Amino Acid-Based Protein-Mimic Hydrogel Incorporating Pro-Regenerative Lipid Mediator and Microvascular Fragments Promotes the Healing of Deep Burn Wounds
Abstract
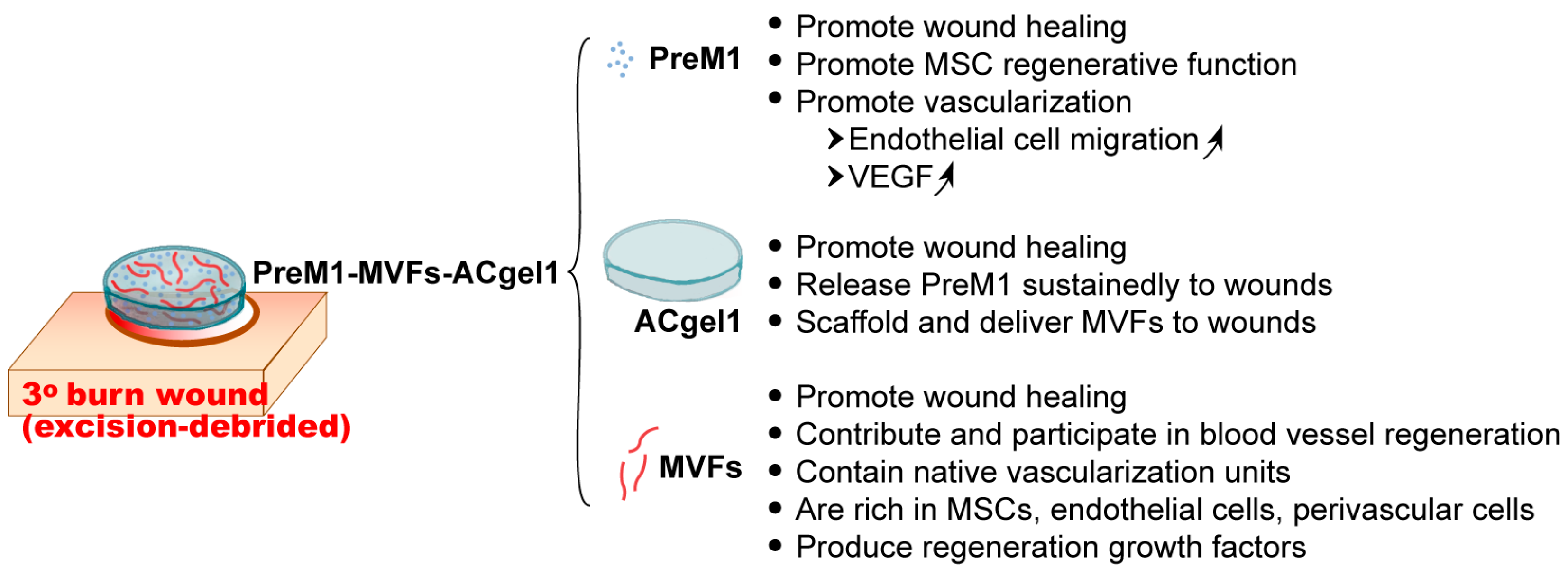
1. Introduction
2. Results
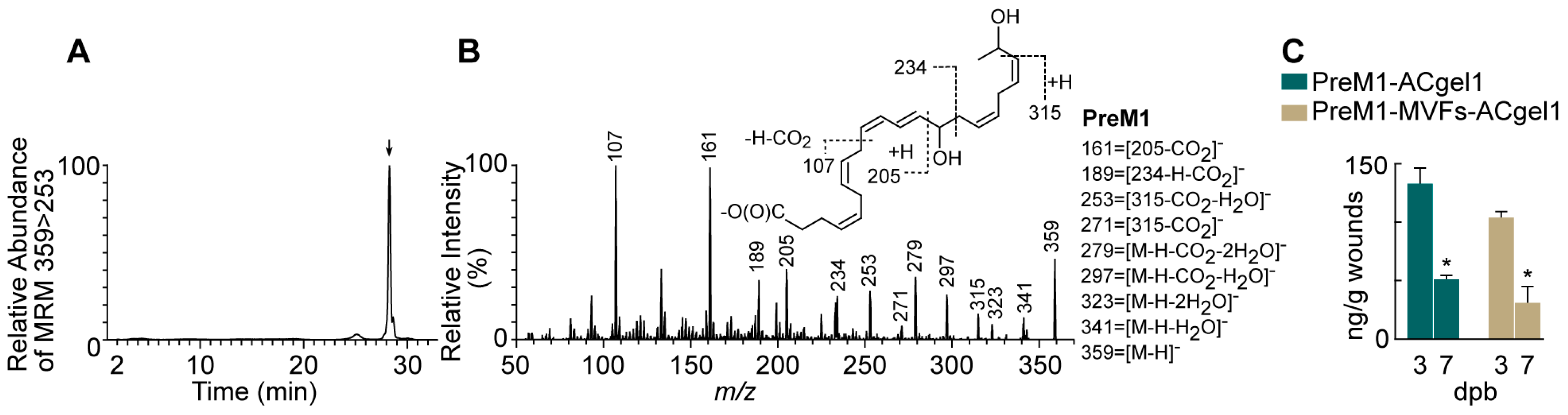
2.1. PreM1 Release to 3° Burn Wounds from ACgel1 with and without MVF Seeding
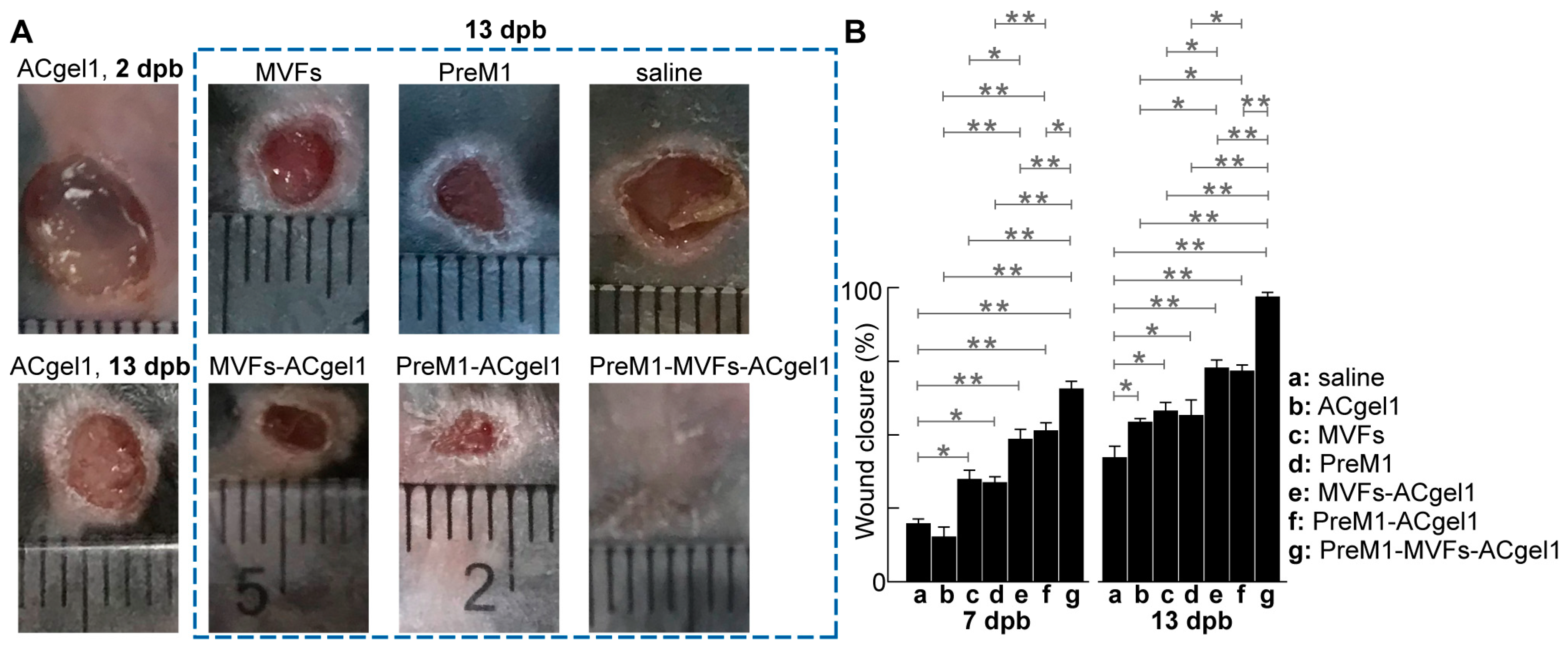
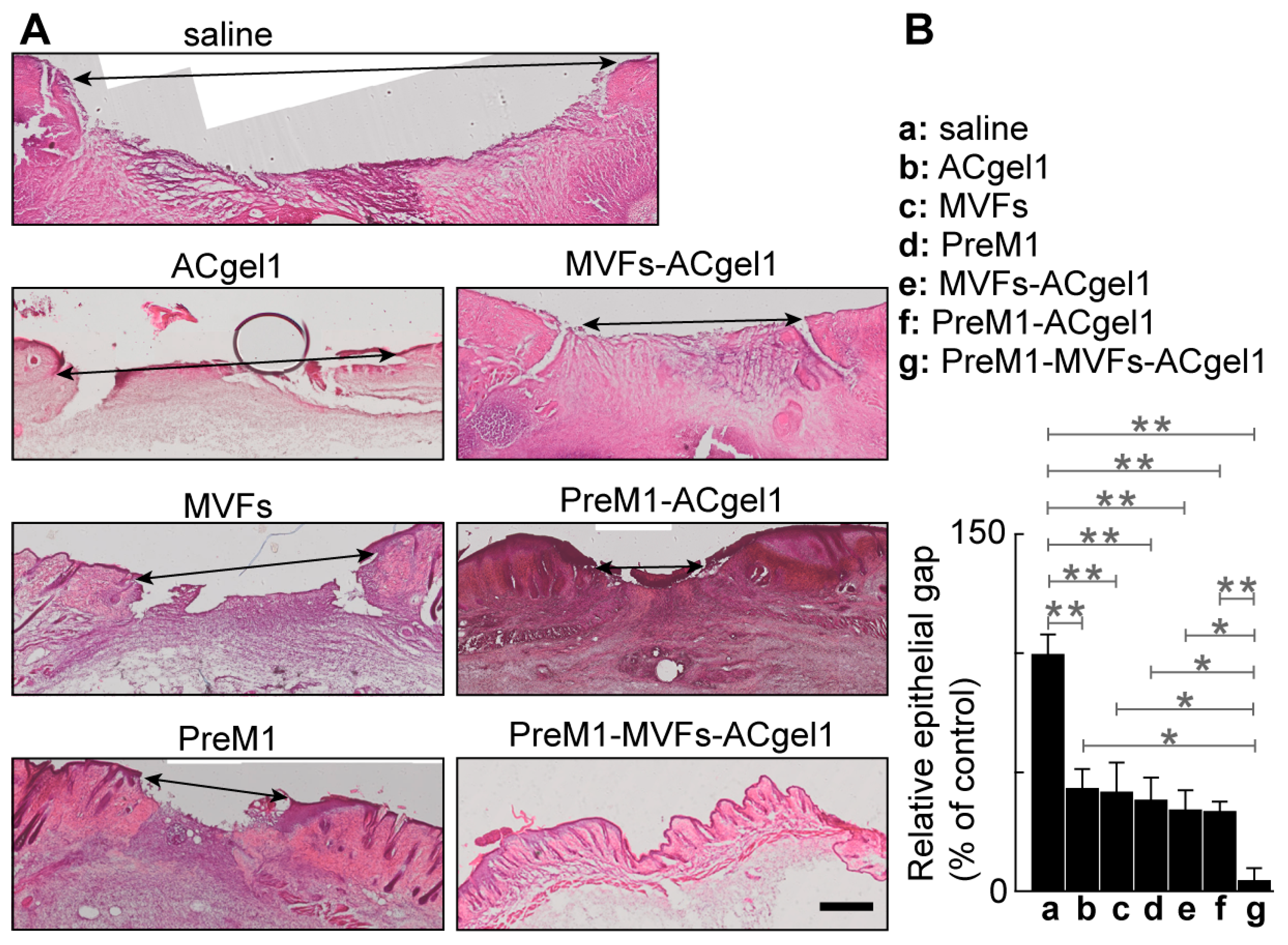
2.2. PreM1-Containing ACgel1 Markedly Enhanced MVF Promotion of Wound Closure and Re-Epithelialization of 3° Burn Wounds
2.3. PreM1 Enhanced MVFs-ACgel1 Promotion and MVFs Enhanced PreM1-Acgel1 Promotion of Blood Vessel Regeneration in 3° Burn Wounds
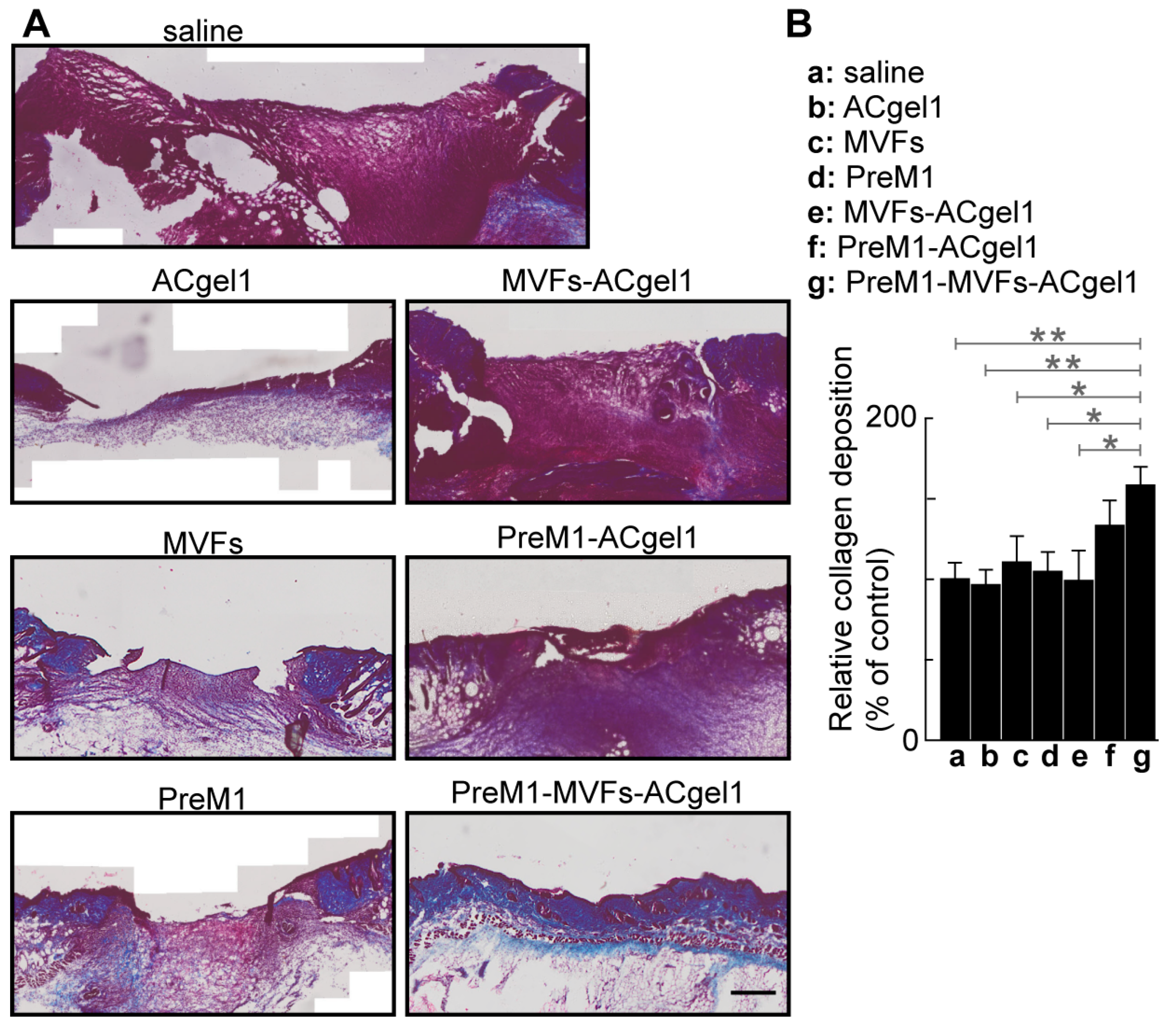
2.4. PreM1-MVFs-ACgel1 Treatment Increased the Collagen Level in 3° Burn Wounds
