Diabetic Mice Spleen Vulnerability Contributes to Decreased Persistence of Antibody Production after SARS-CoV-2 Vaccine
Abstract
:1. Introduction
2. Results
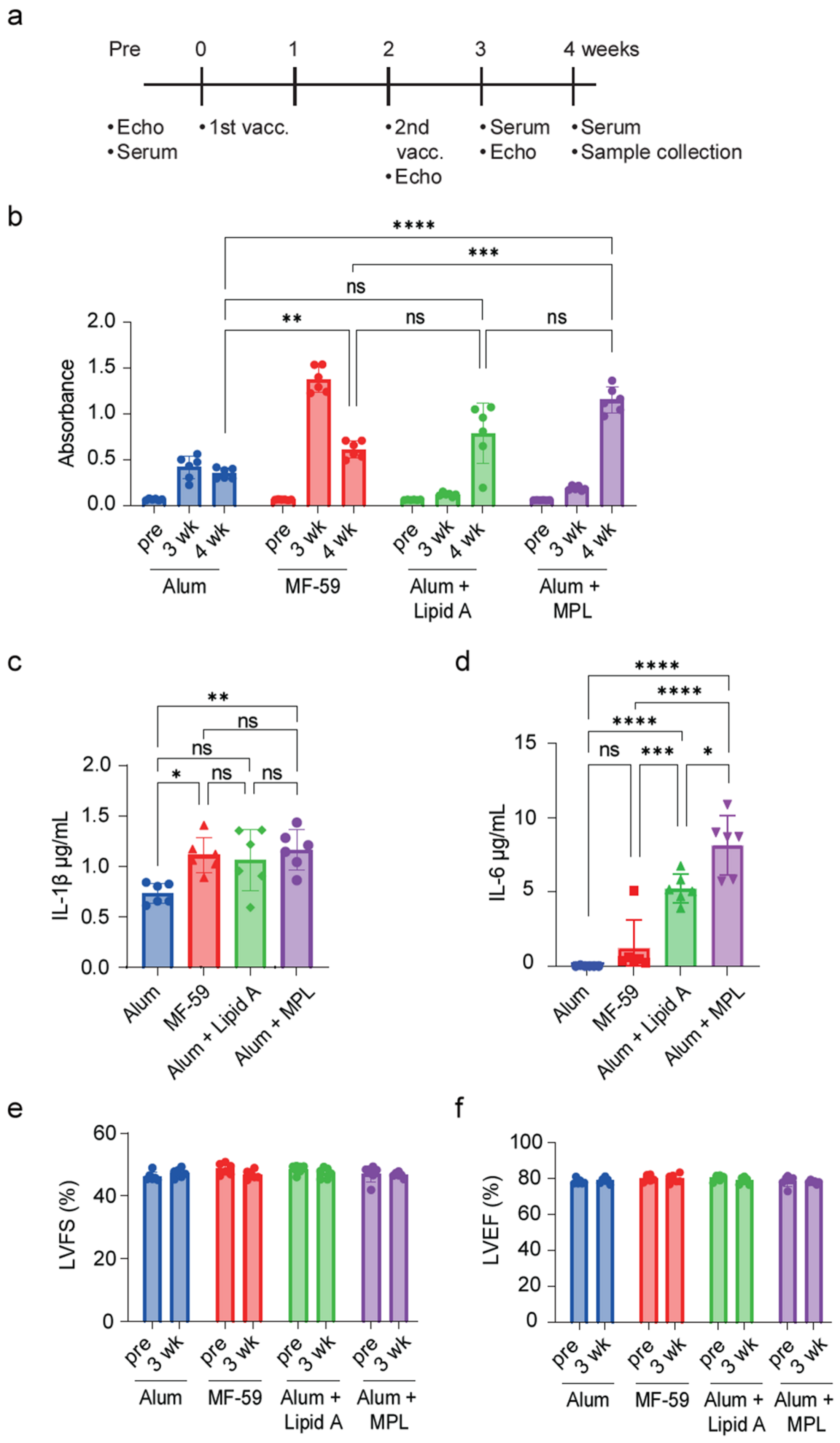
2.1. MF-59 Adjuvant with S-Protein Antigen Mixture Yields the Highest IgG Antibody Titer
2.2. S-Protein Has No Impact on Hyperglycemic Diabetic Cardiomyopathy
2.3. S-Protein Vaccine Has No Effect on Blood Glucose and Serum Insulin in Diabetic Mice
2.4. S-Protein Vaccine Has No Effect on Liver Function in Diabetic Mice
2.5. Vaccinated Diabetic Mice Decreased Persistence of S-Protein-Specific IgG Antibody Production
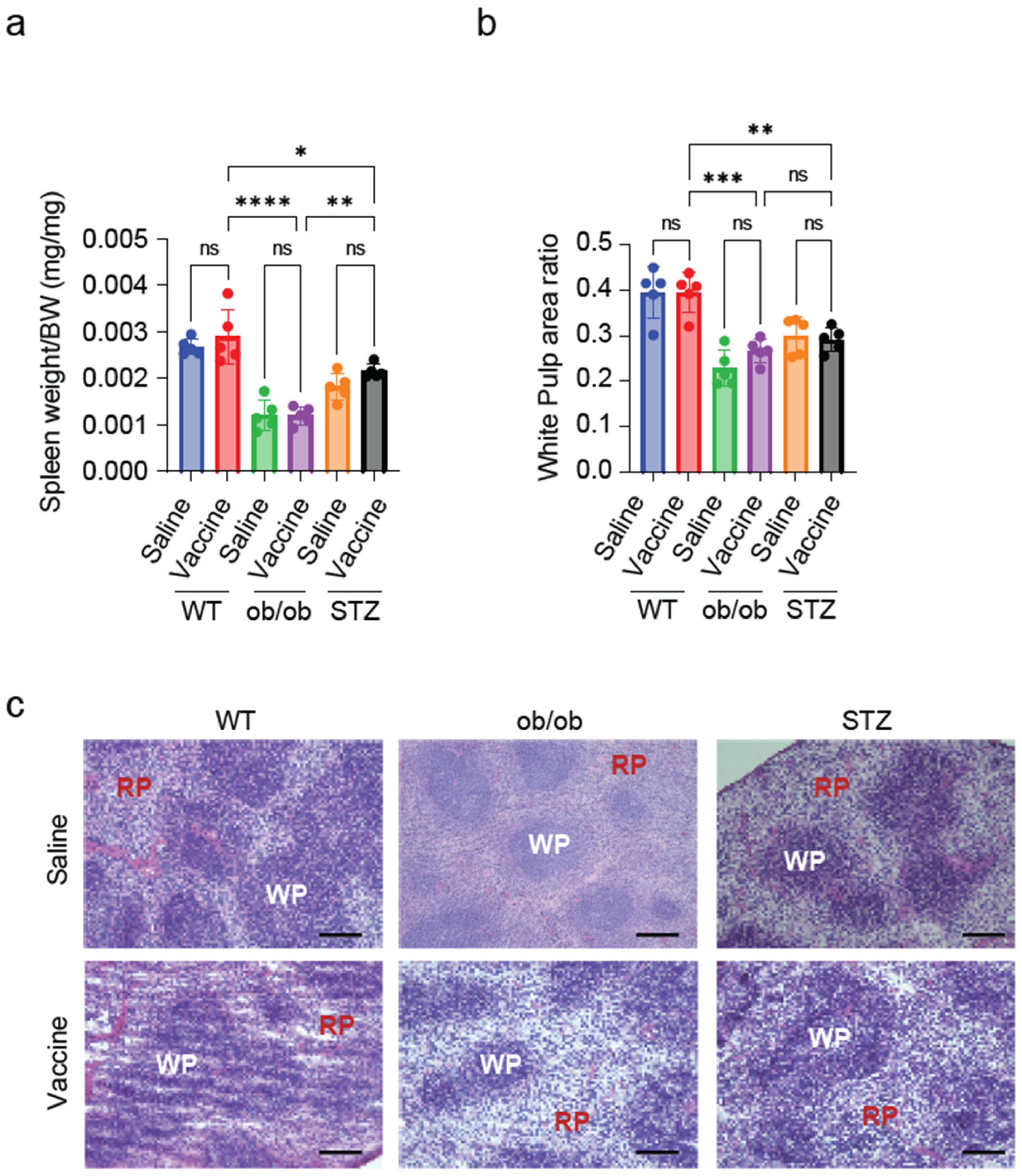
2.6. Splenic White Pulp Is Reduced in Diabetic Mice
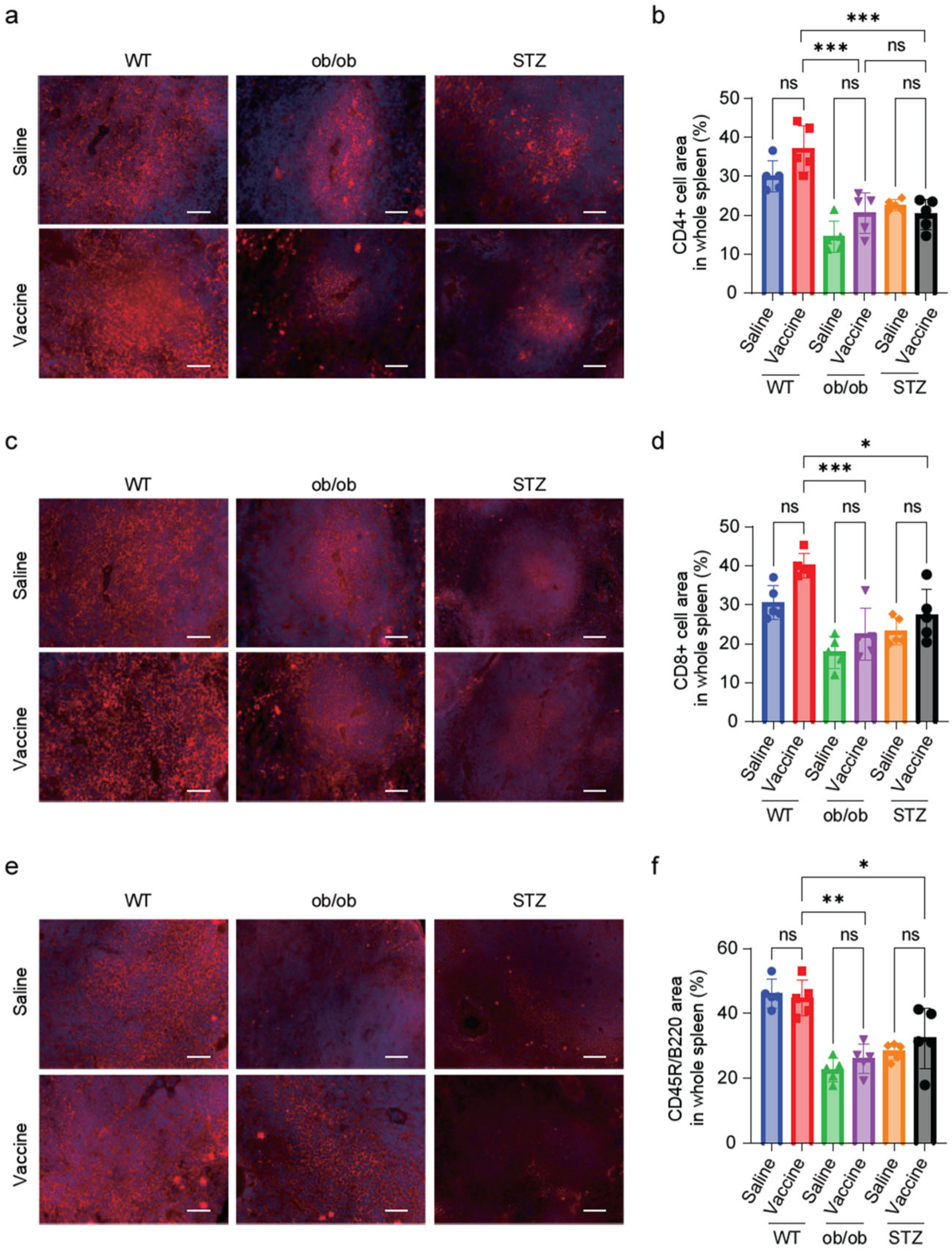
2.7. Diabetic Mice Showed a Reduction in T-Cell and B-Cell Zones in the Splenic Tissue
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Vaccine Preparation
4.3. Study Design
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Echocardiography
4.6. Blood Glucose
4.7. Dry Chem Measurements
4.8. Histological Analysis
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. In National Health Statistics Reports; The Centers for Disease Control and Prevention (CDC): Atlanta, Georgia, 2021. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Blundell, S.; Harris, T.; Cook, D.G.; Critchley, J. Diabetes and Infection: Assessing the Association with Glycaemic Control in Population-Based Studies. Lancet Diabetes Endocrinol. 2016, 4, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Song, Y.; Zhuang, Z.; Ran, J.; Xu, L.; Geng, Y.; Han, L.; Zhao, S.; Qin, J.; He, D.; et al. Obesity and COVID-19 in Adult Patients with Diabetes. Diabetes 2021, 70, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Sophiea, M.K.; Weldegiorgis, M. Diabetes and COVID-19: Population Impact 18 Months into the Pandemic. Diabetes Care 2021, 44, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Bulut, C.; Kato, Y. Epidemiology of COVID-19. Turk. J. Med. Sci. 2020, 50, 563–570. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. Vol. 2020, 5, 947–953. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The Effectiveness of COVID-19 Vaccines in Reducing the Incidence, Hospitalization, and Mortality from COVID-19: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 873596. [Google Scholar] [CrossRef]
- Soetedjo, N.N.M.; Iryaningrum, M.R.; Lawrensia, S.; Permana, H. Antibody Response Following SARS-CoV-2 Vaccination among Patients with Type 2 Diabetes Mellitus: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102406. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Sardu, C.; Prattichizzo, F.; Scisciola, L.; Messina, V.; La Grotta, R.; Luisa Balestrieri, M.; Maggi, P.; Napoli, C.; Ceriello, A.; et al. Glycaemic Control Is Associated with SARS-CoV-2 Breakthrough Infections in Vaccinated Patients with Type 2 Diabetes. Nat. Commun. 2022, 13, 2318. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central Obesity, Smoking Habit, and Hypertension Are Associated with Lower Antibody Titres in Response to COVID-19 MRNA Vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef] [PubMed]
- van der Klaauw, A.A.; Horner, E.C.; Pereyra-Gerber, P.; Agrawal, U.; Foster, W.S.; Spencer, S.; Vergese, B.; Smith, M.; Henning, E.; Ramsay, I.D.; et al. Accelerated Waning of the Humoral Response to COVID-19 Vaccines in Obesity. Nat. Med. 2023, 29, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Alhamar, G.; Briganti, S.; Maggi, D.; Viola, V.; Faraj, M.; Zannella, C.; Galdiero, M.; Franci, G.; Fusco, C.; Isgrò, C.; et al. Prevaccination Glucose Time in Range Correlates with Antibody Response to SARS-CoV-2 Vaccine in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, e474–e479. [Google Scholar] [CrossRef]
