Silicon Nanomaterials Enhance Seedling Growth and Plant Adaptation to Acidic Soil by Promoting Photosynthesis and Antioxidant Activity in Mustard (Brassica campestris L.)
Abstract
:1. Introduction
2. Results
2.1. Effect of Differential Doses of Si-Nanomaterials (Si-NMs) on Seed Germination and Seedling Growth of Mustard under Acidic Conditions
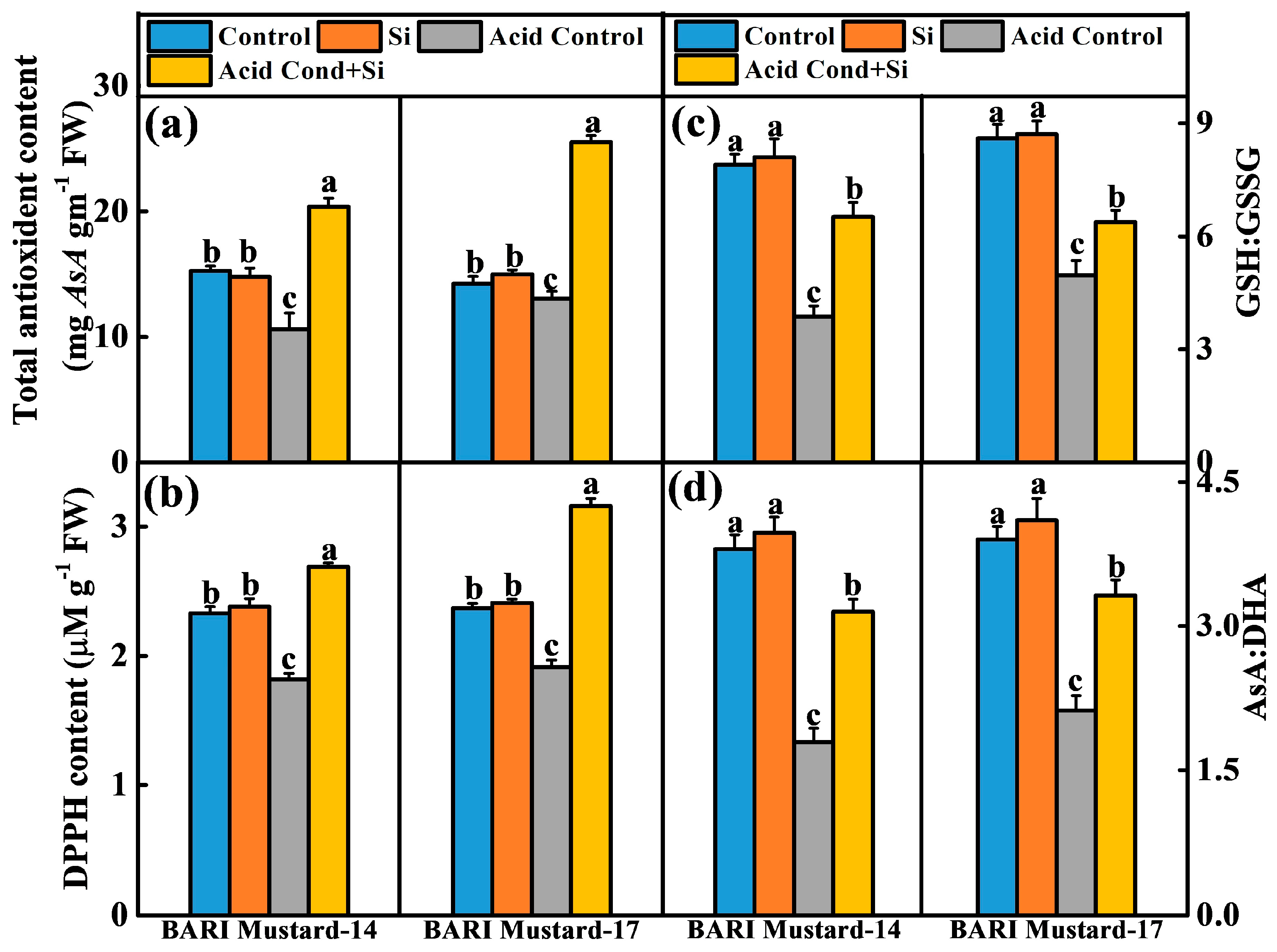
2.2. Si-NM-Mediated Mustard Seedling Growth and Establishment Are Associated with ROS Homeostasis
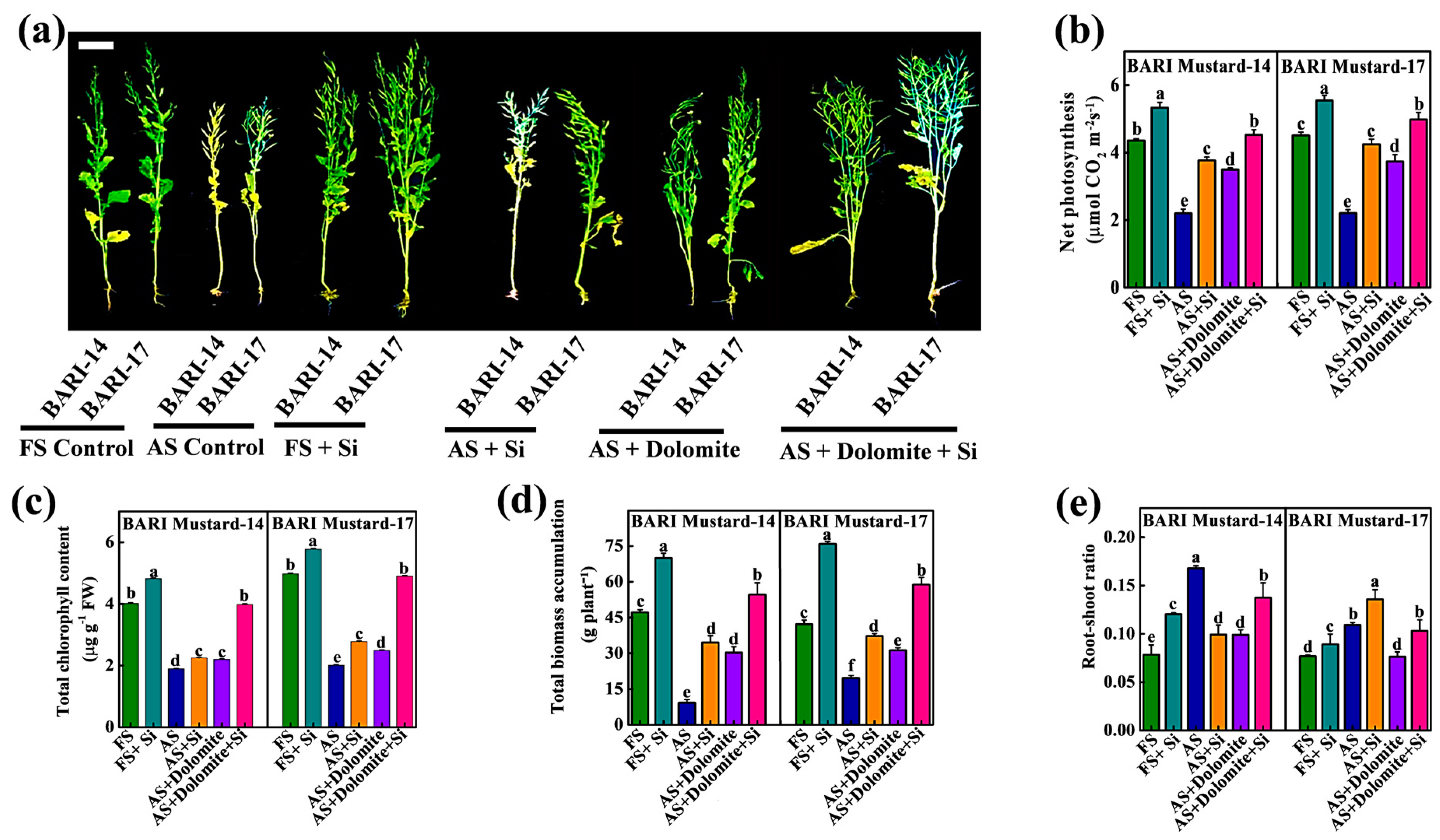
2.3. Exogenous Si-NMs Enhanced Plant Growth and Biomass Accumulation under Acidic Field Soil Condition
2.4. Effects of Si-NMs on Yield Attributes of Mustard Crop under Acidic Field Soil Condition
2.5. Si-NMs Enhanced Seed Yield and Oil Percentage of Mustard Reduced by Acidic Field Soil Condition
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Seed Pretreatment with Silicon Nanomaterials (Si-NMs)
4.2. Description of Field Experiments
4.3. Measurements of Plant Growth and Yield Parameters
4.4. Determination of Chlorophyll Content and Net Photosynthetic Rate
4.5. Histochemical Staining of H2O2 and O2•− and Measurements of H2O2, Lipid Peroxidation and Electrolyte Leakage in Mustard Seedlings
4.6. Antioxidant Assay
4.7. Measurement of Oil Percentage of Mustard Seeds
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agegnehu, G.; Amede, T.; Erkossa, T.; Yirga, C.; Henry, C.; Tyler, R.; Nosworthy, M.G.; Beyene, S.; Sileshi, G.W. Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2021, 71, 852–869. [Google Scholar] [CrossRef]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A. Role of calcium nutrition in plant Physiology: Advances in research and insights into acidic soil conditions-A comprehensive review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Juhász, C.; Labuschagne, M.; Moloi, M.J. The influence of soil acidity on the physiological responses of two bread wheat cultivars. Plants 2020, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Florentino, A.L.; Carvalho, M.E.A.; de Souza Mateus, N.; de Vicente Ferraz, A.; Rossi, M.L.; Gaziola, S.A.; Azevedo, R.A.; Linhares, F.S.; Lavres, J.; de Moraes Gonçalves, J.L. Integrated Ca, Mg, Cu, and Zn supply upregulates leaf anatomy and metabolic adjustments in Eucalyptus seedlings. Plant Physiol. Biochem. 2024, 208, 108446. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Z.; Wang, S.; Ni, B.-J.; Cen, L.; Liu, Q. Electrochemical study characterizations of pyrite weathering in simulated acidic soil: Iron transformation, sulfur conversion and environmental implications. Environ. Technol. Innov. 2024, 35, 103689. [Google Scholar] [CrossRef]
