Nimodipine Used with Vincristine: Protects Schwann Cells and Neuronal Cells from Vincristine-Induced Cell Death but Increases Tumor Cell Susceptibility
Abstract
:1. Introduction
2. Results
2.1. NIM Treatment Affects Cell Morphology and Cell Viability during VCR Treatment
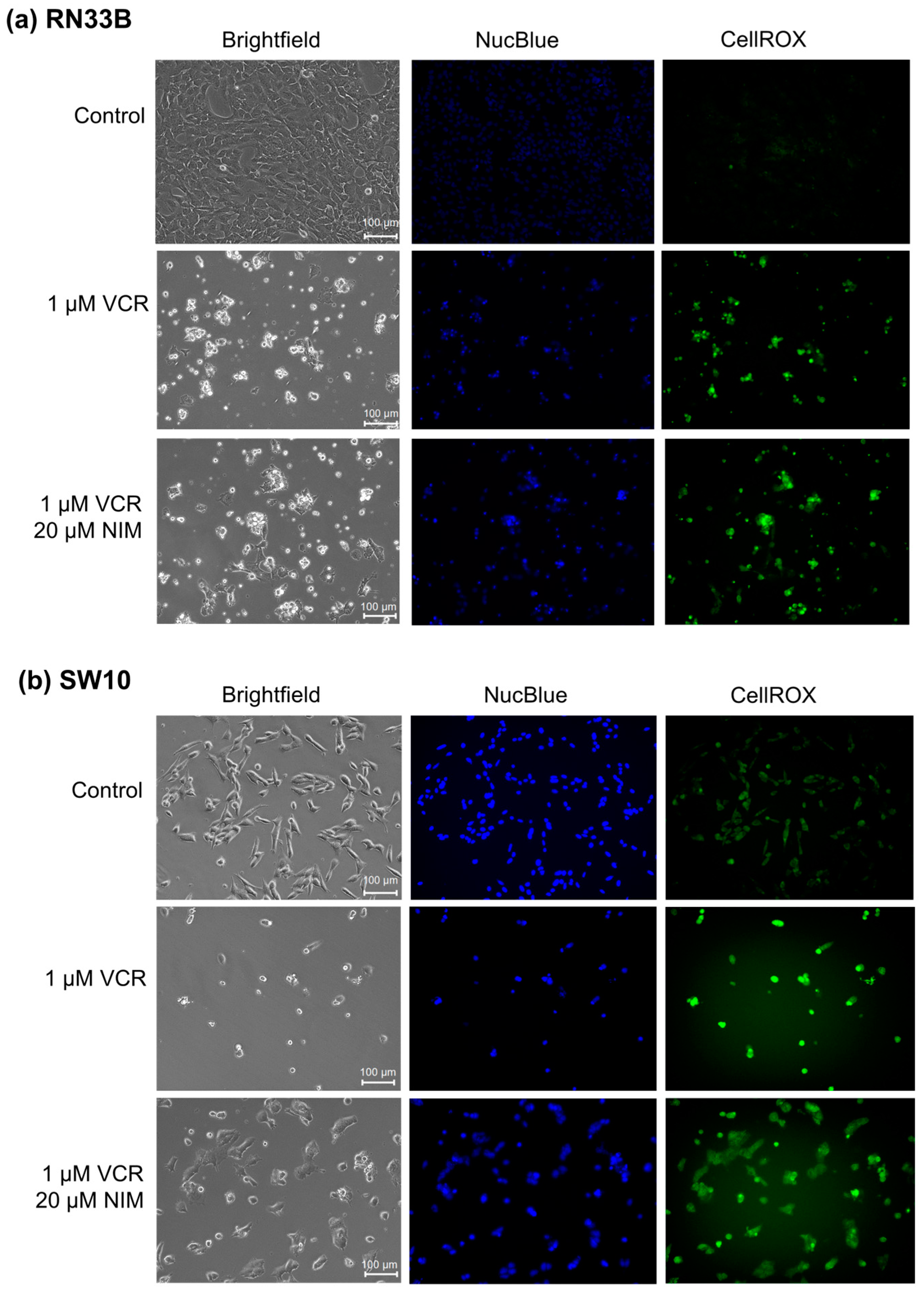
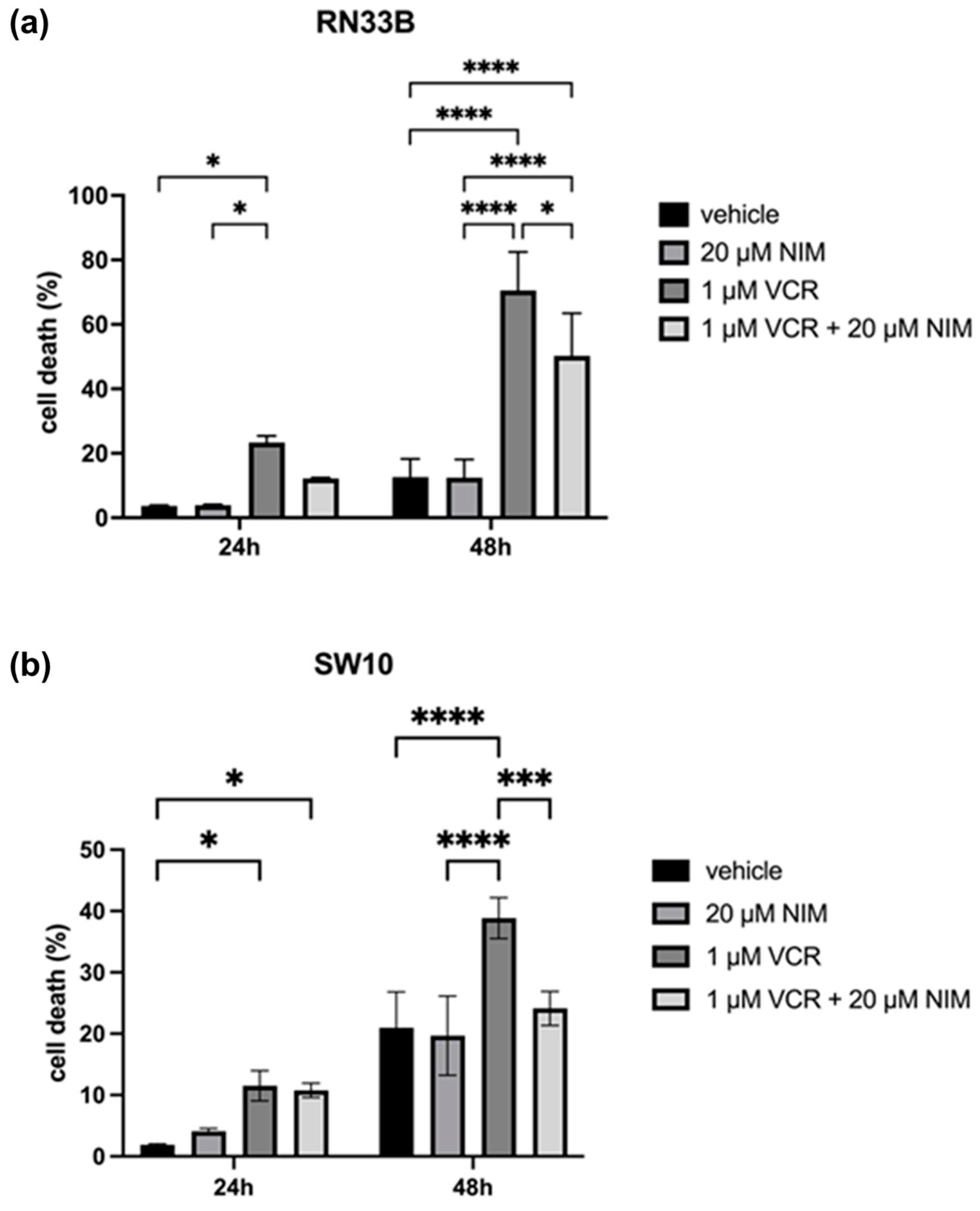
2.2. NIM Protected Neuronal Cells and Schwann Cells during VCR Treatment
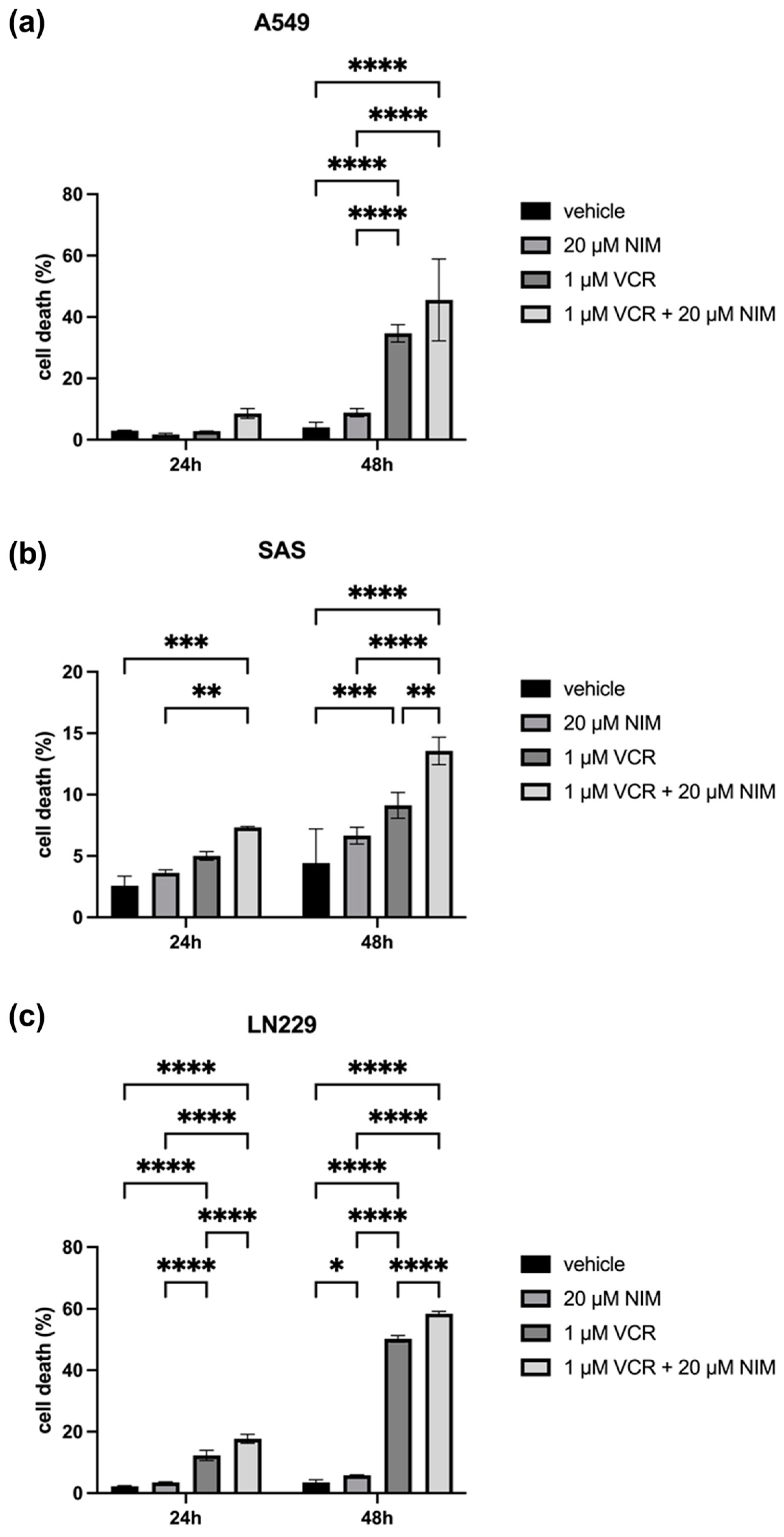
2.3. NIM Pretreatment Increased Susceptibility of Tumor Cells to VCR Application
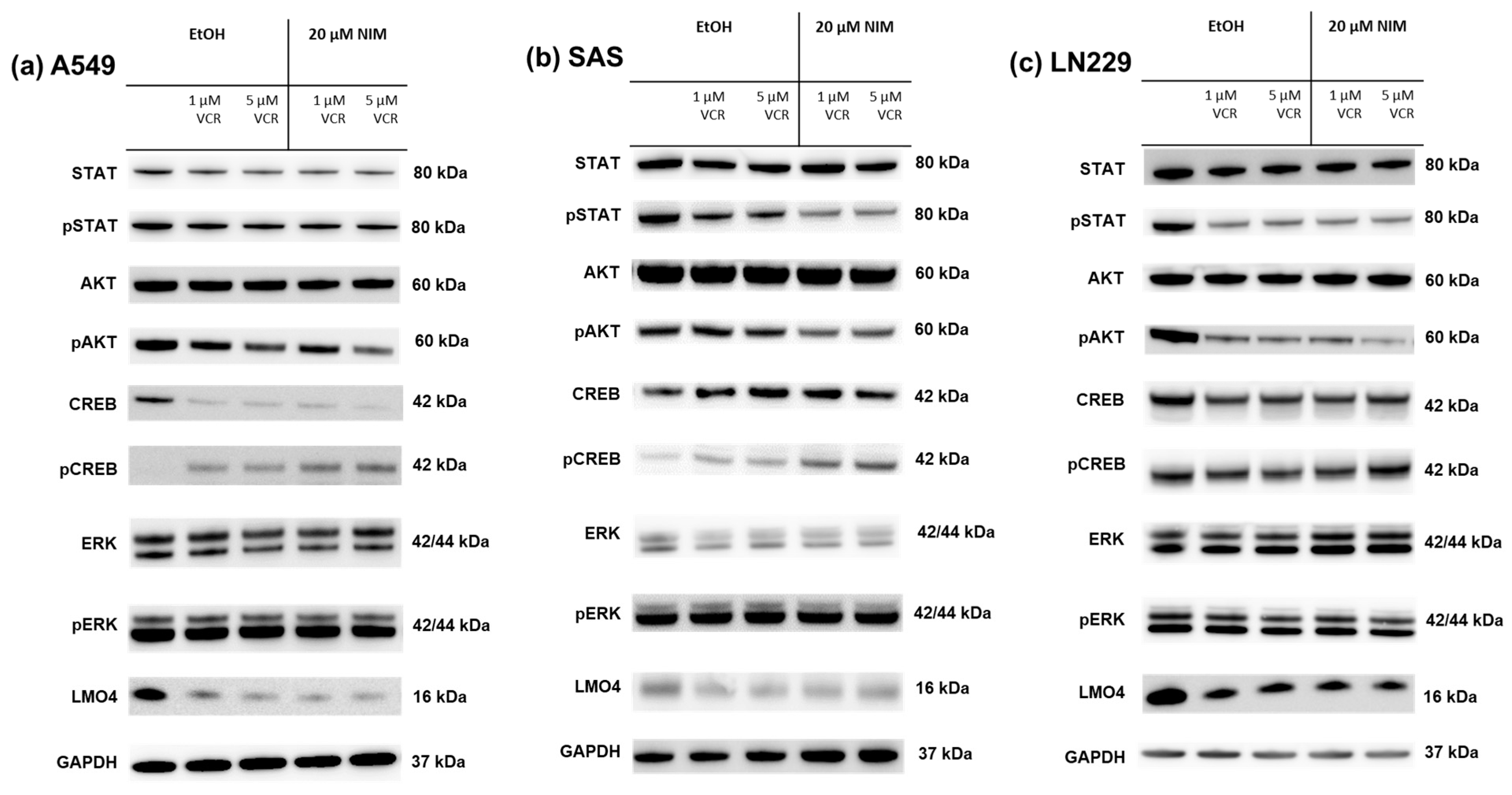
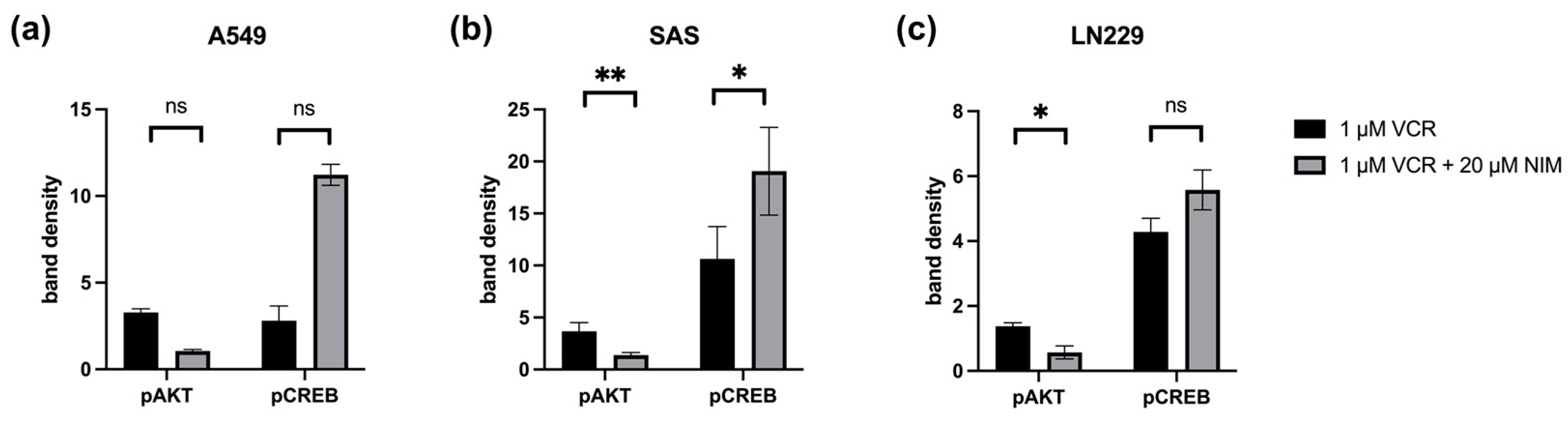
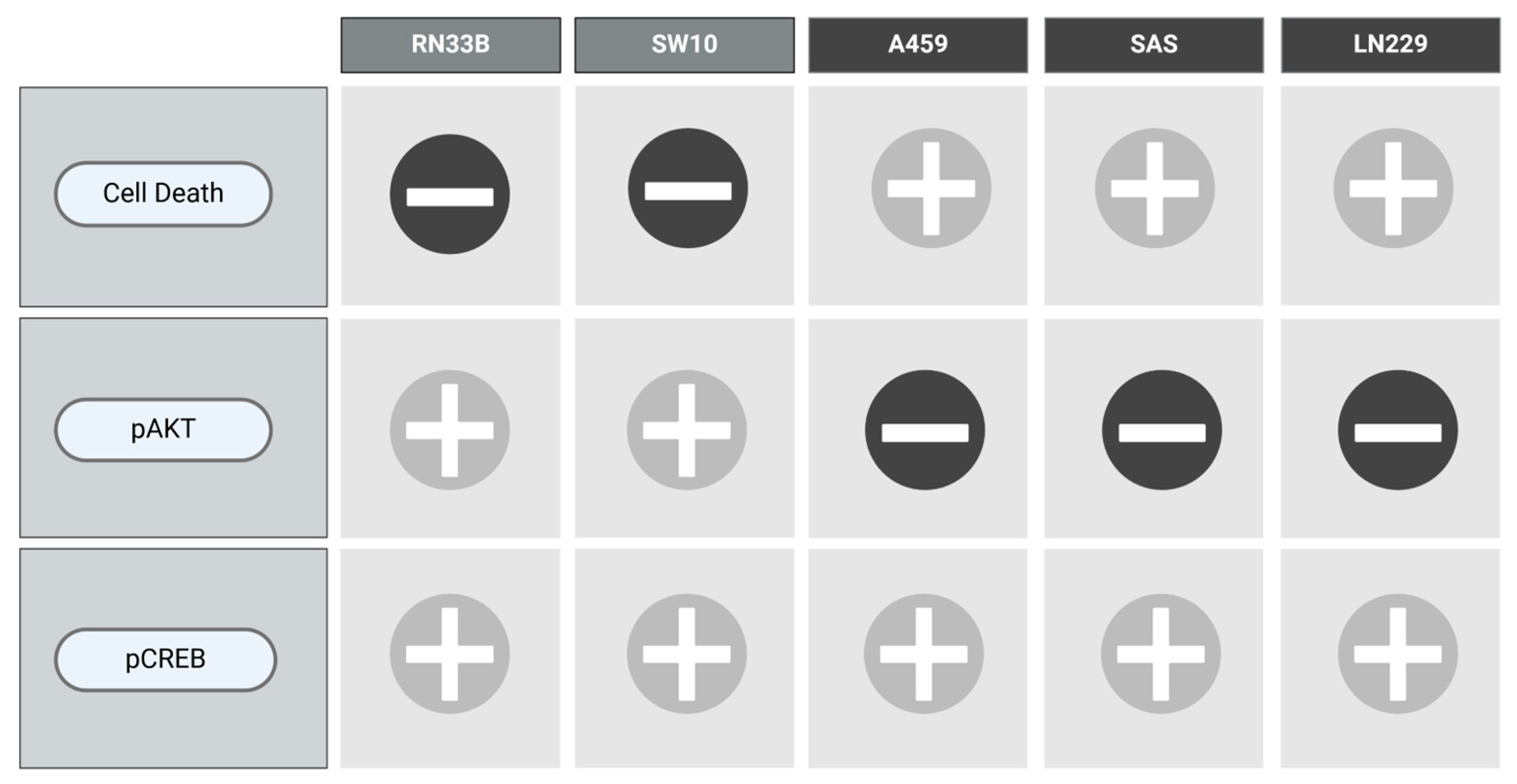
2.4. NIM-Induced Effects Are Associated with Altered Protein Levels of AKT and CREB
3. Discussion
Limitations and Outlook
4. Materials and Methods
4.1. Cell Lines
4.2. NIM and VCR Treatment
4.3. Cytotoxicity Measurement
4.4. Fluorescence Microscopy
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuss, N.; Johnson, I.S.; Armstrong, J.G.; Jansen, C.J., Jr. The Vinca Alkaloids. Adv. Chemother. 1964, 12, 133–174. [Google Scholar] [PubMed]
- Gupta, S.; Chaudhary, K.; Kumar, R.; Gautam, A.; Nanda, J.S.; Dhanda, S.K.; Brahmachari, S.K.; Raghava, G.P.S. Prioritization of anticancer drugs against a cancer using genomic features of cancer cells: A step towards personalized medicine. Sci. Rep. 2016, 6, 23857. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhu, Z.-L.; Qian, Z.-Z.; Hu, G.; Wang, H.-Q.; Liu, W.-H.; Cheng, G. Pharmacokinetic characteristics of vincristine sulfate liposomes in patients with advanced solid tumors. Acta Pharmacol. Sin. 2012, 33, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Olasz, L.; Orsi, E.; Markó, T.; Szalma, J. Induction chemotherapy response and recurrence rates in correlation with N0 or N+ stage in oral squamous cell cancer (OSCC). Cancer Metastasis Rev. 2010, 29, 607–611. [Google Scholar] [CrossRef]
