RNA-Seq-Based Transcriptome Analysis of Chinese Cordyceps Aqueous Extracts Protective Effect against Adriamycin-Induced mpc5 Cell Injury
Abstract
1. Introduction
2. Results
2.1. Polysaccharides Content of WCCs
2.2. Mainly Nucleoside Ingredients in W5CC
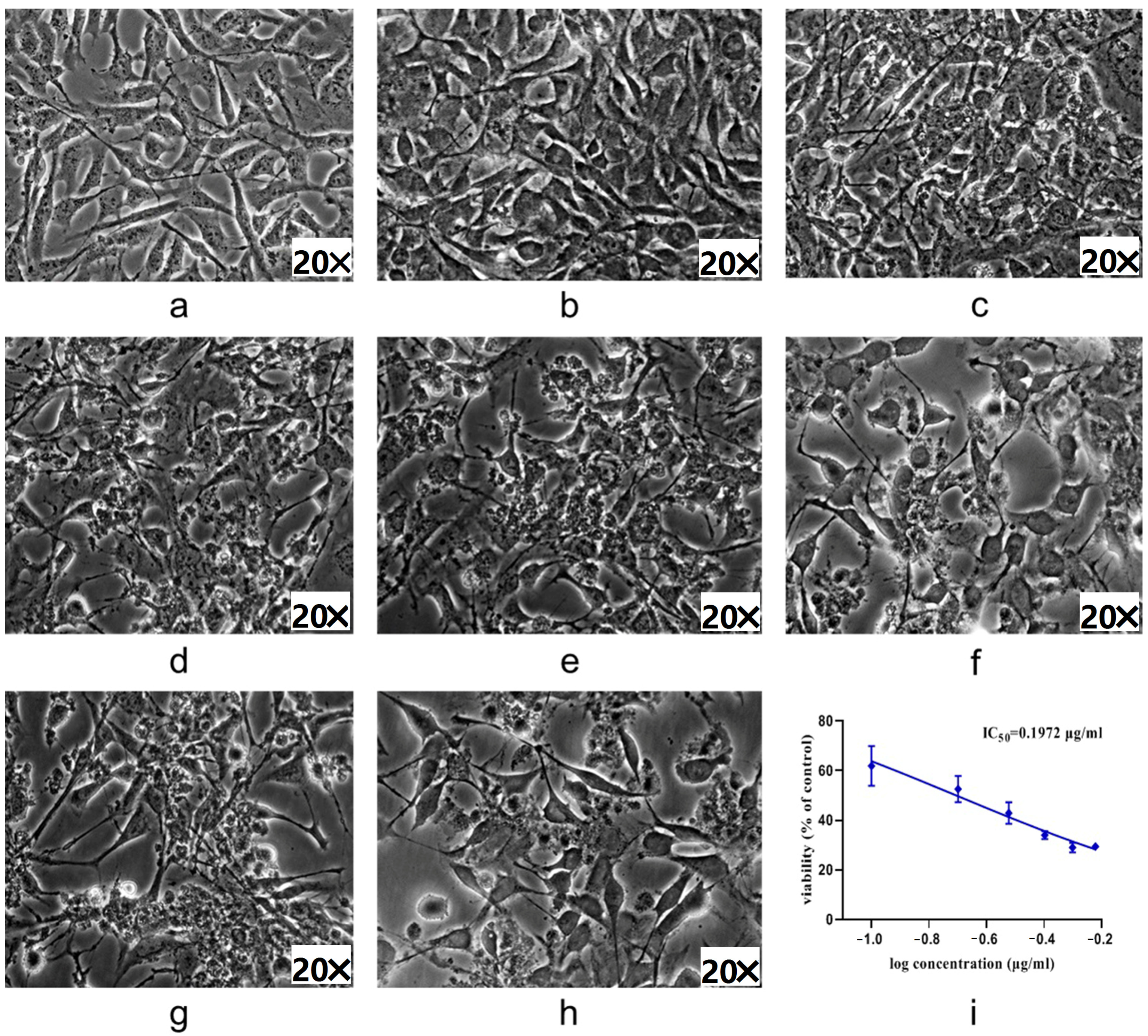
2.3. ADM-Induced Podocyte Injury Cell Model
2.4. Effect of WCCs on mpc5 Cell Viability
2.5. Protective Efficacy of WCCs on ADM-Induced mpc5 Cell
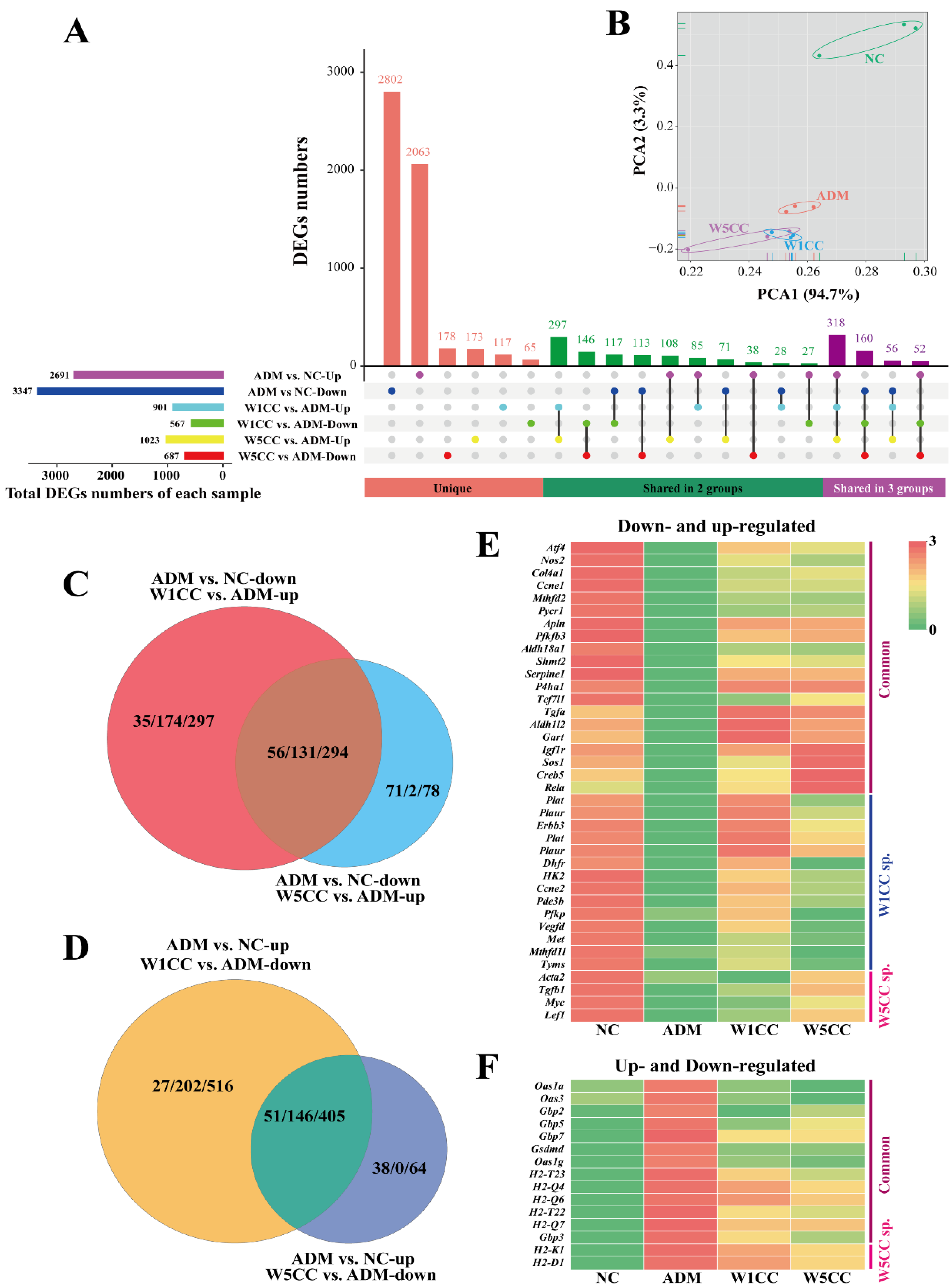
2.6. WCCs Alter RNA Expression Profiles in mpc5 Cell
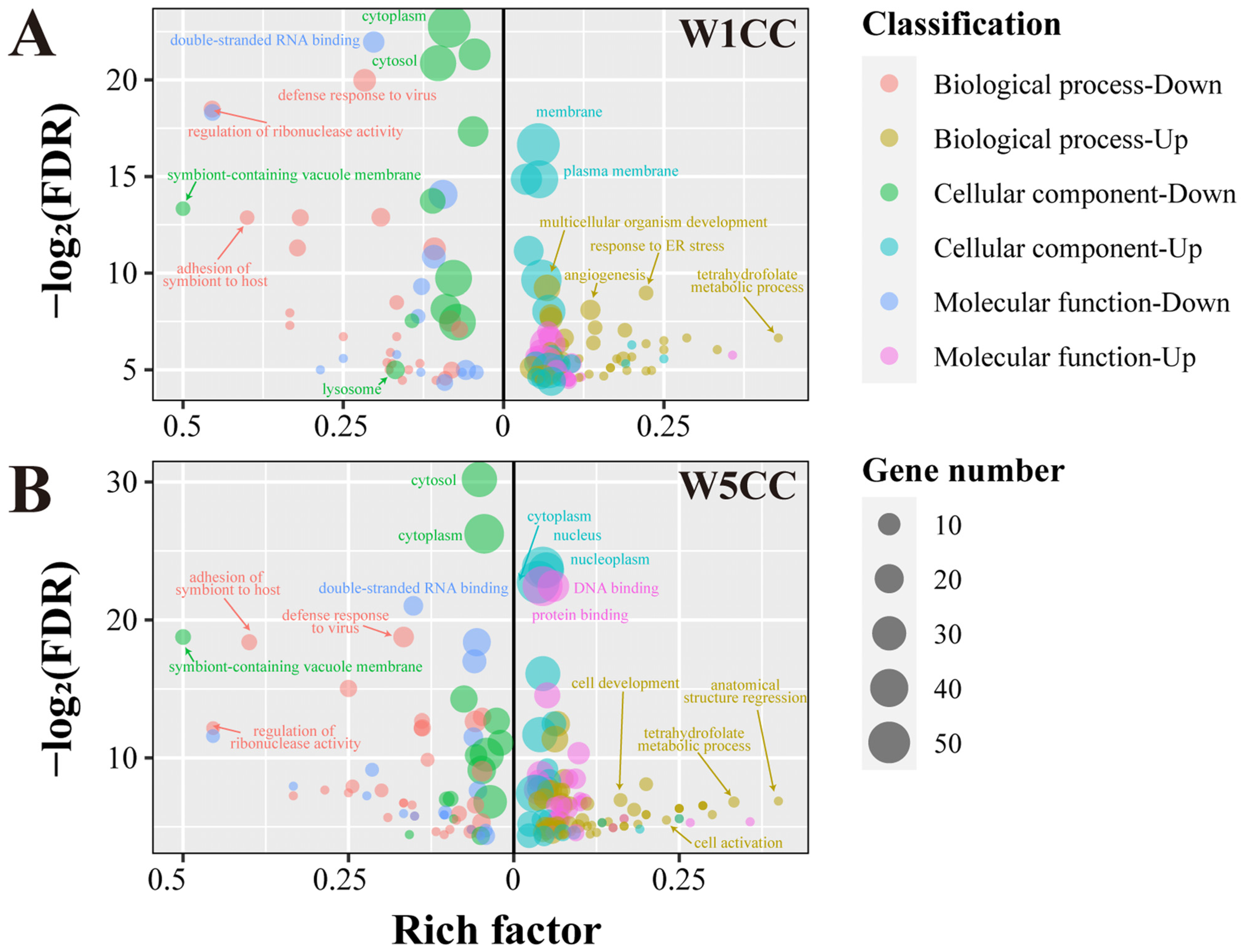
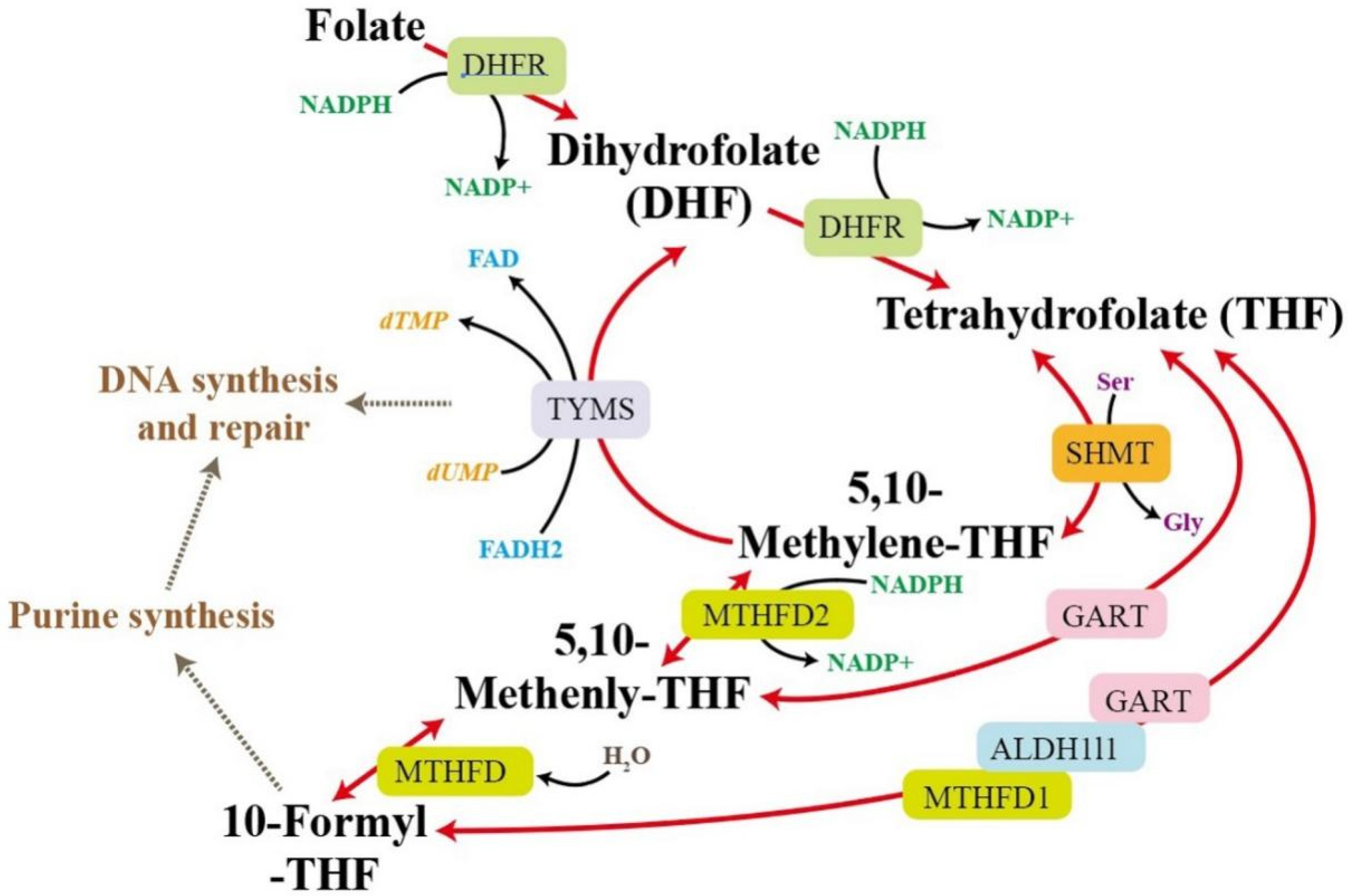
2.7. Enrichment Analysis
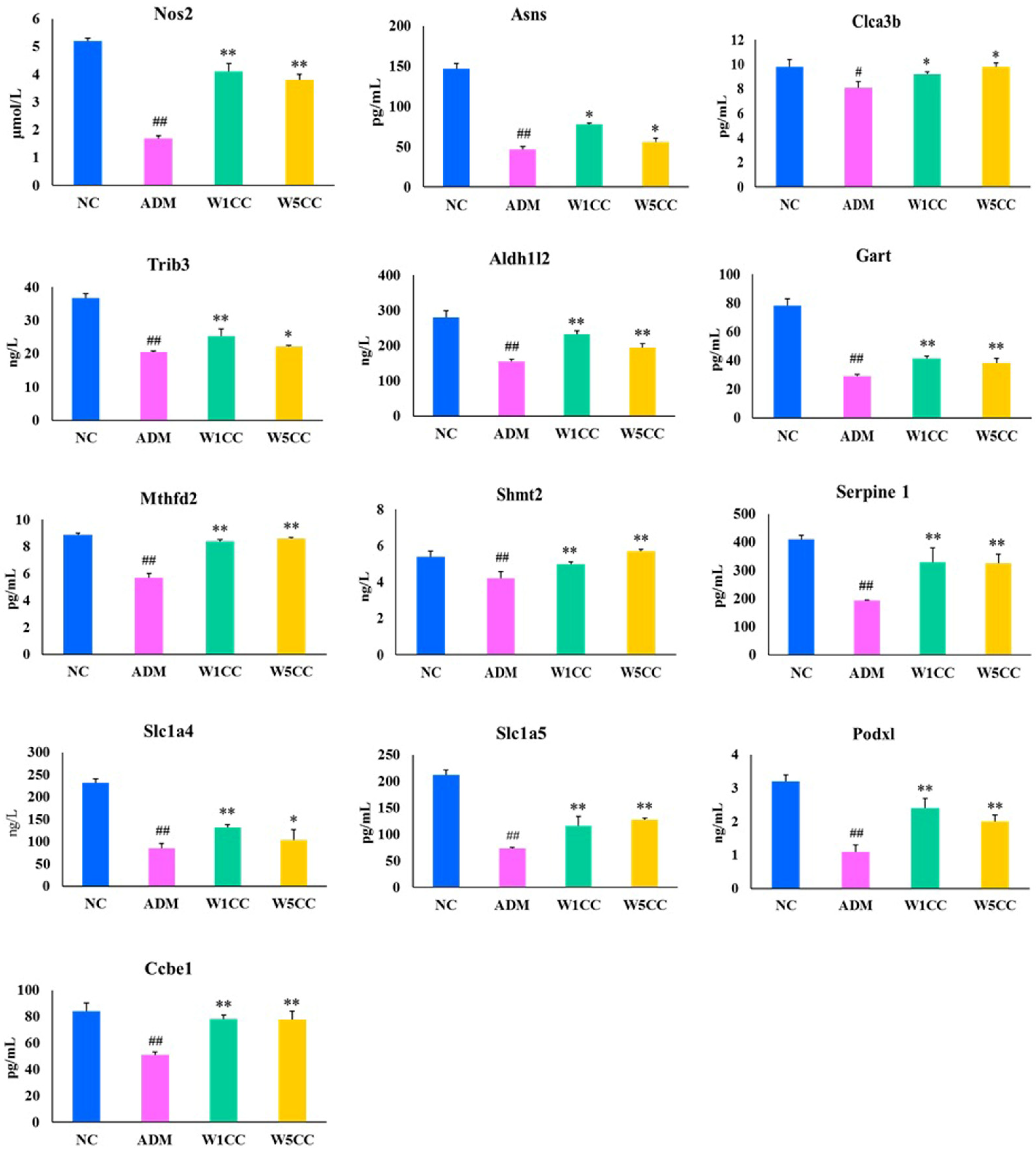
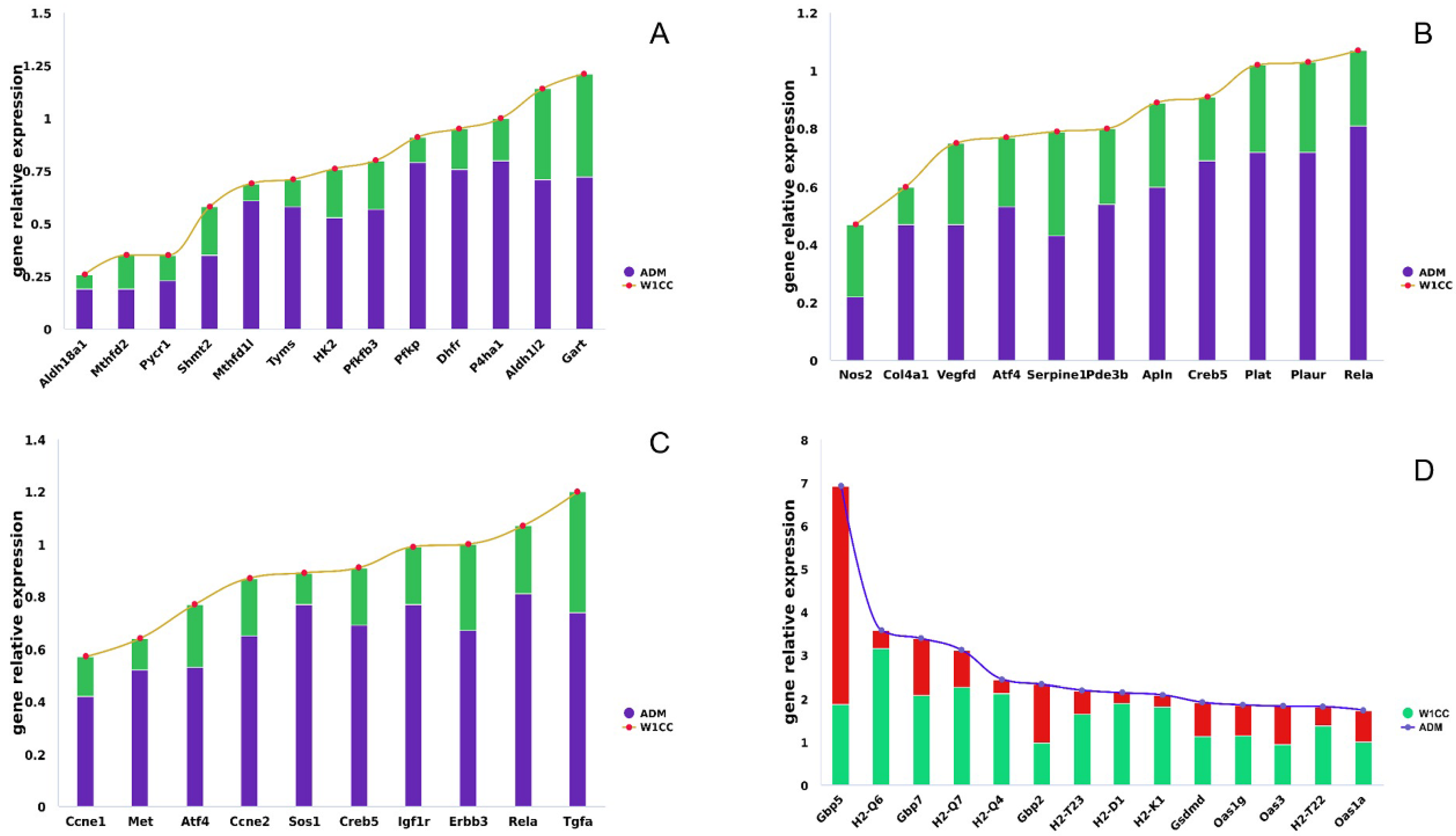
2.8. Validation of the RNA-Seq Results by qRT-PCR and ELISA
3. Discussion
4. Materials and Methods
4.1. Preparation of Sample Solutions
4.2. Quantitative Determination of Polysaccharides of WCCs
4.3. Identification of Nucleoside Ingredients in W5CC
4.4. Establishment of ADM-Induced Podocyte Injury Model
4.5. In Vitro Cytotoxicity of WCCs
4.6. Protective Efficacy of WCCs on ADM-Induced mpc5 Cell Injury
4.7. Total RNA Preparation, cDNA Library Construction, and mRNA Sequencing
4.8. Transcriptome Quality Control and Annotation
4.9. Differentially Expressed Gene Analysis
4.10. Functional Enrichment Analysis
4.11. Confirmation of RNA-Seq Results with qRT-PCR Analysis and ELISA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Shaughnessy, M.M.; Hogan, S.L.; Thompson, B.D.; Coppo, R.; Fogo, A.B.; Jennette, J.C. Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey. Nephrol. Dial. Transplant. 2018, 33, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Politano, S.A.; Colbert, G.B.; Hamiduzzaman, N. Nephrotic Syndrome. Prim. Care 2020, 47, 597–613. [Google Scholar] [CrossRef]
- Hull, R.P.; Goldsmith, D.J.A. Nephrotic syndrome in adults. BMJ 2008, 36, 1185–1189. [Google Scholar] [CrossRef]
- Lombel, R.M.; Gipson, D.S.; Hodson, E.M. Kidney Disease: Improving global outcomes. Treatment of steroid-sensitive nephrotic syndrome: New guidelines from KDIGO. Pediatr. Nephrol. 2013, 28, 415–426. [Google Scholar] [CrossRef]
- Faul, C.; Asanuma, K.; Yanagida-Asanuma, E.; Kim, K.; Mundel, P. Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007, 17, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.H.J.; Neal, C.R.; Harper, S.J. New aspects of glomerular filtration barrier structure and function: Five layers (at least) not three. Curr. Opin. Nephrol. Hypertens. 2009, 18, 197–205. [Google Scholar] [PubMed]
- Sonneveld, R.; Ferrè, S.; Hoenderop, J.G.J.; Dijkman, H.B.; Berden, J.H.M.; Bindels, R.J.M.; Wetzels, J.F.M.; Vlag, J.V.D.; Nijenhuis, T. Vitamin D Down-Regulates TRPC6 Expression in Podocyte Injury and Proteinuric Glomerular Disease. Am. J. Pathol. 2012, 182, 1196–1204. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.J.; Li, J.C. The interpretation of human body in traditional Chinese medicine and its influence on the characteristics of TCM theory. Anat. Rec. 2021, 304, 2559–2565. [Google Scholar] [CrossRef]
- Pan, D.P.; Guo, Y.L.; Fan, Y.F.; Wan, H.T. Development and Application of Traditional Chinese Medicine Using AI Machine Learning and Deep Learning Strategies. Am. J. Chin. Med. 2024, 52, 605–623. [Google Scholar] [CrossRef]