- D’addio, F.; Sabiu, G.; Usuelli, V.; Assi, E.; Abdelsalam, A.; Maestroni, A.; Seelam, A.J.; Nasr, M.B.; Loretelli, C.; Mileto, D.; et al. Immunogenicity and Safety of SARS-CoV-2 MRNA Vaccines in a Cohort of Patients with Type 1 Diabetes. Diabetes 2022, 71, 1800–1806. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef]
- Zhang, N.; Channappanavar, R.; Ma, C.; Wang, L.; Tang, J.; Garron, T.; Tao, X.; Tasneem, S.; Lu, L.; Tseng, C.T.K.; et al. Identification of an Ideal Adjuvant for Receptor-Binding Domain-Based Subunit Vaccines against Middle East Respiratory Syndrome Coronavirus. Cell. Mol. Immunol. 2016, 13, 180–190. [Google Scholar] [CrossRef]
- Han, X.; Alameh, M.-G.; Butowska, K.; Knox, J.J.; Lundgreen, K.; Ghattas, M.; Gong, N.; Xue, L.; Xu, Y.; Lavertu, M.; et al. Nature Nanotechnology Adjuvant Lipidoid-Substituted Lipid Nanoparticles Augment the Immunogenicity of SARS-CoV-2 MRNA Vaccines. Nat. Nanotechnol. 2023, 18, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Thuy, C.; Le, T.; Ahn, S.Y.; Ho, T.L.; Lee, J.; Lee, D.-H.; Hwang, H.S.; Kang, S.-M.; Ko, E.-J. Adjuvant Effects of Combination Monophosphoryl Lipid A and Poly I:C on Antigen-Specific Immune Responses and Protective Efficacy of Influenza Vaccines. Sci. Rep. 2023, 13, 12231. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel Insights into the Genetically Obese (Ob/Ob) and Diabetic (Db/Db) Mice: Two Sides of the Same Coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef]
- Kato, Y.; Ariyoshi, K.; Nohara, Y.; Matsunaga, N.; Shimauchi, T.; Shindo, N.; Nishimura, A.; Mi, X.; Kim, S.G.; Ide, T.; et al. Inhibition of Dynamin-Related Protein 1-Filamin Interaction Improves Systemic Glucose Metabolism. Br. J. Pharmacol. 