Hair Growth-Promoting Effect of Hydrangea serrata (Thunb.) Ser. Extract and Its Active Component Hydrangenol: In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Results
2.1. Effect of WHS and an Active Compound, Hydrangenol, on 5α-Reductase Inhibitory Activity
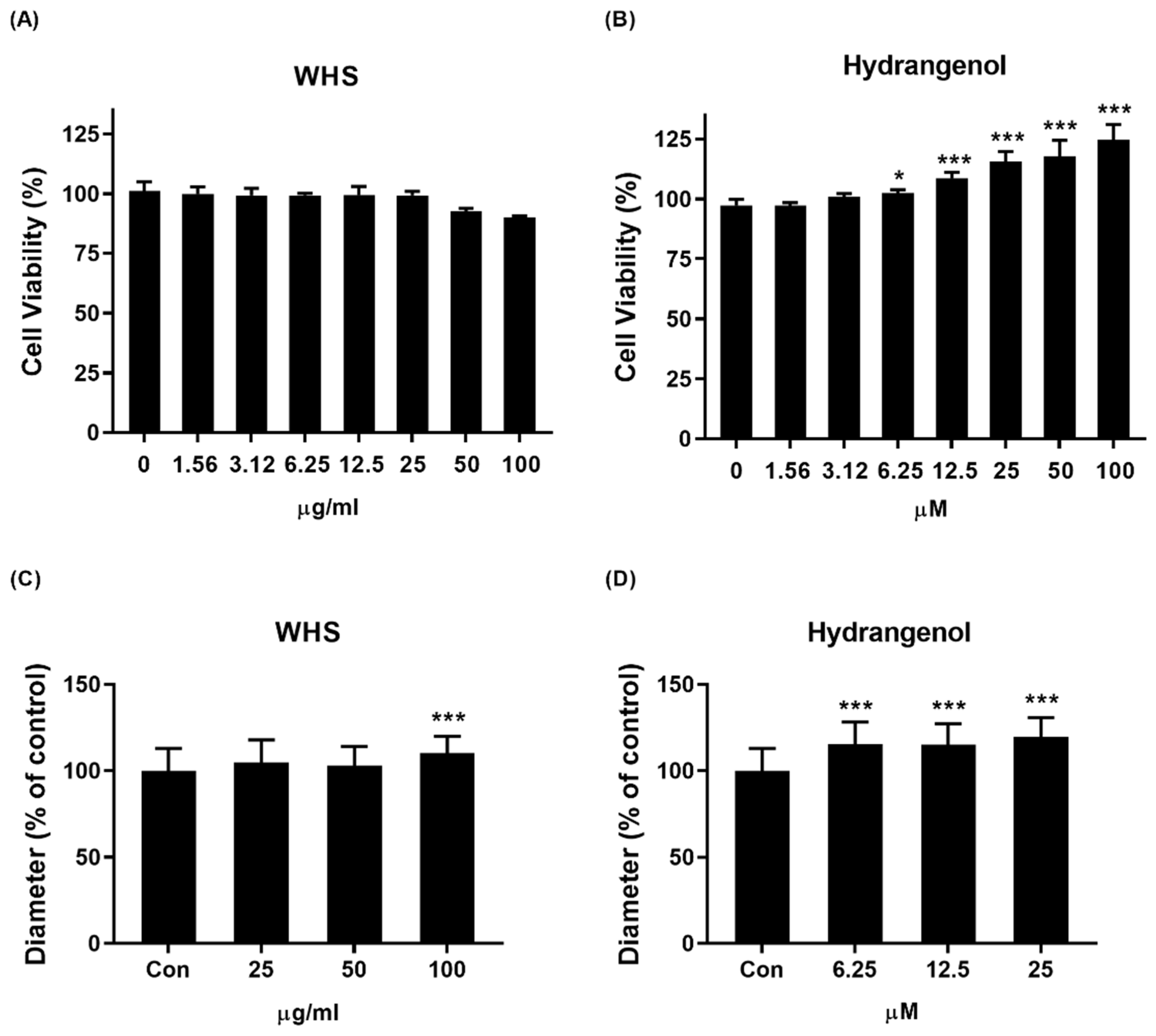
2.2. Effect of WHS and Hydrangenol on DPC Growth
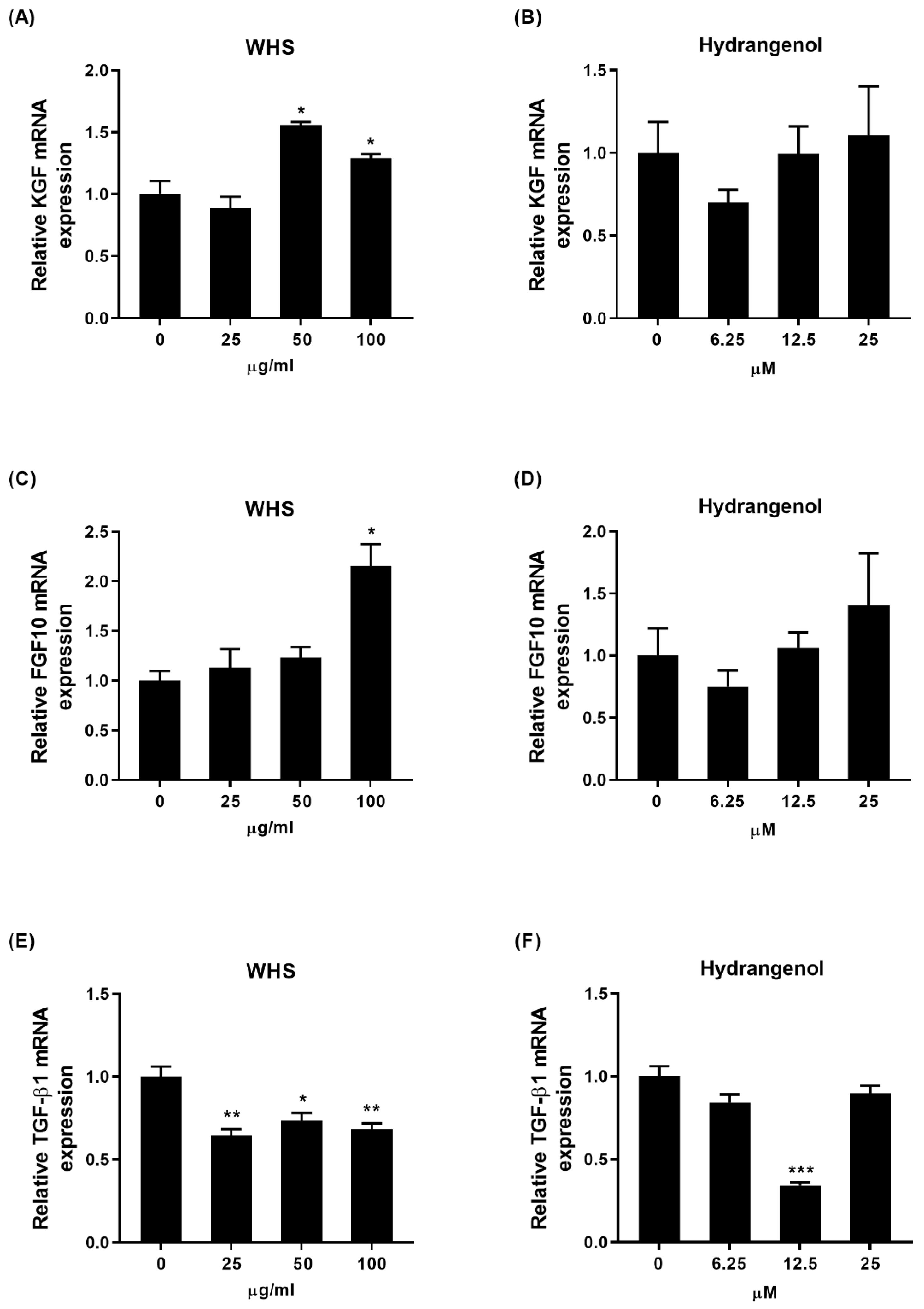
2.3. Effect of WHS and Hydrangenol on Expression of Hair Growth-Related Genes in DPCs
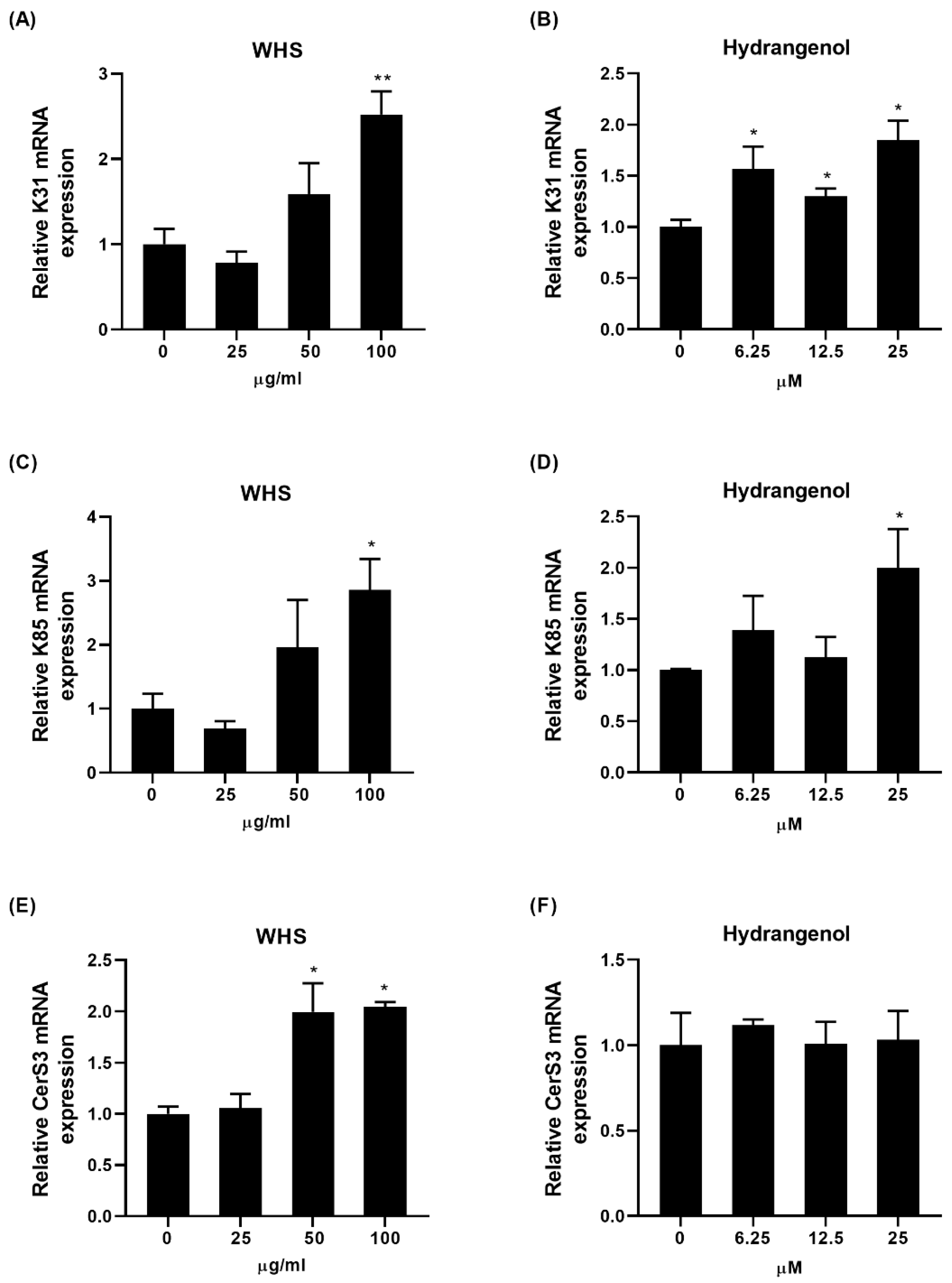
2.4. Effect of WHS and Hydrangenol on Expression of Keratin and Ceramide Synthase Genes in HaCaT
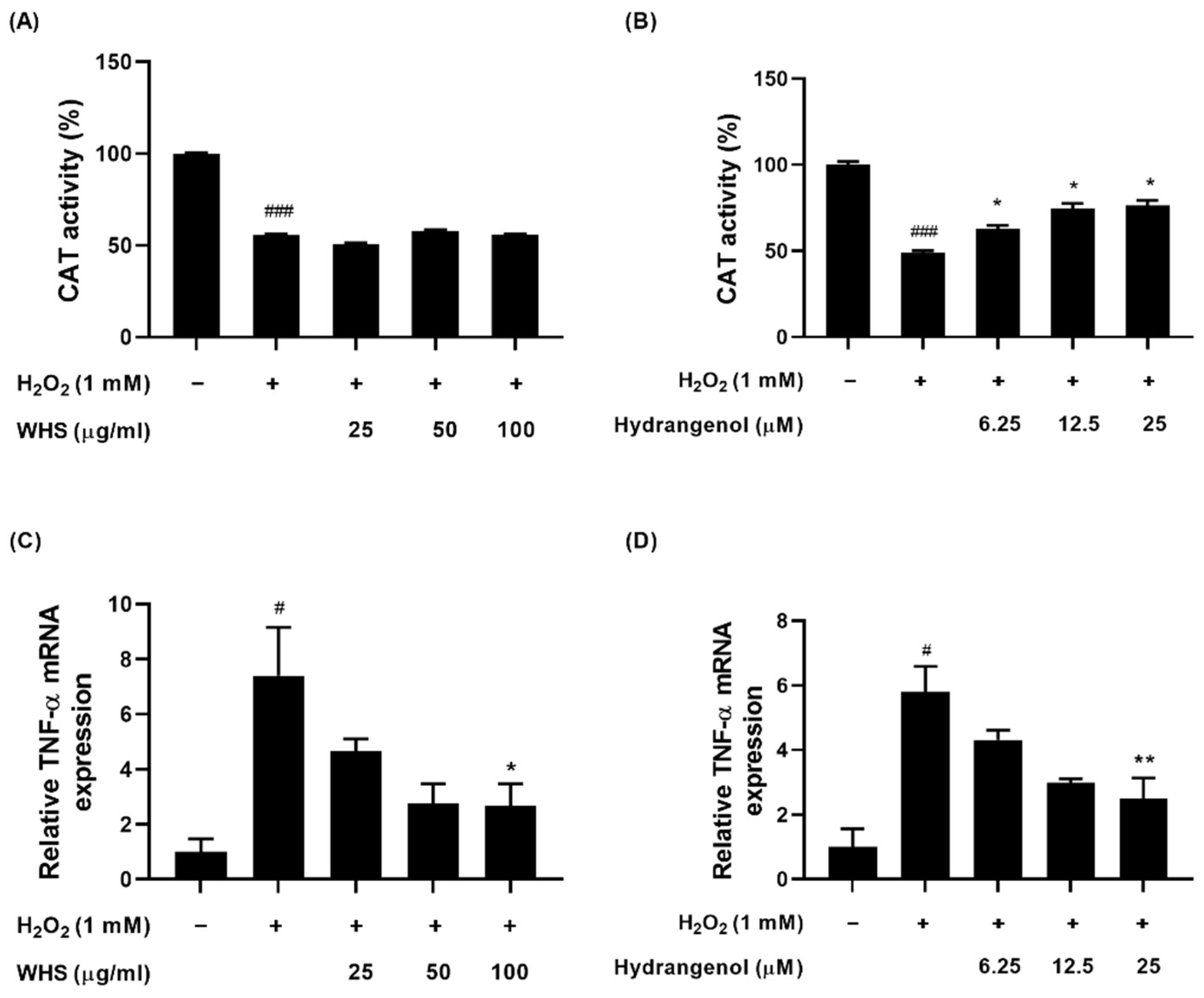
2.5. Effect of WHS and Hydrangenol on Antioxidant Enzyme Activity and Expression of Inflammatory Gene in H2O2-Induced DPCs
2.6. Effect of WHS and Hydrangenol on Androgen Signaling Molecules in DHT-Induced DPCs
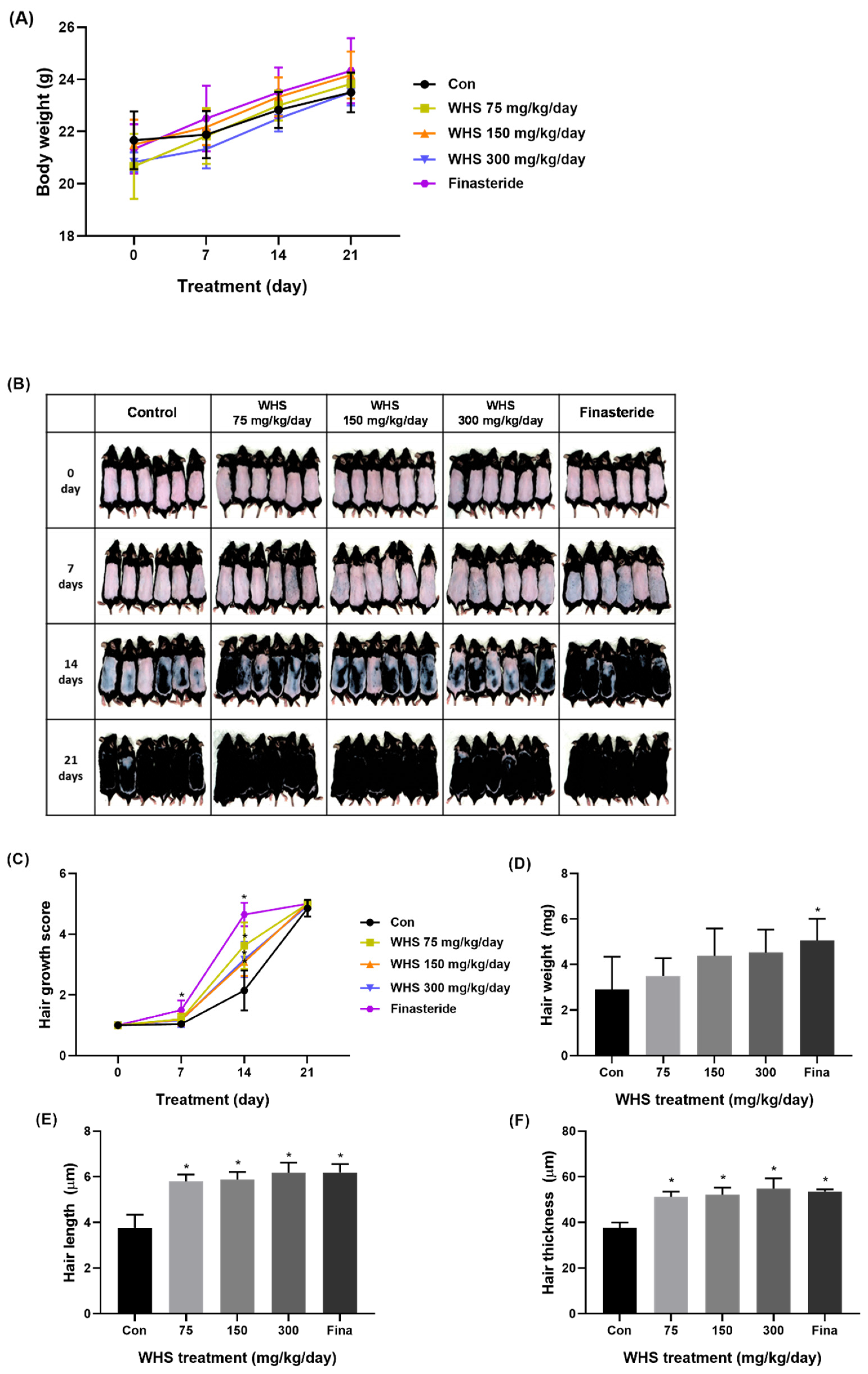
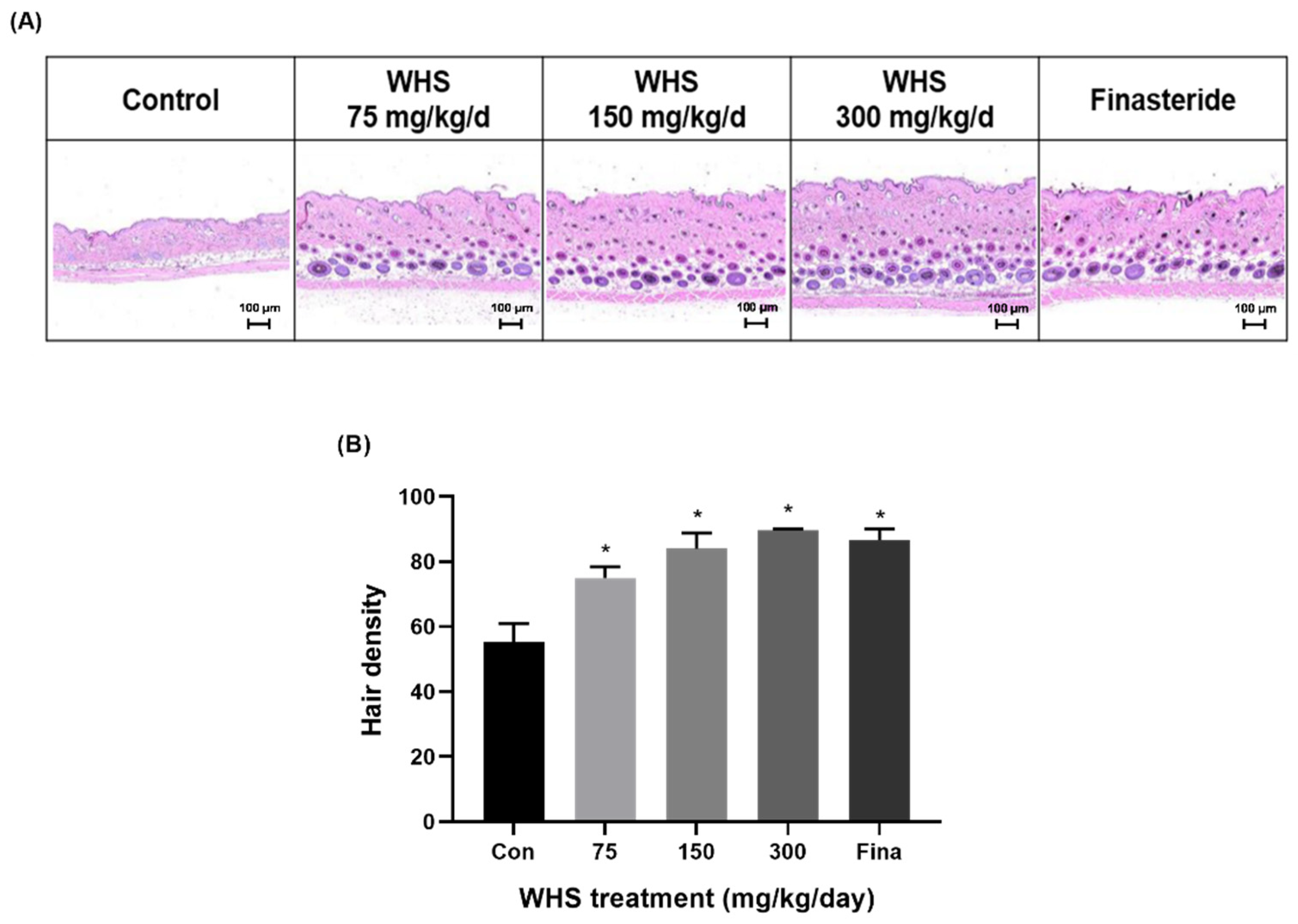
2.7. Effect of WHS on Hair Growth Promotion in C57BL/6 Mice
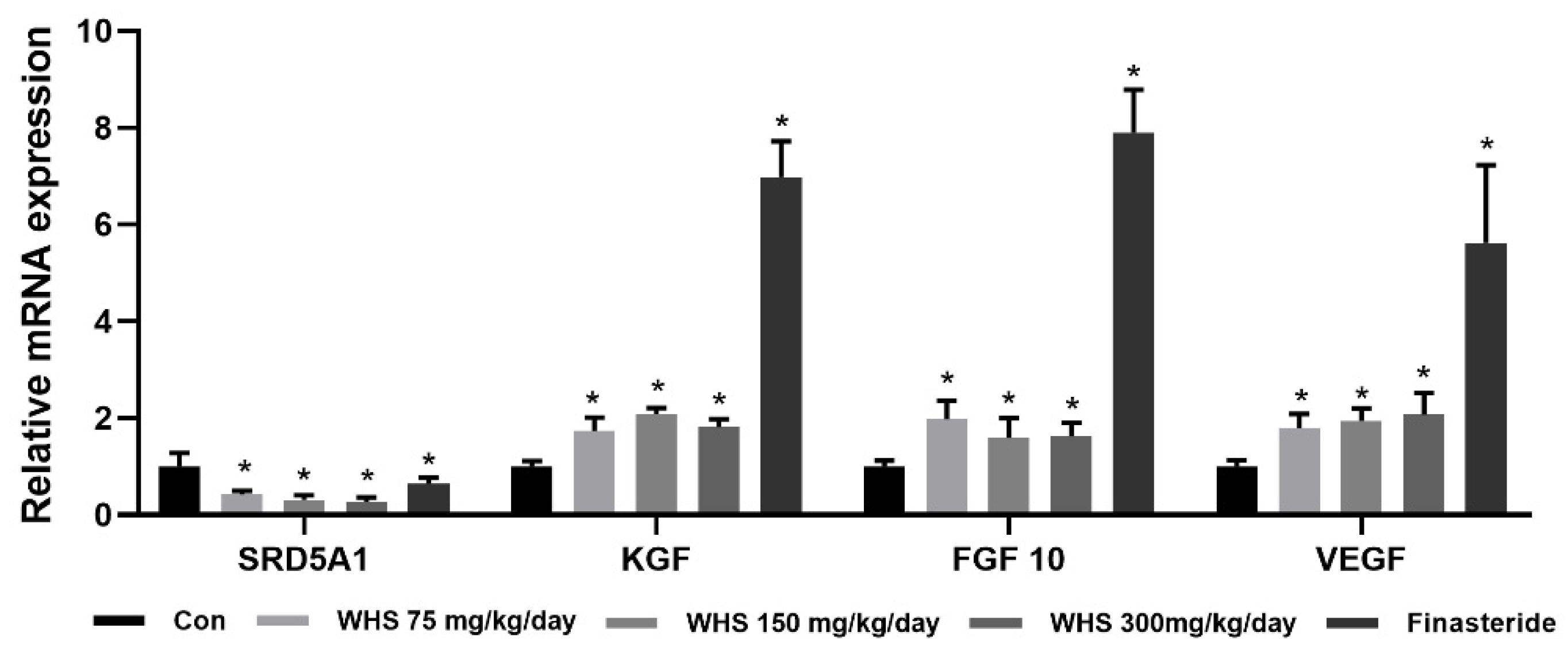
2.8. Effect of WHS on Expression of Growth-Related Genes in C57BL/6 Mice
2.9. Effect of WHS on Enhancing Hair Formation in 3D Human Skin Organoids
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. 5α-Reductase Inhibitory Activity
4.3. Cell Culture
4.4. MTT Assay
4.5. DPC Spheroid (3D) Culture
4.6. RNA Extraction and Quantification Real-Time RT-PCR
4.7. Catalase Activity
4.8. Animal Experiments
4.9. Visual Analysis of Hair Growth
4.10. Measurement of Hair Length and Thickness
4.11. Measurement of Hair Weight
4.12. Measurement of Hair Density