- Li, S.S.; Zhong, X.; Kan, X.T.; Gu, L.; Sun, H.X.; Zhang, G.R.; Liu, X. De novo transcriptome analysis of thitarodes jiachaensis before and after infection by the caterpillar fungus, ophiocordyceps sinensis. Gene 2016, 580, 96–103. [Google Scholar] [CrossRef]
- Qin, Q.L.; Zhou, G.L.; Zhang, H.; Meng, Q.; Zhang, J.H.; Wang, H.T.; Miao, L.; Li, X. Obstacles and approaches in artificial cultivation of Chinese cordyceps. Mycology 2018, 9, 7–9. [Google Scholar] [CrossRef]
- Zhou, X.W.; Li, L.J.; Tian, E.W. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit. Rev. Biotechnol. 2014, 34, 233–243. [Google Scholar] [CrossRef]
- Li, S.P.; Yang, F.Q.; Tsim, K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharmaceut. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef]
- Feng, A.R.; Tian, B.L.; Hu, J.M.; Zhou, P. Recent applications of capillary electrophoresis in the analysis of traditional Chinese medicine. Comb. Chem. High Throughput Screen. 2010, 13, 954–965. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Ma, J.; Liu, Q.Y.; Wu, C.Y.; Yang, Z.W.; Yang, T.T.; Chen, Q.M.; Yue, Y.Y.; Shang, J. Traditional Chinese Medicine formula, Sanwujiao granule, attenuates ischemic stroke by promoting angiogenesis through early administration. J. Ethnopharmacol. 2024, 321, 117418. [Google Scholar] [CrossRef]
- Long, H.L.; Qiu, X.H.; Cao, L.; Han, R.C. Discovery of the signal pathways and major bioactive compounds responsible for the anti-hypoxia effect of Chinese cordyceps. J. Ethnopharmacol. 2021, 277, 114215. [Google Scholar] [CrossRef]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps Sinensis: A review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lin, Z.X.; Tung, Y.S.; Kwan, T.H.; Mok, C.K.; Leung, C.; Chan, L.S. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane. Database. Syst. Rev. 2014, 2014, CD008353. [Google Scholar]
- Han, R.C.; Wu, H.; Tao, H.P.; Qiu, X.H.; Liu, G.Q.; Rao, Z.C.; Cao, L. Research on Chinese cordyceps during the past 70 years in China. Chin. J. Appl. Entom. 2019, 56, 849–883. [Google Scholar]
- Wang, W.; Zhang, X.N.; Yin, H.; Li, X.B.; Hu, X.P.; Liu, H.; Wang, Y.; Zhang, X.D. Effects of bailing capsules for renal transplant recipients: A retrospective clinical study. Chin. Med. J. 2013, 126, 1895–1899. [Google Scholar] [CrossRef]
- Xiao, C.G.; Xiao, P.; Li, X.G.; Li, X.Z.; Li, H.; Chen, Y.; Wang, Y.; Xu, Y.; Huang, G.Q.; Zhou, Q.L. Cordyceps sinensis may inhibit Th22 cell chemotaxis to improve kidney function in IgA nephropathy. Am. J. Transl. Res. 2018, 10, 857–865. [Google Scholar] [PubMed]
- Ren, H.J.; Sun, Y.L.; Yuan, B. Chinese patent medicine bailing capsule for treating lupus nephritis: A protocol for systematic review and metaanalysis. Med. Baltim. 2019, 98, e17041. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.Z.; Yuan, X.N.; Yuan, Q.J.; Chen, W.H.; Peng, Z.Z.; Xiao, X.C.; Zhou, Q.L. A meta-analysis of the clinical efficacy and safety of Bailing capsules in the treatment of nephrotic syndrome. Ann. Palliat. Med. 2020, 9, 3170–3181. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Chen, B.; Butte, A.J. Leveraging big data to transform target selection and drug discovery. Clin. Pharmacol. Ther. 2016, 99, 285–297. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Zhang, W.; Zhang, Y.; Zhan, L.; Wang, N.; Chen, C.; Fu, B.; Zhao, J.; Zhou, X.; et al. Tmnp: A transcriptome-based multi-scale network pharmacology platform for herbal medicine. Brief. Bioinform. 2022, 23, bbab542. [Google Scholar] [CrossRef]