2.5. No Behavior Abnormality Was Observed for Each Treatment Compared to Saline Control
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the PreM1-Containing ACgel1
4.3. Animals: General Information
4.4. Preparation of Adipose Tissue-Derived Microvascular Fragments from Mouse Fat Pads
4.5. MVF Seeding of PreM1-Containing ACgel1
4.6. Mouse Model of 3° Burn Wounds and Treatments
4.7. Postoperative Care, Wound Closure Measurement, and Sampling
4.8. Determination of PreM1 Release to 3° Burn Wounds from ACgel1 with and without MVF Seeding
4.9. Histochemical and Immunohistological Studies of 3° Burn Wounds under Different Treatments
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viswanathan, S.; Keating, A.; Deans, R.; Hematti, P.; Prockop, D.; Stroncek, D.F.; Stacey, G.; Weiss, D.J.; Mason, C.; Rao, M.S. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014, 23, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Veriter, S.; Andre, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.L.; Dufrane, D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different “Hospital Exemption” Clinical Applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Bond, J.; Bergeron, A.; Miller, K.J.; Ehanire, T.; Quiles, C.; Lorden, E.R.; Medina, M.A.; Fisher, M.; Klitzman, B.; et al. A novel immune competent murine hypertrophic scar contracture model: A tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen. 2014, 22, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, X.; Liu, L.; Marti, G.P.; Ghanamah, M.S.; Arshad, M.J.; Strom, L.; Spence, R.; Jeng, J.; Milner, S.; et al. Association of increasing burn severity in mice with delayed mobilization of circulating angiogenic cells. Arch. Surg. 2010, 145, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.J. Stem cell application in acute burn care and reconstruction. J. Wound Care 2013, 22, 7–8, 10, 12–16. [Google Scholar] [CrossRef]
- Chan, R.K.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Renz, E.M.; Christy, R.J.; Natesan, S. Development of a Vascularized Skin Construct Using Adipose-Derived Stem Cells from Debrided Burned Skin. Stem Cells Int. 2012, 2012, 841203. [Google Scholar] [CrossRef]
- American Burn Association. National Burn Repository Report of Data 2006–2015; Version 12.0; American Burn Association: Chicago, IL, USA, 2016; Available online: https://ameriburn.org/wp-content/uploads/2017/05/2016abanbr_final_42816.pdf (accessed on 5 September 2024).