- Clavel, M.; Vermorken, J.B.; Cognetti, F.; Cappelaere, P.; de Mulder, P.; Schornagel, J.H.; Tueni, E.A.; Verweij, J.; Wildiers, J.; Clerico, M.; et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann. Oncol. 1994, 5, 521–526. [Google Scholar]
- Zhang, Y.; Yang, S.-H.; Guo, X.-L. New insights into Vinca alkaloids resistance mechanism and circumvention in lung cancer. Biomed. Pharmacother. 2017, 96, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.; Ju, H.-M.; Song, J.-Y.; Fry, A.M.; Bayliss, R.; Choi, J. A Polytherapy Strategy Using Vincristine and ALK Inhibitors to Sensitise EML4-ALK-Positive NSCLC. Cancers 2022, 14, 779. [Google Scholar] [CrossRef]
- Ghosh, S.; Lalani, R.; Maiti, K.; Banerjee, S.; Patel, V.; Bhowmick, S.; Misra, A. Optimization and efficacy study of synergistic vincristine coloaded liposomal doxorubicin against breast and lung cancer. Nanomedicine 2020, 15, 2585–2607. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef]
- Schmidt, F.; Fischer, J.; Herrlinger, U.; Dietz, K.; Dichgans, J.; Weller, M. PCV chemotherapy for recurrent glioblastoma. Neurology 2006, 66, 587–589. [Google Scholar] [CrossRef]
- Wick, W.; Roth, P.; Hartmann, C.; Hau, P.; Nakamura, M.; Stockhammer, F.; Sabel, M.C.; Wick, A.; Koeppen, S.; Ketter, R.; et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016, 18, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Diouf, B.; Evans, W.E. Pharmacogenomics of Vincristine-Induced Peripheral Neuropathy: Progress Continues. Clin. Pharmacol. Ther. 2019, 105, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.L.; Due, H.; Ejskjær, N.; Jensen, P.; Madsen, J.; Dybkær, K. Aspects of vincristine-induced neuropathy in hematologic malignancies: A systematic review. Cancer Chemother. Pharmacol. 2019, 84, 471–485. [Google Scholar] [CrossRef]