- Wojcikowski, K.; Johnson, D.W.; Gobe, G. Herbs or natural substances as complementary therapies for chronic kidney disease: Ideas for future studies. J. Lab. Clin. Med. 2006, 147, 160–166. [Google Scholar] [CrossRef]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef]
- Muller-Deile, J.; Schiffer, M. Podocyte directed therapy of nephrotic syndrome-can we bring the inside out? Pediatr. Nephrol. 2016, 31, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; He, J.C. The podocyte as a direct target for treatment of glomerular disease? Am. J. Physiol. Renal. Physiol. 2016, 311, F46–F51. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, P.W. The podocyte as a target for therapies-new and old. Nat. Rev. Nephrol. 2011, 8, 52–56. [Google Scholar] [CrossRef]
- Fox, J.T.; Stover, P.J. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008, 79, 1–44. [Google Scholar]
- Lucock, M. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, A.S.; Appling, D.R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. One-carbon metabolism-genome interactions in folate-associated pathologies. J. Nutr. 2009, 139, 2402–2405. [Google Scholar] [CrossRef]
- Tan, V.P.; Miyamoto, S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy 2015, 11, 963–964. [Google Scholar] [CrossRef]
- Cifuente, J.O.; Comino, N.; D’Angelo, C.; Marina, A.; Gil-Carton, D.; Albesa-Jové, D.; Guerin, M.E. The allosteric control mechanism of bacterial glycogen biosynthesis disclosed by cryoEM. Curr. Res. Struct. Biol. 2020, 2, 89–103. [Google Scholar] [CrossRef]
- Bełdowski, P.; Przybyłek, M.; Bełdowski, D.; Dedinaite, A.; Sionkowska, A.; Cysewski, P.; Claesson, P.M. Collagen type II-hyaluronan interactions—The effect of proline hydroxylation: A molecular dynamics study. J. Mater. Chem. B 2022, 10, 9713–9723. [Google Scholar] [CrossRef]
- Maruyama, R.; Shimizu, M.; Li, J.; Inoue, J.; Sato, R. Fibroblast growth factor 21 induction by activating transcription factor 4 is regulated through three amino acid response elements in its promoter region. Biosci. Biotechnol. Biochem. 2016, 80, 929–934. [Google Scholar] [CrossRef]
- Neumann, A.; Walton, E.; Alemany, S.; Cecil, C.; González, J.R.; Jima, D.D.; Lahti, J.; Tuominen, S.T.; Barker, E.D.; Binder, E.; et al. Association between DNA methylation and ADHD symptoms from birth to school age: A prospective meta-analysis. Transl. Psychiatry 2020, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.H.; Li, Y.J.; Reed, E.A.; Odronic, S.I.; Kontos, C.D.; Annex, B.H. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J. Vasc. Surg. 2006, 44, 166–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.; Jiang, S.C.; Zheng, W.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N. RelA/p65 inhibition prevents tendon adhesion by modulating inflammation, cell proliferation, and apoptosis. Cell Death. Dis. 2017, 8, e2710. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.S.; Sharman, S.K.; Lu, C.; Yang, D.F.; Paschall, A.V.; Tulachan, S.S.; Liu, K.B. The NF-κB p65 and p50 homodimer cooperate with IRF8 to activate iNOS transcription. BMC Cancer 2015, 15, 770. [Google Scholar] [CrossRef]