- Rogers, A.D.; Jeschke, M.G. Managing severe burn injuries: Challenges and solutions in complex and chronic wound care. Chrnonic Wound Care Manag. Res. 2016, 3, 59–71. [Google Scholar]
- Liu, A.; Cheong, J.Z.A.; Hassan, S.; Wielgat, M.B.; Meudt, J.J.; Townsend, E.C.; Shanmuganayagam, D.; Kalan, L.R.; Gibson, A. The effect of anatomic location on porcine models of burn injury and wound healing. Wound Repair Regen. 2024, 32, 675–685. [Google Scholar] [CrossRef]
- Karim, A.S.; Yan, A.; Ocotl, E.; Bennett, D.D.; Wang, Z.; Kendziorski, C.; Gibson, A.L.F. Discordance between histologic and visual assessment of tissue viability in excised burn wound tissue. Wound Repair Regen. 2019, 27, 150–161. [Google Scholar] [CrossRef]
- Gibson, A.L.F.; Shatadal, S. A simple and improved method to determine cell viability in burn-injured tissue. J. Surg. Res. 2017, 215, 83–87. [Google Scholar] [CrossRef]
- Gibson, A.L.F.; Carney, B.C.; Cuttle, L.; Andrews, C.J.; Kowalczewski, C.J.; Liu, A.; Powell, H.M.; Stone, R.; Supp, D.M.; Singer, A.J.; et al. Coming to Consensus: What Defines Deep Partial Thickness Burn Injuries in Porcine Models? J. Burn Care Res. 2021, 42, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Willard, J.J.; Supp, D.M.; Roy, S.; Gordillo, G.M.; Sen, C.K.; Powell, H.M. Burn Scar Biomechanics after Pressure Garment Therapy. Plast. Reconstr. Surg. 2015, 136, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Ii, R.S.; Natesan, S.; Kowalczewski, C.J.; Mangum, L.H.; Clay, N.E.; Clohessy, R.M.; Carlsson, A.H.; Tassin, D.H.; Chan, R.K.; Rizzo, J.A.; et al. Advancements in Regenerative Strategies through the Continuum of Burn Care. Front. Pharmacol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, H.; Hong, S. Novel 14,21-dihydroxy-docosahexaenoic acids: Structures, formation pathways, and enhancement of wound healing. J. Lipid Res. 2010, 51, 923–932. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Hong, S. 14S,21R-Dihydroxydocosahexaenoic Acid Remedies Impaired Healing and Mesenchymal Stem Cell Functions in Diabetic Wounds. J. Biol. Chem. 2011, 286, 4443–4453. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Wang, Q.; Hong, S. 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev. 2012, 21, 1187–1199. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Dalli, J.; Vlasakov, I.; Riley, I.R.; Rodriguez, A.R.; Spur, B.W.; Petasis, N.A.; Chiang, N.; Serhan, C.N. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 12232–12237. [Google Scholar] [CrossRef]
- Calandria, J.M.; Bhattacharjee, S.; Kala-Bhattacharjee, S.; Mukherjee, P.K.; Feng, Y.; Vowinckel, J.; Treiber, T.; Bazan, N.G. Elovanoid-N34 modulates TXNRD1 key in protection against oxidative stress-related diseases. Cell Death Dis. 2023, 14, 819. [Google Scholar] [CrossRef]
- Pham, T.L.; Kakazu, A.H.; He, J.; Jun, B.; Bazan, N.G.; Bazan, H.E.P. Novel RvD6 stereoisomer induces corneal nerve regeneration and wound healing post-injury by modulating trigeminal transcriptomic signature. Sci. Rep. 2020, 10, 4582. [Google Scholar] [CrossRef]
- Bazan, N.G.; Musto, A.E.; Knott, E.J. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol. Neurobiol. 2011, 44, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; He, J.; Kakazu, A.H.; Calandria, J.; Do, K.V.; Nshimiyimana, R.; Lam, T.F.; Petasis, N.A.; Bazan, H.E.P.; Bazan, N.G. ELV-N32 and RvD6 isomer decrease pro-inflammatory cytokines, senescence programming, ACE2 and SARS-CoV-2-spike protein RBD binding in injured cornea. Sci. Rep. 2021, 11, 12787. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Calandria, J.M.; Serhan, C.N. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 2010, 51, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.V.; Spite, M.; Yang, R.; Flower, R.J.; Perretti, M.; Serhan, C.N. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011, 186, 5543–5547. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Tang, Y.; Spite, M. Proresolving lipid mediators and diabetic wound healing. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 104–108. [Google Scholar] [CrossRef]