4.13. Generation of Skin Organoids and Assessment of Hair Formation
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, K.; Stephenson, T.J.; Messenger, A.G. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124 Pt 8, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jung, D.M.; Lee, S.S.; Kim, E.M.; Yoon, K.; Kim, K.K. Mangifera Indica leaf extracts promote hair growth via activation of Wnt signaling pathway in human dermal papilla cells. Anim. Cells Syst. 2022, 26, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, X.; Bao, P.; Zhou, X.; Chu, M.; Guo, X.; Liang, C.; Pan, H.; Yan, P. Understanding Mammalian Hair Follicle Ecosystems by Single-Cell RNA Sequencing. Animals 2022, 12, 2409. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Premature senescence of balding dermal papilla cells in vitro is associated with p16INK4a expression. J. Investig. Dermatol. 2008, 128, 1088–1094. [Google Scholar] [CrossRef]
- Huang, W.Y.; Huang, Y.C.; Huang, K.S.; Chan, C.C.; Chiu, H.Y.; Tsai, R.Y.; Chan, J.Y.; Lin, S.J. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J. Dermatol. Sci. 2017, 86, 114–122. [Google Scholar] [CrossRef]
- Zhano, J.; Harada, N.; Okajima, K. Dihydrotestosterone inhibits hair growth in mice by inhibiting insulin-like growth factor-I production in dermal papillae. Growth Horm. IGF Res. 2011, 21, 260–267. [Google Scholar]
- Ma, L.; Shen, H.; Fang, C.; Chen, T.; Wang, J. Camellia seed cake extract supports hair growth by abrogating the effect of dihydrotestosterone in cultured human dermal papilla cells. Molecules 2022, 27, 6443. [Google Scholar] [CrossRef]