- Kumagai, K.; Suzuki, H.; Ichikawa, M.; Jimbo, M.; Murakami, M.; Ryuzaki, M.; Saruta, T. Nitric oxide increases renal blood flow by interacting with the sympathetic nervous system. Hypertension 1994, 24, 220–226. [Google Scholar] [CrossRef]
- Burmakin, M.; Fasching, A.; Kobayashi, H.; Urrutia, A.A.; Damdimopoulos, A.; Palm, F.; Haase, V.H. Pharmacological HIF-PHD inhibition reduces renovascular resistance and increases glomerular filtration by stimulating nitric oxide generation. Acta Physiol. 2021, 233, e13668. [Google Scholar] [CrossRef]
- Therwani, S.A.; Rosenbæk, J.B.; Mose, F.H.; Bech, J.N.; Pedersen, E.B. Effect of tolvaptan on renal water and sodium excretion and blood pressure during nitric oxide inhibition: A dose-response study in healthy subjects. BMC Nephrol. 2017, 18, 86. [Google Scholar] [CrossRef][Green Version]
- Iyer, A.K.V.; Ramesh, V.; Castro, C.A.; Kaushik, V.; Kulkarni, Y.M.; Wright, C.A.; Venkatadri, R.; Rojanasakul, Y.; Azad, N. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J. Cell. Biochem. 2015, 116, 2484–2493. [Google Scholar] [CrossRef]
- Wang, T.; Jin, H.J.; Hu, J.Y.; Li, X.; Ruan, H.Y.; Xu, H.L.; Wei, L.; Dong, W.H.; Teng, F.; Gu, J.R.; et al. COL4A1 promotes the growth and metastasis of hepatocellular carcinoma cells by activating FAK-Src signaling. J. Exp. Clin. Cancer Res. 2020, 39, 148. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, J.Y.; Chen, Y.; Sohel, H.; Ke, X.R.; Chen, J.Q.; Li, Y.X. The correlation and role analysis of COL4A1 and COL4A2 in hepatocarcinogenesis. Aging 2020, 12, 204–223. [Google Scholar] [CrossRef]
- Duan, J.; Wen, P.; Zhao, Y.; Van de Leemput, J.; Lai Yee, J.; Fermin, D.; Warady, B.A.; Furth, S.L.; Ng, D.K.; Sampson, M.G.; et al. A Drosophila model to screen Alport syndrome COL4A5 variants for their functional pathogenicity. bioRxiv 2024. [Google Scholar] [CrossRef]
- Duan, J.L.; Cui, J.; Yang, Z.F.; Guo, C.; Cao, J.Y.; Xi, M.M.; Weng, Y.; Yin, Y.; Wang, Y.H.; Wei, G.; et al. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3β/Nrf2 signaling. J. Neuroinflamm. 2019, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.H.; Wang, Z.; Zhang, R.M.; Sun, W.P.; Chen, X.Y. The Role of Apelin/Apelin Receptor in Energy Metabolism and Water Homeostasis: A Comprehensive Narrative Review. Front. Physiol. 2021, 12, 632886. [Google Scholar] [CrossRef]
- Huvers, F.C.; Leeuw, P.W.D.; Houben, A.J.; Haan, C.H.D.; Hamulyak, K.; Schouten, H.; Wolffenbuttel, B.H.; Schaper, N.C. Endothelium-dependent vasodilatation, plasma markers of endothelial function, and adrenergic vasoconstrictor responses in type 1 diabetes under near-normoglycemic conditions. Diabetes 1999, 48, 1300–1307. [Google Scholar] [CrossRef]
- Trojanowicz, B.; Ulrich, C.; Girndt, M. Uremic Apelin and Leucocytic Angiotensin-Converting Enzyme 2 in CKD Patients. Toxins 2020, 12, 742. [Google Scholar] [CrossRef]
- Zheng, Z.; Nakamura, K.; Gershbaum, S.; Wang, X.B.; Thomas, S.; Bessler, M.; Schrope, B.; Krikhely, A.; Liu, R.M.; Ozcan, L.; et al. Interacting hepatic PAI-1/tPA gene regulatory pathways influence impaired fibrinolysis severity in obesity. J. Clin. Investig. 2020, 130, 4348–4359. [Google Scholar] [CrossRef]