- Panigrahy, D.; Kalish, B.T.; Huang, S.; Bielenberg, D.R.; Le, H.D.; Yang, J.; Edin, M.L.; Lee, C.R.; Benny, O.; Mudge, D.K.; et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13528–13533. [Google Scholar] [CrossRef]
- Kelly, A.G.; Panigrahy, D. Targeting Angiogenesis via Resolution of Inflammation. Cold Spring Harb. Perspect. Med. 2023, 13, a041172. [Google Scholar] [CrossRef]
- Kurihara, T.; Jones, C.N.; Yu, Y.M.; Fischman, A.J.; Watada, S.; Tompkins, R.G.; Fagan, S.P.; Irimia, D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013, 27, 2270–2281. [Google Scholar] [CrossRef]
- Mustafa, M.; Zarrough, A.; Bolstad, A.I.; Lygre, H.; Mustafa, K.; Hasturk, H.; Serhan, C.; Kantarci, A.; Van Dyke, T.E. Resolvin D1 protects periodontal ligament. Am. J. Physiol. Cell Physiol. 2013, 305, C673–C679. [Google Scholar] [CrossRef]
- Bohr, S.; Patel, S.J.; Sarin, D.; Irimia, D.; Yarmush, M.L.; Berthiaume, F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013, 21, 35–43. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Pan, Z.; Wang, Z.; Wolosin, J.M.; Gjorstrup, P.; Reinach, P.S. Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5601–5609. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Lu, Y.; Shah, S.P.; Hong, S. Novel 14S,21-dihydroxy-docosahexaenoic acid rescues wound healing and associated angiogenesis impaired by acute ethanol intoxication/exposure. J. Cell. Biochem. 2010, 111, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Burd, A. An update review of stem cell applications in burns and wound care. Indian J. Plast. Surg. 2012, 45, 229–236. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Adipose tissue-derived microvascular fragments: Natural vascularization units for regenerative medicine. Trends Biotechnol. 2015, 33, 442–448. [Google Scholar] [CrossRef]
- Acosta, F.M.; Gonzalez Porras, M.A.; Stojkova, K.; Pacelli, S.; Rathbone, C.R.; Brey, E.M. Three-Dimensional Culture of Vascularized Thermogenic Adipose Tissue from Microvascular Fragments. J. Vis. Exp. 2023, 192, e64650. [Google Scholar] [CrossRef]
- Frueh, F.S.; Spater, T.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Isolation of Murine Adipose Tissue-derived Microvascular Fragments as Vascularization Units for Tissue Engineering. J. Vis. Exp. 2017, 122, e55721. [Google Scholar] [CrossRef]
- Acosta, F.M.; Stojkova, K.; Zhang, J.; Garcia Huitron, E.I.; Jiang, J.X.; Rathbone, C.R.; Brey, E.M. Engineering Functional Vascularized Beige Adipose Tissue from Microvascular Fragments of Models of Healthy and Type II Diabetes Conditions. J. Tissue Eng. 2022, 13, 20417314221109337. [Google Scholar] [CrossRef]
- Acosta, F.M.; Stojkova, K.; Brey, E.M.; Rathbone, C.R. A Straightforward Approach to Engineer Vascularized Adipose Tissue Using Microvascular Fragments. Tissue Eng. Part A 2020, 26, 905–914. [Google Scholar] [CrossRef]
- Shepherd, B.R.; Chen, H.Y.; Smith, C.M.; Gruionu, G.; Williams, S.K.; Hoying, J.B. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 898–904. [Google Scholar] [CrossRef]
- McDaniel, J.S.; Pilia, M.; Ward, C.L.; Pollot, B.E.; Rathbone, C.R. Characterization and multilineage potential of cells derived from isolated microvascular fragments. J. Surg. Res. 2014, 192, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Frueh, F.S.; Spater, T.; Lindenblatt, N.; Calcagni, M.; Giovanoli, P.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Adipose Tissue-Derived Microvascular Fragments Improve Vascularization, Lymphangiogenesis, and Integration of Dermal Skin Substitutes. J. Investig. Dermatol. 2017, 137, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Spater, T.; Frueh, F.S.; Menger, M.D.; Laschke, M.W. Potentials and limitations of Integra(R) flowable wound matrix seeded with adipose tissue-derived microvascular fragments. Eur. Cells Mater. 2017, 33, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.; Harder, Y.; Schmauss, D.; Menger, M.D.; Laschke, M.W. Microvascular Fragments Protect Ischemic Musculocutaneous Flap Tissue from Necrosis by Improving Nutritive Tissue Perfusion and Suppressing Apoptosis. Biomedicines 2023, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Akhtar, S.; Mohsin, S.; Khan, S.N.; Riazuddin, S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2011, 20, 67–75. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, X.; Wu, Y.; Li, L.; Yin, Q.; Zheng, L.; Zhang, H.; Sun, C. Streptozotocin-induced diabetic rat-derived bone marrow mesenchymal stem cells have impaired abilities in proliferation, paracrine, antiapoptosis, and myogenic differentiation. Transplant. Proc. 2010, 42, 2745–2752. [Google Scholar] [CrossRef]