- Ganzer, C.A.; Jacobs, A.R.; Iqbal, F. Persistent sexual, emotional, and cognitive impairment post-finasteride: A survey of men reporting symptoms. Am. J. Men’s Health 2015, 9, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S.; Kolukula, S. Persistent sexual side effects of finasteride for male pattern hair loss. J. Sex. Med. 2011, 8, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Shin, J.S.; Myung, D.B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.T. Hydrangea serrata (Thunb.) Ser. Extract Attenuate UVB-Induced Photoaging through MAPK/AP-1 Inactivation in Human Skin Fibroblasts and Hairless Mice. Nutrients 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.B.; Han, H.S.; Shin, J.S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice. Nutrients 2019, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, C.H.; Jayasooriya, R.; Dilshara, M.G.; Lee, S.; Choi, Y.H.; Seo, Y.T.; Kim, G.Y. Hydrangenol inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-mediated HO-1 pathway. Int. Immunopharmacol. 2016, 35, 61–69. [Google Scholar] [CrossRef]
- Nozawa, K.; Yamada, M.; Tsuda, Y.; Kawai, K.; Nakajima, S. Antifungal activity of oosponol, oospolactone, phyllodulcin, hydrangenol, and some other related compounds. Chem. Pharm. Bull. 1981, 29, 2689–2691. [Google Scholar] [CrossRef]
- Shin, S.S.; Ko, M.C.; Park, Y.J.; Hwang, B.; Park, S.L.; Kim, W.J.; Moon, S.K. Hydrangenol inhibits the proliferation, migration, and invasion of EJ bladder cancer cells via p21(WAF1)-mediated G1-phase cell cycle arrest, p38 MAPK activation, and reduction in Sp-1-induced MMP-9 expression. EXCLI J. 2018, 17, 531–543. [Google Scholar]