- Chakraborty, N.; Das, A.; Pal, S.; Roy, S.; Sil, S.K.; Adak, M.K.; Hassanzamman, M. Exploring Aluminum Tolerance Mechanisms in Plants with Reference to Rice and Arabidopsis: A Comprehensive Review of Genetic, Metabolic, and Physiological Adaptations in Acidic Soils. Plants 2024, 13, 1760. [Google Scholar] [CrossRef]
- Silva, J.; Aramburu-Merlos, F.; Baudron, F.; Gameda, S.; Sida, T.; Ruganzu, V.; Meliyo, J.; Jaleta, M.; Chamberlin, J.; Hijmans, R. The burden of acid soils for crop production in sub-Saharan Africa. Nature, 2024; under review. [Google Scholar] [CrossRef]
- Singh, V.K.; Chander, S.; Sheoran, R.K.; Sheoran, O.P.; Garcia Oliveira, A.L. Genetic variability for aluminium tolerance in sunflower (Helianthus annuus L.). Czech J. Genet. Plant Breed. 2022, 58, 201–209. [Google Scholar] [CrossRef]
- Baloch, S.B.; Ali, S.; Bernas, J.; Moudrý, J.; Konvalina, P.; Mushtaq, Z.; Murindangabo, Y.T.; Onyebuchi, E.F.; Baloch, F.B.; Ahmad, M. Wood ash application for crop production, amelioration of soil acidity and contaminated environments. Chemosphere 2024, 357, 141865. [Google Scholar] [CrossRef]
- Kumar, V.; Raha, P.; Kohali, A.; Patel, C.; Chandra, V. Biostimulants and its influence on soil properties and crop production: Biostimulants for sustainable crop production. J. AgriSearch 2024, 11, 81–87. [Google Scholar]
- Imtiaz, M.; Rizwan, M.S.; Mushtaq, M.A.; Ashraf, M.; Shahzad, S.M.; Yousaf, B.; Saeed, D.A.; Rizwan, M.; Nawaz, M.A.; Mehmood, S. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: A review. J. Environ. Manag. 2016, 183, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Q.; de Vries, W.; Ros, G.H.; Chen, X.; Muneer, M.A.; Zhang, F.; Wu, L. Effects of soil amendments on soil acidity and crop yields in acidic soils: A world-wide meta-analysis. J. Environ. Manag. 2023, 345, 118531. [Google Scholar] [CrossRef] [PubMed]
- Rachappanavar, V.; Kumar, M.; Negi, N.; Chowdhury, S.; Kapoor, M.; Singh, S.; Rustagi, S.; Rai, A.K.; Shreaz, S.; Negi, R. Silicon derived benefits to combat biotic and abiotic stresses in fruit crops: Current research and future challenges. Plant Physiol. Biochem. 2024, 211, 108680. [Google Scholar] [CrossRef]