- Haider, H.; Ashraf, M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J. Mol. Cell. Cardiol. 2008, 45, 554–566. [Google Scholar] [CrossRef]
- Kirker, K.R.; Luo, Y.; Nielson, J.H.; Shelby, J.; Prestwich, G.D. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 2002, 23, 3661–3671. [Google Scholar] [CrossRef]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Kiyozumi, T.; Kanatani, Y.; Ishihara, M.; Saitoh, D.; Shimizu, J.; Yura, H.; Suzuki, S.; Okada, Y.; Kikuchi, M. The effect of chitosan hydrogel containing DMEM/F12 medium on full-thickness skin defects after deep dermal burn. Burns 2007, 33, 642–648. [Google Scholar] [CrossRef]
- Kim, K.L.; Han, D.K.; Park, K.; Song, S.H.; Kim, J.Y.; Kim, J.M.; Ki, H.Y.; Yie, S.W.; Roh, C.R.; Jeon, E.S.; et al. Enhanced dermal wound neovascularization by targeted delivery of endothelial progenitor cells using an RGD-g-PLLA scaffold. Biomaterials 2009, 30, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Armes, S.P.; Bertal, K.; Lomas, H.; Macneil, S.; Lewis, A.L. Biocompatible wound dressings based on chemically degradable triblock copolymer hydrogels. Biomacromolecules 2008, 9, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Sarker, P.; Rimmer, S.; Swanson, L.; MacNeil, S.; Douglas, I. Hyperbranched poly(NIPAM) polymers modified with antibiotics for the reduction of bacterial burden in infected human tissue engineered skin. Biomaterials 2011, 32, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Baravkar, S.B.; Lu, Y.; Masoud, A.R.; Zhao, Q.; He, J.; Hong, S. Development of a Novel Covalently Bonded Conjugate of Caprylic Acid Tripeptide (Isoleucine-Leucine-Aspartic Acid) for Wound-Compatible and Injectable Hydrogel to Accelerate Healing. Biomolecules 2024, 14, 94. [Google Scholar] [CrossRef]
- Rustad, K.C.; Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Major, M.R.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012, 33, 80–90. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef]
- Vriend, L.; van der Lei, B.; Harmsen, M.C.; van Dongen, J.A. Adipose Tissue-Derived Components: From Cells to Tissue Glue to Treat Dermal Damage. Bioengineering 2023, 10, 328. [Google Scholar] [CrossRef]
- Liu, H.; Xing, F.; Yu, P.; Zhe, M.; Duan, X.; Liu, M.; Xiang, Z.; Ritz, U. A review of biomacromolecule-based 3D bioprinting strategies for structure-function integrated repair of skin tissues. Int. J. Biol. Macromol. 2024, 268, 131623. [Google Scholar] [CrossRef]
- Kammona, O.; Tsanaktsidou, E.; Kiparissides, C. Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings. Gels 2024, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Alapure, B.V.; Lu, Y.; He, M.; Chu, C.C.; Peng, H.; Muhale, F.; Brewerton, Y.L.; Bunnell, B.; Hong, S. Accelerate Healing of Severe Burn Wounds by Mouse Bone Marrow Mesenchymal Stem Cell-Seeded Biodegradable Hydrogel Scaffold Synthesized from Arginine-Based Poly(ester amide) and Chitosan. Stem Cells Dev. 2018, 27, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Potuck, A.; Zhang, Y.; Chu, C.C. Arginine-based polyester amide/polysaccharide hydrogels and their biological response. Acta Biomater. 2014, 10, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C. Novel Biodegradable Functional Amino Acid-based Poly(ester amide) Biomaterials: Design, Synthesis, Property and Biomedical Applications. J. Fiber Bioeng. Inform. 2012, 5, 1–31. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, X.; Wu, D.; Chu, C.C. Development of a biocompatible and biodegradable hybrid hydrogel platform for sustained release of ionic drugs. J. Mater. Chem. B 2014, 2, 6660–6668. [Google Scholar] [CrossRef]