2024; early view. [Google Scholar] [CrossRef]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V.; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 MRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Rodent Models of Diabetic Cardiomyopathy. Dis. Model. Mech. 2009, 2, 454–466. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and Function of the Immune System in the Spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ji, Q.; Liu, Z.; Tang, K.; Xie, Y.; Li, K.; Zhou, J.; Li, S.; Shang, H.; Shi, Z.; et al. Effect of Different Adjuvants on Immune Responses Elicited by Protein-Based Subunit Vaccines against SARS-CoV-2 and Its Delta Variant. Viruses 2022, 14, 501. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, S.; Aggarwal, R.; Chauhan, M.K. Diabetes and Cardiovascular Disease: Inter-Relation of Risk Factors and Treatment. Future J. Pharm. Sci. 2020, 6, 130. [Google Scholar] [CrossRef]
- Ssentongo, P.; Zhang, Y.; Witmer, L.; Chinchilli, V.M.; Ba, D.M. Association of COVID-19 with Diabetes: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 20191. [Google Scholar] [CrossRef]
- Xiong, X.; Lui, D.T.W.; Chung, M.S.H.; Au, I.C.H.; Lai, F.T.T.; Wan, E.Y.F.; Chui, C.S.L.; Li, X.; Cheng, F.W.T.; Cheung, C.L.; et al. Incidence of Diabetes Following COVID-19 Vaccination and SARS-CoV-2 Infection in Hong Kong: A Population-Based Cohort Study. PLoS Med. 2023, 20, e1004274. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Fu, Z.; Liu, D. Development of a Nongenetic Mouse Model of Type 2 Diabetes. Exp. Diabetes Res. 2011, 2011, 416254. [Google Scholar] [CrossRef]
- Mahfoz, A.M.; Gawish, A.Y. Insight into the Hepatoprotective, Hypolipidemic, and Antidiabetic Impacts of Aliskiren in Streptozotocin-Induced Diabetic Liver Disease in Mice. Diabetol. Metab. Syndr. 2022, 14, 163. [Google Scholar] [CrossRef]