- Haruhara, K.; Tsuboi, N.; Koike, K.; Kanzaki, G.; Okabayashi, Y.; Sasaki, T.; Fukui, A.; Miyazaki, Y.; Kawamura, T.; Ogura, M.; et al. Circadian blood pressure abnormalities in patients with primary nephrotic syndrome. Clin. Exp. Hypertens. 2017, 39, 155–159. [Google Scholar] [CrossRef]
- Brunet, A.; Datta, S.R.; Greenberg, M.E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001, 11, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front. Cell. Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, J.B.; Wei, G.B.; Yang, K.; Wang, G.H.; Sun, X.J. miR-148a inhibits cell proliferation and migration through targeting ErbB3 in colorectal cancer. Oncol. Lett. 2019, 18, 2530–2536. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhou, H.; Chen, Y.Y.; Zhong, D.M.; Su, P.Q.; Yuan, H.D.; Yang, X.M.; Liao, Z.H.; Qiu, X.J.; Wang, X.D.; et al. MET promotes the proliferation and differentiation of myoblasts. Exp. Cell Res. 2020, 388, 111838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.C.; Cai, A.; Ma, C.X.; Cai, S.; Wang, H.; Que, Y.K.; Xu, S.L.; Xu, T.B.; Hu, Y. Cisplatin inhibits the proliferation of Saos-2 osteosarcoma cells via the miR-376c/TGFA pathway. Bosn. J. Basic Med. Sci. 2021, 21, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Calvo, I.; Martinez-Salgado, C. Sos1 Modulates Extracellular Matrix Synthesis, Proliferation, and Migration in Fibroblasts. Front. Physiol. 2021, 12, 645044. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhou, P.; Wang, W.J.; Sun, A.N.; Guo, F. RelB, together with RelA, sustains cell survival and confers proteasome inhibitor sensitivity of chronic lymphocytic leukemia cells from bone marrow. J. Mol. Med. 2014, 92, 77–92. [Google Scholar] [CrossRef]
- Pathria, G.; Scott, D.A.; Feng, Y.M.; Lee, J.S.; Fujita, Y.; Zhang, G.; Sahu, A.D.; Ruppin, E.; Herlyn, M.; Osterman, A.L.; et al. Targeting the Warburg effect via LDHA inhibition engages ATF4 signaling for cancer cell survival. EMBO J. 2018, 37, e99735. [Google Scholar] [CrossRef]
- Zhang, M.B.; Li, Y.; Wang, H.B.; Yu, W.H.; Lin, S.; Guo, J.Q. LncRNA SNHG5 affects cell proliferation, metastasis and migration of colorectal cancer through regulating miR-132-3p/CREB5. Cancer Biol. Ther. 2019, 20, 524–536. [Google Scholar] [CrossRef]
- Fisch, D.; Bando, H.; Clough, B.; Hornung, V.; Yamamoto, M.; Shenoy, A.R.; Frickel, E.M. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO. J. 2019, 38, e100926. [Google Scholar] [CrossRef]
- Shi, J.J.; Gao, W.Q.; Shao, F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Frankiw, L.; Mann, M.; Li, G.D.; Joglekar, A.; Baltimore, D. Alternative splicing coupled with transcript degradation modulates OAS1g antiviral activity. RNA 2020, 26, 126–136. [Google Scholar] [CrossRef]
- Wang, K.L.; Li, L.; Jin, J.; An, Y.L.; Wang, Z.J.; Zhou, S.Y.; Zhang, J.Y.; Abuduaini, B.; Cheng, C.; Li, N. Fatty acid synthase (Fasn) inhibits the expression levels of immune response genes via alteration of alternative splicing in islet cells. J. Diabetes Complicat. 2022, 36, 108159. [Google Scholar] [CrossRef]
