz, M.; Das, S.; Cook, D.; Creamer, R. Phylogenetic Comparison of Swainsonine Biosynthetic Gene Clusters among Fungi. J Fungi 2022, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Hong, S.; Chen, B.; Yin, Y.; Tang, G.; Hu, F.; Zhang, H.; Wang, C. Unveiling of Swainsonine Biosynthesis via a Multibranched Pathway in Fungi. ACS Chem. Biol. 2020, 15, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Wickwire, B.M.; Harris, C.M.; Harris, T.M.; Broquist, H.P. Pipecolic Acid Biosynthesis in Rhizoctonia leguminicola. I. The Lysine Saccharopine, Delta 1-Piperideine-6-Carboxylic Acid Pathway. J. Biol. Chem. 1990, 265, 14742–14747. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.L.; Perry, D. Analysis of Swainsonine and Its Early Metabolic Precursors in Cultures of Metarhizium anisopliae. Glycoconj. J. 1997, 14, 661–668. [Google Scholar] [CrossRef]
- Li, X.; Lu, P. Transcriptome Profiles of Alternaria oxytropis Provides Insights into Swainsonine Biosynthesis. Sci. Rep. 2019, 9, 6021. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, Z.; Hao, B.; Cheng, F.; Song, X.; Shang, X.; Zhao, H.; Shang, R.; Wang, X.; Liang, J.; et al. Screening of Endophytic Fungi in Locoweed Induced by Heavy-Ion Irradiation and Study on Swainsonine Biosynthesis Pathway. J Fungi 2022, 8, 951. [Google Scholar] [CrossRef]
- Harris, T.M.; Harris, C.M.; Hill, J.E.; Ungemach, F.S.; Broquist, H.P.; Wickwire, B.M. (1S,2R,8aS)-1,2-Dihydroxyindolizidine Formation by Rhizoctonia leguminicola, the Fungus That Produces Slaframine and Swainsonine. J. Org. Chem. 1987, 52, 3094–3098. [Google Scholar] [CrossRef]
- Harris, C.M.; Schneider, M.J.; Ungemach, F.S.; Hill, J.E.; Harris, T.M. Biosynthesis of the Toxic Indolizidine Alkaloids Slaframine and Swainsonine in Rhizoctonia leguminicola: Metabolism of 1-Hydroxyindolizidines. J. Am. Chem. Soc. 1988, 110, 940–949. [Google Scholar] [CrossRef]
- Hu, J.; Lu, P.; Niu, Y. Preparation and Regeneration of Protoplasts Isolated from Embellisia Fungal Endophyte of Oxytropis glabra. Biotechnol. Bull. 2015, 31, 134–139. [Google Scholar]
- Sarula; Xi, L.; Lu, P.; Gao, F.; Du, L. The Influence of Different Additives on the Swainsonine Biosynthesis in Saccharopine Reductase Gene Disruption Mutant M1 of an Endophytic Fungus from Oxytropis glabra. Life Sci. Res. 2018, 22, 298–304. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; Yang, R. An Assessment of AcquireX and Compound Discoverer Software 3.3 for Non-Targeted Metabolomics. Sci. Rep. 2024, 14, 4841. [Google Scholar] [CrossRef]