- Kumar, N.; Samota, S.R.; Venkatesh, K.; Tripathi, S. Global trends in use of nano-fertilizers for crop production: Advantages and constraints–A review. Soil Tillage Res. 2023, 228, 105645. [Google Scholar] [CrossRef]
- Ma, C.; Han, L.; Shang, H.; Hao, Y.; Xu, X.; White, J.C.; Wang, Z.; Xing, B. Nanomaterials in agricultural soils: Ecotoxicity and application. Curr. Opin. Environ. Sci. Health 2023, 31, 100432. [Google Scholar] [CrossRef]
- Hasan, M.K.; Shopan, J.; Ahammed, G.J. Nanomaterials and soil health for agricultural crop production: Current status and future prospects. Nanomater. Agric. For. Appl. 2020, 289–312. [Google Scholar] [CrossRef]
- Maleki, K.; Soltani, E.; Seal, C.E.; Colville, L.; Pritchard, H.W.; Lamichhane, J.R. The seed germination spectrum of 486 plant species: A global meta-regression and phylogenetic pattern in relation to temperature and water potential. Agric. For. Meteorol. 2024, 346, 109865. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Zhang, Q.; Wei, J.; Zhao, X.; Zheng, Z.; Chen, B.; Xu, Z. Nanopriming Boost Seed Vigor: Deeper Insights Into The Effect Mechanism. Plant Physiol. Biochem. 2024, 214, 108895. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Li, P.; Xia, Y.; Song, K.; Liu, D. The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects. Plants 2024, 13, 984. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, K.; Rathore, S.; Premi, O.; Kandpal, B.; Chauhan, J. Advances in agronomic management of Indian mustard (Brassica juncea (L.) Czernj. Cosson): An overview. Int. J. Agron. 2012, 2012, 408284. [Google Scholar] [CrossRef]
- Dawud, K.M.; Devi, C.A.; Pandey, A.K. Genetic Improvement of Mustard. In Genetic Engineering of Crop Plants for Food and Health Security: Volume 1; Springer: Berlin/Heidelberg, Germany, 2024; pp. 331–354. [Google Scholar]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Khan, M.N.; Fu, C.; Li, J.; Tao, Y.; Li, Y.; Hu, J.; Chen, L.; Khan, Z.; Wu, H.; Li, Z. Seed nanopriming: How do nanomaterials improve seed tolerance to salinity and drought? Chemosphere 2023, 310, 136911. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef]