- Gho, Y.; Shin, S.S.; Choi, Y.H.; Ko, K.; Kim, W.J.; Moon, S.K. Hydrangenol suppresses VEGF-stimulated angiogenesis by targeting p27KIP1-dependent G1-cell cycle arrest, VEGFR-2-mediated signaling, and MMP-2 expression. Anim. Cells Syst. 2019, 23, 72–81. [Google Scholar] [CrossRef]
- Han, H.S.; Chung, K.S.; Shin, Y.K.; Yu, J.S.; Kang, S.H.; Lee, S.H.; Lee, K.T. Effect of Standardized Hydrangea serrata (Thunb.) Ser. Leaves Extract on Body Weight and Body Fat Reduction in Overweight or Obese Humans: A Randomized Double-Blind Placebo-Controlled Study. Nutrients 2022, 14, 208. [Google Scholar] [CrossRef]
- Myung, D.B.; Lee, J.H.; Han, H.S.; Lee, K.Y.; Ahn, H.S.; Shin, Y.K.; Song, E.; Kim, B.H.; Lee, K.H.; Lee, S.H.; et al. Oral Intake of Hydrangea serrata (Thunb.) Ser. Leaves Extract Improves Wrinkles, Hydration, Elasticity, Texture, and Roughness in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1588. [Google Scholar] [CrossRef]
- Liu, X.; Ji, T.; Hu, H.; Lv, X.; Zhu, G. The Hair Growth-Promoting Effect of Gardenia florida Fruit Extract and Its Molecular Regulation. Evid. Based Complement. Altern. Med. 2022, 2022, 8498974. [Google Scholar] [CrossRef] [PubMed]
- Pi, L.Q.; Lee, W.S.; Min, S.H. Hot water extract of oriental melon leaf promotes hair growth and prolongs anagen hair cycle: In vivo and in vitro evaluation. Food Sci. Biotechnol. 2016, 25, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Wakame, K.; Okawa, H.; Komatsu, K.I.; Nakata, A.; Sato, K.; Ingawa, H.; Kohchi, C.; Nishizawa, T.; Soma, G. Immunopotentiator from Pantoea agglomerans 1 (IP-PA1) Promotes Murine Hair Growth and Human Dermal Papilla Cell Gene Expression. Anticancer Res. 2016, 36, 3687–3692. [Google Scholar] [PubMed]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Shipley, G.D.; Pittelkow, M.R.; Wille, J.J., Jr.; Scott, R.E.; Moses, H.L. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986, 46 Pt 2, 2068–2071. [Google Scholar]
- Thibaut, S.; de Becker, E.; Bernard, B.A.; Huart, M.; Fiat, F.; Baghdadli, N.; Luengo, G.S.; Leroy, F.; Angevin, P.; Kermoal, A.M.; et al. Chronological ageing of human hair keratin fibres. Int. J. Cosmet. Sci. 2010, 32, 422–434. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-based cosmetics for hair care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Nagase, S. Hair structures affecting hair appearance. Cosmetics 2019, 6, 43. [Google Scholar] [CrossRef]
- Cruz, C.; Martins, M.; Egipto, J.; Osório, H.; Ribeiro, A.; Cavaco-Paulo, A. Changing the shape of hair with keratin peptides. RSC Adv. 2017, 7, 51581–51592. [Google Scholar] [CrossRef]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.; Rogers, M.A. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef]
- Schweizer, J.; Langbein, L.; Rogers, M.A.; Winter, H. Hair follicle-specific keratins and their diseases. Exp. Cell Res. 2007, 313, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Zhang, G.; Bíró, T.; Lisztes, E.; Funk, W.; Ingber, A.; Langbein, L.; Paus, R. TSH is a novel neuroendocrine regulator of selected keratins in the human hair follicle. J. Dermatol. Sci. 2011, 64, 67. [Google Scholar] [CrossRef]