- Nazir, H.F.; AlFutaisi, A.; Zacharia, M.; Elshinawy, M.; Mevada, S.T.; Alrawas, A.; Khater, D.; Jaju, D.; Wali, Y. Vincristine-induced neuropathy in pediatric patients with acute lymphoblastic leukemia in Oman: Frequent autonomic and more severe cranial nerve involvement. Pediatr. Blood Cancer 2017, 64, e26677. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Feldman, E.L. Immune-mediated vincristine-induced neuropathy: Unlocking therapies. J. Exp. Med. 2021, 218, e20210286. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Singh, A.; Singh, K.K. Vincristine-based nanoformulations: A preclinical and clinical studies overview. Drug Deliv. Transl. Res. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Lavoie Smith, E.M.; Li, L.; Chiang, C.; Thomas, K.; Hutchinson, R.J.; Wells, E.M.; Ho, R.H.; Skiles, J.; Chakraborty, A.; Bridges, C.M.; et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J. Peripher. Nerv. Syst. 2015, 20, 37–46. [Google Scholar] [CrossRef]
- Triarico, S.; Romano, A.; Attinà, G.; Capozza, M.A.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Vincristine-Induced Peripheral Neuropathy (VIPN) in Pediatric Tumors: Mechanisms, Risk Factors, Strategies of Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 4112. [Google Scholar] [CrossRef]
- Schloss, J.; Colosimo, M.; Vitetta, L. New Insights into Potential Prevention and Management Options for Chemotherapy-Induced Peripheral Neuropathy. Asia Pac. J. Oncol. Nurs. 2016, 3, 73–85. [Google Scholar] [CrossRef]
- Haar, C.P.; Hebbar, P.; Wallace, G.C.T.; Das, A.; Vandergrift, W.A., 3rd; Smith, J.A.; Giglio, P.; Patel, S.J.; Ray, S.K.; Banik, N.L. Drug resistance in glioblastoma: A mini review. Neurochem. Res. 2012, 37, 1192–1200. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed]
- Delou, J.M.A.; Souza, A.S.O.; Souza, L.C.M.; Borges, H.L. Highlights in Resistance Mechanism Pathways for Combination Therapy. Cells 2019, 8, 1013. [Google Scholar] [CrossRef]
- Carlson, A.P.; Hänggi, D.; Macdonald, R.L.; Shuttleworth, C.W. Nimodipine Reappraised: An Old Drug With a Future. Curr. Neuropharmacol. 2019, 18, 65–82. [Google Scholar] [CrossRef]