- De Jesus, D.F.; Zhang, Z.J.; Brown, N.K.; Li, X.L.; Gaffrey, M.J.; Kahraman, S.; Wei, J.B.; Hu, J.; Basile, G.; Xiao, L.; et al. Redox Regulation of m6A methyltransferase METTL3 in β-cells controls the innate immune response in type 1 diabetes. Nat. Cell Biol. 2024, 26, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Ishina, I.A.; Zakharova, M.Y.; Kurbatskaia, I.N.; Mamedov, A.E.; Belogurov, A.A., Jr.; Gabibov, A.G. MHC Class II Presentation in Autoimmunity. Cells 2023, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fan, Z.; Rauh, M.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 2017, 36, 5593–5608. [Google Scholar] [CrossRef]
- Wu, M.H.; Skaug, B.; Bi, X.J.; Mills, T.T.; Salazar, G.; Zhou, X.D.; Reveille, J.; Agarwal, S.K.; Blackburn, M.R.; Mayes, M.D.; et al. Interferon regulatory factor 7 (IRF7) represents a link between inflammation and fibrosis in the pathogenesis of systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 1583–1591. [Google Scholar] [CrossRef]
- Su, H.H.; Xie, J.Z.; Wen, L.J.; Wang, S.Y.; Chen, S.; Li, J.C.; Qi, C.L.; Zhang, Q.Q.; He, X.D.; Zheng, L.Y.; et al. LncRNA Gas5 regulates Fn1 deposition via Creb5 in renal fibrosis. Epigenomics 2021, 13, 699–713. [Google Scholar] [CrossRef]
- Liang, Q.E.; Liu, T.H.; Guo, T.T.; Tao, W.C.; Chen, X.D.; Chen, W.H.; Chen, L.G.; Xiao, Y. ATF4 promotes renal tubulointerstitial fibrosis by suppressing autophagy in diabetic nephropathy. Life Sci. 2021, 264, 118686. [Google Scholar] [CrossRef]
- He, J.; Li, X.Y.; Yu, M. Bioinformatics Analysis Identifies Potential Ferroptosis Key Genes in the Pathogenesis of Pulmonary Fibrosis. Front. Genet. 2022, 12, 788417. [Google Scholar] [CrossRef]
- Wu, S.S.; Yin, L.J.; Han, K.; Jiang, B.; Meng, Q.; Aschner, M.; Li, X.; Chen, R. NAT10 accelerates pulmonary fibrosis through N4-acetylated TGFB1-initiated epithelial-to-mesenchymal transition upon ambient fine particulate matter exposure. Environ. Pollut. 2023, 322, 121149. [Google Scholar]
- Milbank, E.; Díaz-Trelles, R.; Dragano, N.; Jiang, B.; Meng, Q.T.; Aschner, M.; Li, X.B.; Chen, R. Liver lipopolysaccharide binding protein prevents hepatic inflammation in physiological and pathological non-obesogenic conditions. Pharmacol. Res. 2023, 187, 106562. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Delgado, M.D.; León, J. MYC Oncogene Contributions to Release of Cell Cycle Brakes. Genes 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.N.; George, E.; Kinose, Y.; Kim, H.; Shah, J.B.; Peake, J.D.; Ferman, B.; Medvedev, S.; Murtha, T.; Barger, C.J.; et al. CCNE1 copy number is a biomarker for response to combination WEE1-ATR inhibition in ovarian and endometrial cancer models. Cell Rep. Med. 2021, 2, 100394. [Google Scholar] [CrossRef]
- Bezamat, M.; Souza, J.F.; Silva, F.M.F.; Corrêa, E.G.; Fatturi, A.L.; Brancher, J.A.; Carvalho, F.M.; Cavallari, T.; Bertolazo, L.; Machado-Souza, C.; et al. Gene-environment interaction in molar-incisor hypomineralization. PLoS ONE 2021, 16, e0241898. [Google Scholar] [CrossRef] [PubMed]
- Han, L. miR-99a inhibits proliferation and migration of cervical cancer cells by targeting IGF1R. J. Buon. 2021, 26, 1782–1788. [Google Scholar]
- Kuwahara, A.; Sakai, H.; Xu, Y.J.; Itoh, Y.; Hirabayashi, Y.; Gotoh, Y. Tcf3 represses Wnt-β-catenin signaling and maintains neural stem cell population during neocortical development. PLoS ONE 2014, 9, e94408. [Google Scholar] [CrossRef]