- Chen, M.; Jiao, S.-q.; Xie, L.; Geng, X.; Qi, S.; Fan, J.; Cheng, S.; Shi, J.; Cao, X. Integrated physiological, transcriptomic, and metabolomic analyses of drought stress alleviation in Ehretia macrophylla Wall. seedlings by SiO2 NPs (silica nanoparticles). Front. Plant Sci. 2024, 15, 1260140. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Hasan, M.K.; Liu, C.; Wang, F.; Ahammed, G.J.; Zhou, J.; Xu, M.-X.; Yu, J.-Q.; Xia, X.-J. Glutathione-mediated regulation of nitric oxide, S-nitrosothiol and redox homeostasis confers cadmium tolerance by inducing transcription factors and stress response genes in tomato. Chemosphere 2016, 161, 536–545. [Google Scholar] [CrossRef]
- Fan, N.; Zhao, C.; Yue, L.; Ji, H.; Wang, X.; Xiao, Z.; Rasmann, S.; Wang, Z. Nanosilicon alters oxidative stress and defence reactions in plants: A meta-analysis, mechanism and perspective. Environ. Sci. Nano 2022, 9, 3742–3755. [Google Scholar] [CrossRef]
- de Vargas, J.P.; dos Santos, D.R.; Bastos, M.C.; Schaefer, G.; Parisi, P.B. Application forms and types of soil acidity corrective: Changes in depth chemical attributes in long term period experiment. Soil Tillage Res. 2019, 185, 47–60. [Google Scholar] [CrossRef]
- Kalousek, P.; Holátko, J.; Schreiber, P.; Pluháček, T.; Širůčková Lónová, K.; Radziemska, M.; Tarkowski, P.; Vyhnánek, T.; Hammerschmiedt, T.; Brtnický, M. The effect of chelating agents on the Zn-phytoextraction potential of hemp and soil microbial activity. Chem. Biol. Technol. Agric. 2024, 11, 23. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.S.; Mondal, A.K.; Sharma, V.; Puniya, R.; Bhanwaria, R.; Yadav, N.K.; Jhajhra, S. Effect of Organic Manures and Boron Application on Yield Attributes and Yield of Mustard (Brassica junciea L.) under Jammu Region. Commun. Soil Sci. Plant Anal. 2023, 54, 1024–1041. [Google Scholar] [CrossRef]
- Dhakate, P.; Kandhol, N.; Raturi, G.; Ray, P.; Bhardwaj, A.; Srivastava, A.; Kaushal, L.; Singh, A.; Pandey, S.; Chauhan, D.K. Silicon nanoforms in crop improvement and stress management. Chemosphere 2022, 305, 135165. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Priya, R.S.; Jagathjothi, N.; Saranya, M.; Suganthi, N.; Sharmila, R.; Cyriac, J.; Anitha, R.; Subramanian, K. Silicon nanoparticles (SiNPs): Challenges and perspectives for sustainable agriculture. Physiol. Mol. Plant Pathol. 2023, 128, 102161. [Google Scholar] [CrossRef]