- Maher, M.; Schweizer, T.A.; Macdonald, R.L. Treatment of Spontaneous Subarachnoid Hemorrhage: Guidelines and Gaps. Stroke 2020, 51, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Leisz, S.; Simmermacher, S.; Prell, J.; Strauss, C.; Scheller, C. Nimodipine-Dependent Protection of Schwann Cells, Astrocytes and Neuronal Cells from Osmotic, Oxidative and Heat Stress Is Associated with the Activation of AKT and CREB. Int. J. Mol. Sci. 2019, 20, 4578. [Google Scholar] [CrossRef]
- Scheller, C.; Rampp, S.; Leisz, S.; Tatagiba, M.; Gharabaghi, A.; Ramina, K.F.; Ganslandt, O.; Matthies, C.; Westermaier, T.; Antoniadis, G.; et al. Prophylactic nimodipine treatment improves hearing outcome after vestibular schwannoma surgery in men: A subgroup analysis of a randomized multicenter phase III trial. Neurosurg. Rev. 2021, 44, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Zech, J.; Leisz, S.; Göttel, B.; Syrowatka, F.; Greiner, A.; Strauss, C.; Knolle, W.; Scheller, C.; Mäder, K. Electrospun Nimodipine-loaded fibers for nerve regeneration: Development and in vitro performance. Eur. J. Pharm. Biopharm. 2020, 151, 116–126. [Google Scholar] [CrossRef]
- Fritzsche, S.; Strauss, C.; Scheller, C.; Leisz, S. Nimodipine Treatment Protects Auditory Hair Cells from Cisplatin-Induced Cell Death Accompanied by Upregulation of LMO4. Int. J. Mol. Sci. 2022, 23, 5780. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Adhikari, S.; Dongol, R.M.; Hewett, Y.; Shah, B.K. Vincristine-induced blindness: A case report and review of literature. Anticancer. Res. 2014, 34, 6731–6733. [Google Scholar] [PubMed]
- Boyle, F.M.; Wheeler, H.R.; Shenfield, G.M. Glutamate ameliorates experimental vincristine neuropathy. J. Pharmacol. Exp. Ther. 1996, 279, 410–415. [Google Scholar] [PubMed]