- Papaspyropoulos, A.; Bradley, L.; Thapa, A.; Leung, C.Y.; Toskas, K.; Koennig, D.; Pefani, D.E.; Raso, C.; Grou, C.; Hamilton, G.; et al. RASSF1A uncouples Wnt from Hippo signalling and promotes YAP mediated differentiation via p73. Nat. Commun. 2018, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.X.; He, Y.J.; Zhang, X. Identification and Characterization of Copy Number-Associated Driver Genes in Esophageal Squamous Cell Carcinoma. BioMed Res. Int. 2020, 2020, 6387519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Q.N.; Sun, Q.X.; Li, L.D.; Xu, S.Y.; Li, C.Q. Transcriptomic Analysis Reveals a Link Between Hippo Signaling Pathway and Macrophages in Lungs of Mice with OVA-Induced Allergic Asthma. J. Inflamm. Res. 2022, 15, 423–437. [Google Scholar] [CrossRef]
- Yu, B.; Su, J.; Shi, Q.Q.; Liu, Q.; Ma, J.; Ru, G.Q.; Zhang, L.; Zhang, J.; Hu, X.C.; Tang, J.M. KMT5A-methylated SNIP1 promotes triple-negative breast cancer metastasis by activating YAP signaling. Nat. Commun. 2022, 13, 2192. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Sun, J.; Zhu, X.F.; Yang, D.J.; Zeng, Y.X. ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells by suppressing the c-Myc signaling pathway. J. Transl. Med. 2009, 7, 74. [Google Scholar] [CrossRef]
- Chiaro, C.; Lazarova, D.L.; Bordonaro, M. Tcf3 and cell cycle factors contribute to butyrate resistance in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2012, 428, 121–126. [Google Scholar]
- Shang, D.H.; Bi, R.M.; Han, T.D.; Wang, D.Y.; Tian, Y.; Liu, Y.T. Expression and proliferation-promoting role of lymphoid enhancer-binding factor 1 in human clear cell renal carcinoma. Cancer Investig. 2014, 32, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Han, R.C.; Cao, L. Artificial cultivation of the Chinese cordyceps from injected ghost moth larvae. Environ. Entomol. 2019, 48, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Long, H.L.; Qiu, X.H.; Cao, L.; Liu, G.Q.; Rao, Z.C.; Han, R.C. Toxicological safety evaluation of the cultivated Chinese cordyceps. J. Ethnopharmacol. 2021, 268, 113600. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yan, W.; Wang, M.H.; Cui, X.; Hu, Y.; Chen, Q.Q.; Zhang, Y.; Qi, X.W.; Jiang, J. Huaier polysaccharide inhibits the stem-like characteristics of ERα-36high triple negative breast cancer cells via inactivation of the ERα-36 signaling pathway. Int. J. Biol. Sci. 2019, 15, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Cock, P.J.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.P.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A. The Benjamini-Hochberg method in the case of discrete test statistics. Int. J. Biostat. 2007, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.F. p Value fetishism and use of the Bonferroni adjustment. Evid. Based Ment. Health 2007, 10, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Sample Name | Total Reads | Clean Reads | Clean Bases (bp) | CleanGC (%) | CleanQ20 (%) | CleanQ30 (%) | Total Mapped |
|---|---|---|---|---|---|---|---|
| NC-1 | 43,857,918 | 43,982,656 | 6,597,398,400 | 50.57;50.83 | 97.63;96.38 | 93.71;90.65 | 96.06% |