- Sirisuntornlak, N.; Ullah, H.; Sonjaroon, W.; Anusontpornperm, S.; Arirob, W.; Datta, A. Interactive effects of silicon and soil pH on growth, yield and nutrient uptake of maize. Silicon 2021, 13, 289–299. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M. Acidification with nitric acid improves chemical characteristics and reduces phytotoxicity of alkaline chars. J. Environ. Manag. 2017, 191, 237–243. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Fahim, A.K.F.; Kamal, A.M.; Shahid, S. Spatiotemporal change in groundwater sustainability of Bangladesh and its major causes. Stoch. Environ. Res. Risk Assess. 2023, 37, 665–680. [Google Scholar] [CrossRef]
- Xing, Q.; Hasan, M.K.; Li, Z.; Yang, T.; Jin, W.; Qi, Z.; Yang, P.; Wang, G.; Ahammed, G.J.; Zhou, J. Melatonin-induced plant adaptation to cadmium stress involves enhanced phytochelatin synthesis and nutrient homeostasis in Solanum lycopersicum L. J. Hazard. Mater. 2023, 456, 131670. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Shopan, J.; Rahman, M.M.; Sarkar, A.; Baset, M.A.; Zhang, Z.; Li, X.; Ahammed, G.J.; Hasan, M.K. Long-term traditional fertilization alters tea garden soil properties and tea leaf quality in Bangladesh. Agronomy 2022, 12, 2128. [Google Scholar] [CrossRef]
- Velioglu, S.D.; Temiz, H.T.; Ercioglu, E.; Velioglu, H.M.; Topcu, A.; Boyaci, I.H. Use of Raman spectroscopy for determining erucic acid content in canola oil. Food Chem. 2017, 221, 87–90. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.K.; Shopan, J.; Jahan, I.; Suravi, T.I. Silicon Nanomaterials Enhance Seedling Growth and Plant Adaptation to Acidic Soil by Promoting Photosynthesis and Antioxidant Activity in Mustard (Brassica campestris L.). Int. J. Mol. Sci. 2024, 25, 10318. https://doi.org/10.3390/ijms251910318
Hasan MK, Shopan J, Jahan I, Suravi TI. Silicon Nanomaterials Enhance Seedling Growth and Plant Adaptation to Acidic Soil by Promoting Photosynthesis and Antioxidant Activity in Mustard (Brassica campestris L.). International Journal of Molecular Sciences. 2024; 25(19):10318. https://doi.org/10.3390/ijms251910318
Chicago/Turabian StyleHasan, Md. Kamrul, Jannat Shopan, Israt Jahan, and Tonima Islam Suravi. 2024. "Silicon Nanomaterials Enhance Seedling Growth and Plant Adaptation to Acidic Soil by Promoting Photosynthesis and Antioxidant Activity in Mustard (Brassica campestris L.)" International Journal of Molecular Sciences 25, no. 19: 10318. https://doi.org/10.3390/ijms251910318