- Van Helleputte, L.; Kater, M.; Cook, D.P.; Eykens, C.; Rossaert, E.; Haeck, W.; Jaspers, T.; Geens, N.; Vanden Berghe, P.; Gysemans, C.; et al. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol. Dis. 2018, 111, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Wurm, F.; Haller, H.; Strauss, C.; Scheller, C.; Gnanapragassam, V.S.; Horstkorte, R. Neuroprotective and neuroregenerative effects of nimodipine in a model system of neuronal differentiation and neurite outgrowth. Molecules 2015, 20, 1003–1013. [Google Scholar] [CrossRef]
- Durmaz, R.; Deliorman, S.; Uyar, R.; Işiksoy, S.; Erol, K.; Tel, E. The effects of anticancer drugs in combination with nimodipine and verapamil on cultured cells of glioblastoma multiforme. Clin. Neurol. Neurosurg. 1999, 101, 238–244. [Google Scholar] [CrossRef]
- Kiwit, J.C.; Hertel, A.; Matuschek, A.E. Reversal of chemoresistance in malignant gliomas by calcium antagonists: Correlation with the expression of multidrug-resistant p-glycoprotein. J. Neurosurg. 1994, 81, 587–594. [Google Scholar] [CrossRef]
- Kunert-Radek, J.; Stepien, H.; Radek, A.; Lyson, K.; Pawlikowski, M. Inhibitory effect of calcium channel blockers on proliferation of human glioma cells in vitro. Acta Neurol. Scand. 1989, 79, 166–169. [Google Scholar] [CrossRef]
- Taghizadehghalehjoughi, A.; Sezen, S.; Hacimuftuoglu, A.; Güllüce, M. Vincristine combination with Ca+2 channel blocker increase antitumor effects. Mol. Biol. Rep. 2019, 46, 2523–2528. [Google Scholar] [CrossRef]
- Honn, K.V.; Onoda, J.M.; Diglio, C.A.; Carufel, M.M.; Taylor, J.D.; Sloane, B.F. Inhibition of tumor cell-platelet interactions and tumor metastasis by the calcium channel blocker, nimodipine. Clin. Exp. Metastasis 1984, 2, 61–72. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Z.; Lv, P.; Wang, H.; Zhu, Y.; Qi, Q.; Xu, J. Autophagy and Akt/CREB signalling play an important role in the neuroprotective effect of nimodipine in a rat model of vascular dementia. Behav. Brain Res. 2017, 325(Pt A), 79–86. [Google Scholar] [CrossRef]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-κB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Hasegawa, Y. Nimodipine promotes neurite outgrowth and protects against neurotoxicity in PC12 cells. Iran. J. Basic. Med. Sci. 2021, 24, 51–57. [Google Scholar] [PubMed]