| NC-2 | 52,689,236 | 52,742,126 | 7,911,318,900 | 50.34;50.30 | 97.93;96.74 | 94.38;91.70 | 96.67% |
| NC-3 | 39,438,362 | 39,522,550 | 5,928,382,500 | 51.04;51.17 | 97.72;97.25 | 93.91;92.66 | 96.50% |
| ADM-1 | 49,934,498 | 49,970,138 | 7,495,520,700 | 50.39;50.34 | 98.16;97.54 | 94.87;93.36 | 96.70% |
| ADM-2 | 44,982,290 | 45,062,288 | 6,759,343,200 | 50.10;50.34 | 97.51;96.96 | 93.33;91.96 | 96.16% |
| ADM-3 | 40,773,188 | 40,856,468 | 6,128,470,200 | 51.19;51.40 | 97.70;95.72 | 93.84;89.20 | 95.84% |
| W1CC-1 | 48,806,762 | 48,885,248 | 7,332,787,200 | 50.37;50.32 | 97.78;97.23 | 94.13;92.94 | 96.20% |
| W1CC-2 | 39,302,792 | 39,379,476 | 5,906,921,400 | 50.60;50.80 | 97.59;97.14 | 93.58;92.40 | 96.26% |
| W1CC-3 | 49,761,814 | 49,850,608 | 7,477,591,200 | 50.85;50.84 | 97.89;96.55 | 94.26;91.23 | 96.40% |
| W5CC-1 | 40,728,338 | 40,764,416 | 6,114,662,400 | 50.06;49.95 | 97.63;97.14 | 93.79;93.06 | 96.19% |
| W5CC-2 | 43,624,828 | 43,707,238 | 6,556,085,700 | 50.71;50.94 | 97.67;96.05 | 93.78;89.97 | 95.84% |
| W5CC-3 | 40,124,848 | 40,188,070 | 6,028,210,500 | 51.00;51.25 | 97.59;96.18 | 93.58;90.31 | 95.93% |
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|
| Nos2 | CCTGCTTTGTGCGAAGTGTC | CCCTTTGTGCTGGGAGTCAT |
| Slc1a4 | GGCGAGACCAACGGCTAC | GATTTGCCCATCGCCGTCT |
| Slc1a5 | TTCGCTATCGTCTTTGGTGTG | ATGGTGGCATCATTGAAGGAG |
| Asns | GACTGCAACCTGCTACCCAA | AAGGGAAACTTCTGGGAGGC |
| Clca3b | CCACCACACTCCCAGTCATC | AACAGGCAAAAACCCTTGGC |
| Trib3 | ACCTTCAGAGCGACTTGTGG | TCTCCCTTCGGTCAGACTGT |
| Podxl | CTCTCCCATCTGGGCCTTTG | ACACAGCAGTTCCACGAGTT |
| Aldh1l2 | CTAGAGGCGGGAACGGTTTT | TTCAGGGCCTCCTCACCTAA |
| Gart | TCACTGCTGCCCATCATACG | CACGAGAAGACCTTGGGGAC |
| Mthfd2 | TATCACTCCCGTCCCTGGTG | GCGCTGTTTGGACTTGAACA |
| Shmt2 | GTACGAACCGTAGACCCCAAG | CCCTGTAGGGATGGGAACAC |
| Ccbe1 | GAAACAGAAGATGGCGCTGC | TGGAAGGTAGGCGTTTGAGG |
| Serpine1 | TCTTTCCGACCAAGAGCAGC | AAAGGCTGTGGAGGAAGACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, H.; Liu, M.; Rao, Z.; Guan, S.; Chen, X.; Huang, X.; Cao, L.; Han, R. RNA-Seq-Based Transcriptome Analysis of Chinese Cordyceps Aqueous Extracts Protective Effect against Adriamycin-Induced mpc5 Cell Injury. Int. J. Mol. Sci. 2024, 25, 10352. https://doi.org/10.3390/ijms251910352
Long H, Liu M, Rao Z, Guan S, Chen X, Huang X, Cao L, Han R. RNA-Seq-Based Transcriptome Analysis of Chinese Cordyceps Aqueous Extracts Protective Effect against Adriamycin-Induced mpc5 Cell Injury. International Journal of Molecular Sciences. 2024; 25(19):10352. https://doi.org/10.3390/ijms251910352
Chicago/Turabian StyleLong, Hailin, Mengzhen Liu, Zhongchen Rao, Shanyue Guan, Xiaotian Chen, Xiaoting Huang, Li Cao, and Richou Han. 2024. "RNA-Seq-Based Transcriptome Analysis of Chinese Cordyceps Aqueous Extracts Protective Effect against Adriamycin-Induced mpc5 Cell Injury" International Journal of Molecular Sciences 25, no. 19: 10352. https://doi.org/10.3390/ijms251910352
APA StyleLong, H., Liu, M., Rao, Z., Guan, S., Chen, X., Huang, X., Cao, L., & Han, R. (2024). RNA-Seq-Based Transcriptome Analysis of Chinese Cordyceps Aqueous Extracts Protective Effect against Adriamycin-Induced mpc5 Cell Injury. International Journal of Molecular Sciences, 25(19), 10352. https://doi.org/10.3390/ijms251910352