- Rammohan, R.; Joy, M.; Saggar, T.; Magam, S.G.; Sinha, A.; Natt, D.; Mehta, V.; Bunting, S.; Anand, P.; Mustacchia, P.; et al. Investigating the Impact of COVID-19 Vaccines on Liver Function: Insights from a Single-Institute Study. Cureus 2023, 15, e36588. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The Generation of Antibody-Secreting Plasma Cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Muller, Y.D.; Golshayan, D.; Ehirchiou, D.; Wyss, J.C.; Giovannoni, L.; Meier, R.; Serre-Beinier, V.; Yung, G.P.; Morel, P.; Bühler, L.H.; et al. Immunosuppressive Effects of Streptozotocin-Induced Diabetes Result in Absolute Lymphopenia and a Relative Increase of T Regulatory Cells. Diabetes 2011, 60, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, J.; Li, B.; Lu, M.; Le, G.; Xie, Y. Metabolomics Based On1 H-Nmr Reveal the Regulatory Mechanisms of Dietary Methionine Restriction on Splenic Metabolic Dysfunction in Obese Mice. Foods 2021, 10, 2439. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.-J.; Varkey, P.; Chen, P.-Y.; Chang, S.-Y.; Huang, L.L.H. Counting CD4+ and CD8+ T Cells in the Spleen: A Novel In Vivo Method for Assessing Biomaterial Immunotoxicity. Regen. Biomater. 2014, 1, 11–16. [Google Scholar] [CrossRef]
- Qu, X.; Li, J.; Baldwin, H.S. Postnatal Lethality and Abnormal Development of Foregut and Spleen in Ndrg4 Mutant Mice. Biochem. Biophys. Res. Commun. 2016, 470, 613–619. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.J.; Lee, I.K.; Hong, C.W.; Jeon, J.H. Impact of Hyperglycemia on Immune Cell Function: A Comprehensive Review. In Diabetology International; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–16. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I.M. Immune Dysfunction in Patients with Diabetes Mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Alexander, M.; Cho, E.; Gliozheni, E.; Salem, Y.; Cheung, J.; Ichii, H. Pathology of Diabetes-Induced Immune Dysfunction. Int. J. Mol. Sci. 2024, 25, 7105. [Google Scholar] [CrossRef]