- Connelly, J.A.; Zhang, X.; Chen, Y.; Chao, Y.; Shi, Y.; Jacob, T.C.; Wang, Q.J. Protein kinase D2 confers neuroprotection by promoting AKT and CREB activation in ischemic stroke. Neurobiol. Dis. 2023, 187, 106305. [Google Scholar] [CrossRef]
- Molina-Salinas, G.; Rodríguez-Chávez, V.; Langley, E.; Cerbon, M. Prolactin-induced neuroprotection against excitotoxicity is mediated via PI3K/AKT and GSK3β/NF-κB in primary cultures of hippocampal neurons. Peptides 2023, 166, 171037. [Google Scholar] [CrossRef]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef]
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert. Opin. Investig. Drugs 2019, 28, 977–988. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Pu, T. MicroRNA-1179 suppresses the proliferation and enhances vincristine sensitivity of oral cancer cells via induction of apoptosis and modulation of MEK/ERK and PI3K/AKT signalling pathways. AMB Express 2020, 10, 149. [Google Scholar] [CrossRef]
- Liu, Q.; Turner, K.M.; Yung, W.K.A.; Chen, K.; Zhang, W. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro Oncol. 2014, 16, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dong, W.; Wang, F.; Liu, Q.; Yang, Y.; Guo, P.; Li, X.; Wei, B. Downregulation of miRNA-14669 Reverses Vincristine Resistance in Colorectal Cancer Cells through PI3K/AKT Signaling Pathway. Recent Pat. Anticancer Drug Discov. 2022, 17, 178–186. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Tang, H.; Hu, X.; Hubert, S.M.; Li, Q.; Su, D.; Xu, H.; Fan, Y.; Yu, X.; et al. Activation of PI3K/AKT Pathway Is a Potential Mechanism of Treatment Resistance in Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 526–539. [Google Scholar] [CrossRef]