- Lee, W.-S.; Oh, T.H.; Chun, S.H.; Jeon, S.Y.; Lee, E.Y.; Lee, S.; Park, W.-S.; Hwang, S. Integral lipid in human hair follicle. J. Investig. Dermatol. Symp. 2005, 10, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Alonso, C.; García, M.T.; Pérez, L.; Martí, M. Hair lipid structure: Effect of surfactants. Cosmetics 2023, 10, 107. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichol. 2009, 1, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R. Understanding hair loss due to air pollution and the approach to management. Hair Ther. Transpl. 2015, 5, 2. [Google Scholar]
- Ohn, J.; Kim, S.J.; Choi, S.-J.; Choe, Y.S.; Kwon, O.; Kim, K.H. Hydrogen peroxide (H2O2) suppresses hair growth through downregulation of β-catenin. J. Dermatol. Sci. 2018, 89, 91–94. [Google Scholar] [CrossRef]
- Yenin, J.; Serarslan, G.; Yönden, Z.; Ulutaş, K. Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hsieh, J.-H.; Wang, I.-T.; Jheng, P.-R.; Yeh, Y.-Y.; Lee, J.-W.; Bolouki, N.; Chuang, E.-Y. Transferred cold atmospheric plasma treatment on melanoma skin cancer cells with/without catalase enzyme in vitro. Appl. Sci. 2021, 11, 6181. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar]
- Philpott, M.; Sanders, D.; Bowen, J.; Kealey, T. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: A possible role for interleukin-1 and tumour necrosis factor-α in alopecia areata. Br. J. Dermatol. 1996, 135, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dai, J.; Ai, H.; Du, W.; Ji, H. β-Nicotinamide Mononucletodie Promotes Cell Proliferation and Hair Growth by Reducing Oxidative Stress. Molecules 2024, 29, 798. [Google Scholar] [CrossRef] [PubMed]