- Nishimura, K.; Sakaguchi, T.; Nanba, Y.; Suganuma, Y.; Morita, M.; Hong, S.; Lu, Y.; Jun, B.; Bazan, N.G.; Arita, M.; et al. Stereoselective Total Synthesis of Macrophage-Produced Prohealing 14,21-Dihydroxy Docosahexaenoic Acids. J. Org. Chem. 2018, 83, 154–166. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Hong, S. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. Am. J. Pathol. 2011, 179, 1780–1791. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Singer, A.J.; Boyce, S.T. Burn Wound Healing and Tissue Engineering. J. Burn Care Res. 2017, 38, e605–e613. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E. The basis of fibrosis and wound healing disorders following thermal injury. J. Trauma Acute Care Surg. 2007, 62, S69. [Google Scholar] [CrossRef] [PubMed]
- Alapure, B.V.; Lu, Y.; Peng, H.; Hong, S. Surgical Denervation of Specific Cutaneous Nerves Impedes Excisional Wound Healing of Small Animal Ear Pinnae. Mol. Neurobiol. 2018, 55, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Ocotl, E.; Karim, A.; Wolf, J.J.; Cox, B.L.; Eliceiri, K.W.; Gibson, A.L.F. Modeling early thermal injury using an ex vivo human skin model of contact burns. Burns 2021, 47, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Cox, B.; Karim, A.; Liu, A.; Gibson, A.L.F.; Eliceiri, K. Burn Device for Reproducible Wound Generation on Ex-Vivo Human Skin Models. Morgridge Institute for Research. Available online: https://morgridge.org/research/labs/fablab/designs/burn-device-for-reproducible-wound-generation/ (accessed on 1 September 2024).
- Hong, S.; Baravkar, S.B.; Lu, Y. Amphiphilic Conjugates of Fatty Acids or Their Derivatives. U.S. Provisional Patent No: 63617303, 3 January 2024. [Google Scholar]
- Chung, T.Y.; Peplow, P.V.; Baxter, G.D. Laser photobiomodulation of wound healing in diabetic and non-diabetic mice: Effects in splinted and unsplinted wounds. Photomed. Laser Surg. 2010, 28, 251–261. [Google Scholar] [CrossRef]
- Bae, S.H.; Bae, Y.C.; Nam, S.B.; Choi, S.J. A skin fixation method for decreasing the influence of wound contraction on wound healing in a rat model. Arch. Plast. Surg. 2012, 39, 457–462. [Google Scholar] [CrossRef]
- Yao, Z.; Huang, Y.; Luo, G.; Wu, J.; He, W. A biological membrane-based novel excisional wound-splinting model in mice (with video). Burn. Trauma 2014, 2, 196–200. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y.; Tian, H.; Alapure, B.V.; Wang, Q.; Bunnell, B.A.; Laborde, J.M. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem. Biol. 2014, 21, 1318–1329. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Su, S.; Chu, C.-C.; Kobayashi, Y.; Masoud, A.-R.; Peng, H.; Lien, N.; He, M.; Vuong, C.; Tran, R.; et al. Amino Acid-Based Protein-Mimic Hydrogel Incorporating Pro-Regenerative Lipid Mediator and Microvascular Fragments Promotes the Healing of Deep Burn Wounds. Int. J. Mol. Sci. 2024, 25, 10378. https://doi.org/10.3390/ijms251910378
Lu Y, Su S, Chu C-C, Kobayashi Y, Masoud A-R, Peng H, Lien N, He M, Vuong C, Tran R, et al. Amino Acid-Based Protein-Mimic Hydrogel Incorporating Pro-Regenerative Lipid Mediator and Microvascular Fragments Promotes the Healing of Deep Burn Wounds. International Journal of Molecular Sciences. 2024; 25(19):10378. https://doi.org/10.3390/ijms251910378
Chicago/Turabian StyleLu, Yan, Shanchun Su, Chih-Chang Chu, Yuichi Kobayashi, Abdul-Razak Masoud, Hongying Peng, Nathan Lien, Mingyu He, Christopher Vuong, Ryan Tran, and et al. 2024. "Amino Acid-Based Protein-Mimic Hydrogel Incorporating Pro-Regenerative Lipid Mediator and Microvascular Fragments Promotes the Healing of Deep Burn Wounds" International Journal of Molecular Sciences 25, no. 19: 10378. https://doi.org/10.3390/ijms251910378
APA StyleLu, Y., Su, S., Chu, C.-C., Kobayashi, Y., Masoud, A.-R., Peng, H., Lien, N., He, M., Vuong, C., Tran, R., & Hong, S. (2024). Amino Acid-Based Protein-Mimic Hydrogel Incorporating Pro-Regenerative Lipid Mediator and Microvascular Fragments Promotes the Healing of Deep Burn Wounds. International Journal of Molecular Sciences, 25(19), 10378. https://doi.org/10.3390/ijms251910378