- Tantai, J.; Pan, X.; Chen, Y.; Shen, Y.; Ji, C. TRIM46 activates AKT/HK2 signaling by modifying PHLPP2 ubiquitylation to promote glycolysis and chemoresistance of lung cancer cells. Cell Death Dis. 2022, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Mellado, M.J.; Aguilar, C.; Rocha-Zavaleta, L. Erythropoietin protects neuroblastoma cells against etoposide and vincristine by activating ERK and AKT pathways but has no effect in kidney cells. Life Sci. 2015, 137, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bai, X.; Feng, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer 2019, 19, 618. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Sun, Y.-J.; Wu, Y.-J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J. Exp. Clin. Cancer Res. 2019, 38, 265. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Volakakis, N.; Kadkhodaei, B.; Joodmardi, E.; Wallis, K.; Panman, L.; Silvaggi, J.; Spiegelman, B.M.; Perlmann, T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc. Natl. Acad. Sci. USA 2010, 107, 12317–12322. [Google Scholar] [CrossRef]
- Al-Saedi, H.F.; Panahi, Y.; Ghanimi, H.A.; Abdolmaleki, A.; Asadi, A. Enhancement of nerve regeneration with nimodipine treatment after sciatic nerve injury. Fundam. Clin. Pharmacol. 2023, 37, 107–115. [Google Scholar] [CrossRef]
- Koskimäki, J.; Matsui, N.; Umemori, J.; Rantamäki, T.; Castrén, E. Nimodipine activates TrkB neurotrophin receptors and induces neuroplastic and neuroprotective signaling events in the mouse hippocampus and prefrontal cortex. Cell Mol. Neurobiol. 2015, 35, 189–196. [Google Scholar] [CrossRef]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- Sapio, L.; Salzillo, A.; Ragone, A.; Illiano, M.; Spina, A.; Naviglio, S. Targeting CREB in Cancer Therapy: A Key Candidate or One of Many? An Update. Cancers 2020, 12, 3166. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Frank, D.A. CREB in the pathophysiology of cancer: Implications for targeting transcription factors for cancer therapy. Clin. Cancer Res. 2009, 15, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Conkright, M.D.; Montminy, M. CREB: The unindicted cancer co-conspirator. Trends Cell Biol. 2005, 15, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Jung, W.Y.; Kang, Y.; Lee, H.; Kim, A.; Kim, B.