- Jafar, N.; Edriss, H.; Nugent, K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Masuda, A.; Lee, J.M.; Miyata, T.; Mon, H.; Sato, K.; Oyama, K.; Sakurai, Y.; Yasuda, J.; Takahashi, D.; Ueda, T.; et al. Optimization of SARS-CoV-2 Spike Protein Expression in the Silkworm and Induction of Efficient Protective Immunity by Inoculation with Alum Adjuvants. Front. Immunol. 2022, 12, 803647. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atef, Y.; Ito, T.; Masuda, A.; Kato, Y.; Nishimura, A.; Kanda, Y.; Kunisawa, J.; Kusakabe, T.; Nishida, M. Diabetic Mice Spleen Vulnerability Contributes to Decreased Persistence of Antibody Production after SARS-CoV-2 Vaccine. Int. J. Mol. Sci. 2024, 25, 10379. https://doi.org/10.3390/ijms251910379
Atef Y, Ito T, Masuda A, Kato Y, Nishimura A, Kanda Y, Kunisawa J, Kusakabe T, Nishida M. Diabetic Mice Spleen Vulnerability Contributes to Decreased Persistence of Antibody Production after SARS-CoV-2 Vaccine. International Journal of Molecular Sciences. 2024; 25(19):10379. https://doi.org/10.3390/ijms251910379
Chicago/Turabian StyleAtef, Yara, Tomoya Ito, Akitsu Masuda, Yuri Kato, Akiyuki Nishimura, Yasunari Kanda, Jun Kunisawa, Takahiro Kusakabe, and Motohiro Nishida. 2024. "Diabetic Mice Spleen Vulnerability Contributes to Decreased Persistence of Antibody Production after SARS-CoV-2 Vaccine" International Journal of Molecular Sciences 25, no. 19: 10379. https://doi.org/10.3390/ijms251910379
APA StyleAtef, Y., Ito, T., Masuda, A., Kato, Y., Nishimura, A., Kanda, Y., Kunisawa, J., Kusakabe, T., & Nishida, M. (2024). Diabetic Mice Spleen Vulnerability Contributes to Decreased Persistence of Antibody Production after SARS-CoV-2 Vaccine. International Journal of Molecular Sciences, 25(19), 10379. https://doi.org/10.3390/ijms251910379