- Haspel, J.A.; Chettimada, S.; Shaik, R.S.; Chu, J.-H.; Raby, B.A.; Cernadas, M.; Carey, V.; Process, V.; Hunninghake, G.M.; Ifedigbo, E.; et al. Circadian Rhythm Reprogramming during Lung Inflammation. Nat. Commun. 2014, 5, 4753. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic Profiles Delineate Potential Role for Sarcosine in Prostate Cancer Progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, P.; Du, L.; Niu, Y. Selection of Reference Genes in Endophytic Fungi Alternaria oxytropis in Oxytropis glabra and Expression Analysis of Its. Sac. Genom. Appl. Biol. 2019, 38, 5091–5098. [Google Scholar]
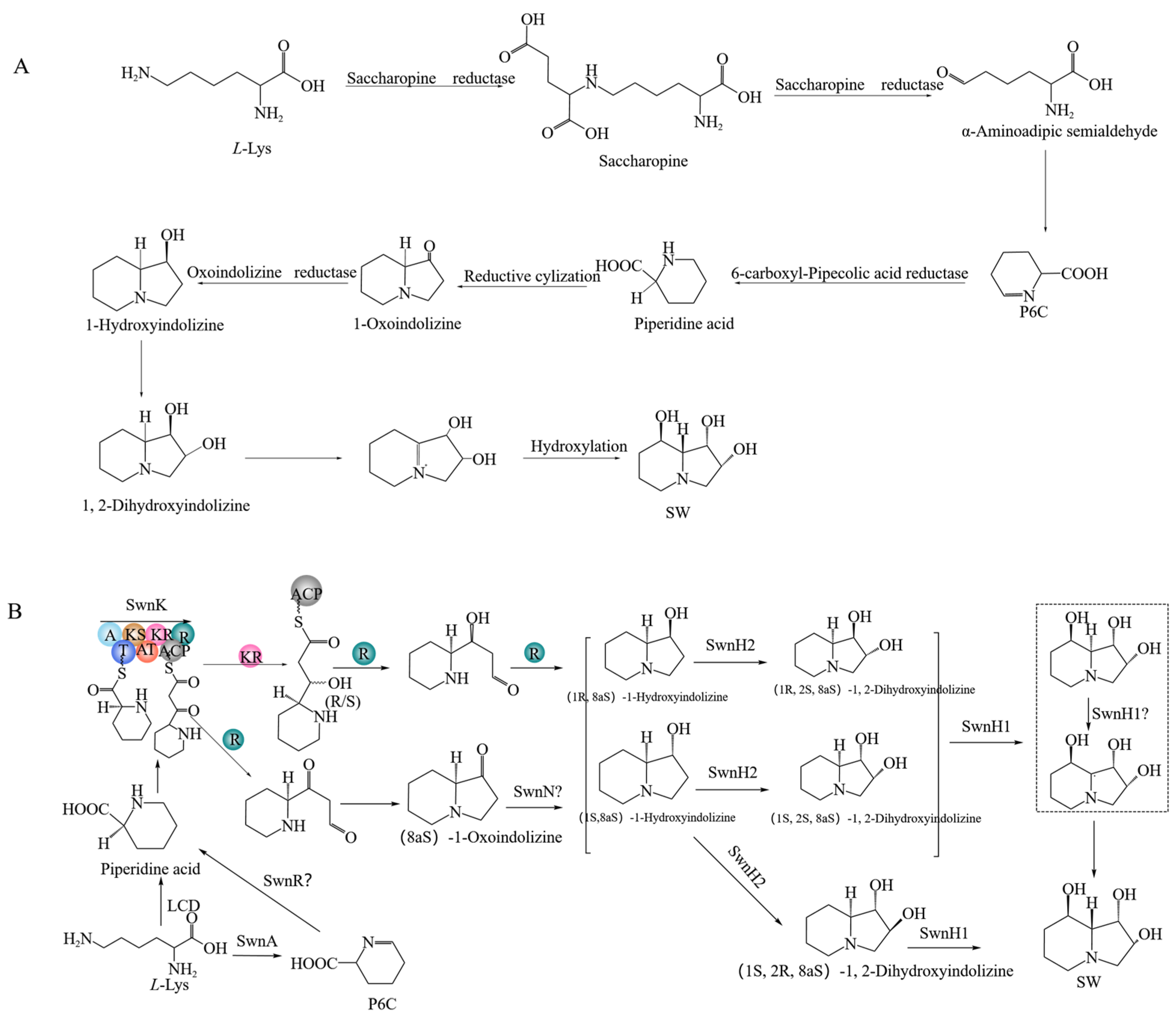








| Gene | Encoding Product | Function Prediction |
|---|---|---|
| swnA | Aminotransferase | Catalyzing the synthesis of Pyrroline-6-carboxylate (P6C) from L-Lysine |
| swnR | Dehydrogenase or reductase | Catalyzing the synthesis of L-PA from P6C |
| swnK | Multifunctional protein | Catalyzing the synthesis of 1-Oxoindolizidine (or 1-Hydroxyindolizine) from L-PA |
| swnN | Dehydrogenase or reductase | Catalyzing the synthesis of 1-Hydroxyindolizine from 1-Oxoindolizidine |
| swnH1 | Fe (II)/α-Ketoglutarate-dependent dioxygenase | Catalyzing the synthesis of SW from 1,2-Dihydroxyindolizine |
| swnH2 | Fe (II)/α-Ketoglutarate-dependent dioxygenase | Catalyzing the synthesis of 1,2-Dihydroxyindolizine form 1-Hydroxyindolizine |
| swnT | Transmembrane transporter | Transport of SW |
| Screening Mode | Total of Metabolites | Total of DEMs | Up-Regulated | Down-Regulated |
|---|---|---|---|---|
| Positive | 801 | 462 | 258 | 204 |
| Negative | 472 | 271 | 172 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ding, N.; Lu, P.; Yuan, B.; Li, Y.; Jiang, K. The Effects of swnN Gene Function of Endophytic Fungus Alternaria oxytropis OW 7.8 on Its Swainsonine Biosynthesis. Int. J. Mol. Sci. 2024, 25, 10310. https://doi.org/10.3390/ijms251910310
Liu C, Ding N, Lu P, Yuan B, Li Y, Jiang K. The Effects of swnN Gene Function of Endophytic Fungus Alternaria oxytropis OW 7.8 on Its Swainsonine Biosynthesis. International Journal of Molecular Sciences. 2024; 25(19):10310. https://doi.org/10.3390/ijms251910310
Chicago/Turabian StyleLiu, Chang, Ning Ding, Ping Lu, Bo Yuan, Yuling Li, and Kai Jiang. 2024. "The Effects of swnN Gene Function of Endophytic Fungus Alternaria oxytropis OW 7.8 on Its Swainsonine Biosynthesis" International Journal of Molecular Sciences 25, no. 19: 10310. https://doi.org/10.3390/ijms251910310
APA StyleLiu, C., Ding, N., Lu, P., Yuan, B., Li, Y., & Jiang, K. (2024). The Effects of swnN Gene Function of Endophytic Fungus Alternaria oxytropis OW 7.8 on Its Swainsonine Biosynthesis. International Journal of Molecular Sciences, 25(19), 10310. https://doi.org/10.3390/ijms251910310