- Aguchem, R.N.; Okagu, I.U.; Okagu, O.D.; Ndefo, J.C.; Udenigwe, C.C. A review on the techno-functional, biological, and health-promoting properties of hempseed-derived proteins and peptide. J. Food Biochem. 2022, 46, e14127. [Google Scholar] [CrossRef]
- Liu, L.-P.; Wariboko, M.A.; Hu, X.; Wang, Z.-H.; Wu, Q.; Li, Y.-M. Factors associated with early-onset androgenetic alopecia: A scoping review. PLoS ONE 2024, 19, e0299212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Rungseevijitprapa, W.; Narkkhong, N.-A.; Suttajit, M.; Chaiyasut, C. 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment. J. Ethnopharmacol. 2012, 139, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gao, W.V.; Endo, H.; McElwee, K.J. Experimental and early investigational drugs for androgenetic alopecia. Expert Opin. Investig. Drugs 2017, 26, 917–932. [Google Scholar] [CrossRef]
- Leiros, G.J.; Attorresi, A.I.; Balaña, M.E. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2012, 166, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Jia, K.; Xu, X.; Li, Y.; Zhao, Y.; Zhang, X.; Zhang, J.; Liu, G.; Deng, S. Crosstalk between androgen and Wnt/β-catenin leads to changes of wool density in FGF5-knockout sheep. Cell Death Dis. 2020, 11, 407. [Google Scholar] [CrossRef]
- Chung, M.S.; Bae, W.J.; Choi, S.W.; Lee, K.W.; Jeong, H.C.; Bashraheel, F.; Jeon, S.H.; Jung, J.W.; Yoon, B.I.; Kwon, E.B. An Asian traditional herbal complex containing Houttuynia cordata Thunb, Perilla frutescens Var. acuta and green tea stimulates hair growth in mice. BMC Complement. Altern. Med. 2017, 17, 515. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Shin, J.S.; Han, H.S.; Lee, S.B.; Myung, D.B.; Lee, K.S.; Lee, S.H.; Kim, H.J.; Lee, K.T. Chemical Constituents from Leaves of Hydrangea serrata and Their Anti-photoaging Effects on UVB-Irradiated Human Fibroblasts. Biol. Pharm. Bull. 2019, 42, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Adelman, M.J.; Bedford, L.M.; Potts, G.A. Clinical efficacy of popular oral hair growth supplement ingredients. Int. J. Dermatol. 2021, 60, 1199–1210. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, H.; Ahn, H.S.; Na, C.; Shin, Y.-K. Hair Growth-Promoting Effect of Hydrangea serrata (Thunb.) Ser. Extract and Its Active Component Hydrangenol: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2024, 25, 10370. https://doi.org/10.3390/ijms251910370
Park S, Kim H, Ahn HS, Na C, Shin Y-K. Hair Growth-Promoting Effect of Hydrangea serrata (Thunb.) Ser. Extract and Its Active Component Hydrangenol: In Vitro and In Vivo Study. International Journal of Molecular Sciences. 2024; 25(19):10370. https://doi.org/10.3390/ijms251910370
Chicago/Turabian StylePark, Soyoon, Hyunjae Kim, Hye Shin Ahn, Changseon Na, and Yu-Kyong Shin. 2024. "Hair Growth-Promoting Effect of Hydrangea serrata (Thunb.) Ser. Extract and Its Active Component Hydrangenol: In Vitro and In Vivo Study" International Journal of Molecular Sciences 25, no. 19: 10370. https://doi.org/10.3390/ijms251910370
APA StylePark, S., Kim, H., Ahn, H. S., Na, C., & Shin, Y. -K. (2024). Hair Growth-Promoting Effect of Hydrangea serrata (Thunb.) Ser. Extract and Its Active Component Hydrangenol: In Vitro and In Vivo Study. International Journal of Molecular Sciences, 25(19), 10370. https://doi.org/10.3390/ijms251910370