-H. Expression of ROR1, pAkt, and pCREB in gastric adenocarcinoma. Ann. Diagn. Pathol. 2015, 19, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-C.; Mitton, B.; Sakamoto, K.M. CREB and leukemogenesis. Crit. Rev. Oncog. 2011, 16, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lin, K.K.; Lu, Z.; Lam, K.S.; Newton, R.; Xu, X.; Yu, Z.; Gill, G.N.; Andersen, B. The LIM-only factor LMO4 regulates expression of the BMP7 gene through an HDAC2-dependent mechanism, and controls cell proliferation and apoptosis of mammary epithelial cells. Oncogene 2007, 26, 6431–6441. [Google Scholar] [CrossRef]
- Sum, E.Y.M.; Segara, D.; Duscio, B.; Bath, M.L.; Field, A.S.; Sutherland, R.L.; Lindeman, G.J.; Visvader, J.E. Overexpression of LMO4 induces mammary hyperplasia, promotes cell invasion, and is a predictor of poor outcome in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 7659–7664. [Google Scholar] [CrossRef]
- Wang, N.; Dong, Q.; Zhou, X.-N. LMO4 promotes the invasion and proliferation of gastric cancer by activating PI3K-Akt-mTOR signaling. Am. J. Transl. Res. 2019, 11, 6534–6543. [Google Scholar]
- Wang, W.; Wu, S.; Guo, M.; He, J. LMO4 is a prognostic marker involved in cell migration and invasion in non-small-cell lung cancer. J. Thorac. Dis. 2016, 8, 3682–3690. [Google Scholar] [CrossRef]
- Ruiz-Aparicio, P.F.; Uribe, G.I.; Linares-Ballesteros, A.; Vernot, J.-P. Sensitization to Drug Treatment in Precursor B-Cell Acute Lymphoblastic Leukemia Is Not Achieved by Stromal NF-κB Inhibition of Cell Adhesion but by Stromal PKC-Dependent Inhibition of ABC Transporters Activity. Molecules 2021, 26, 5366. [Google Scholar] [CrossRef]
- Luo, Y.; Alexander, M.; Gadina, M.; O’sHea, J.J.; Meylan, F.; Schwartz, D.M. JAK-STAT signaling in human disease: From genetic syndromes to clinical inhibition. J. Allergy Clin. Immunol. 2021, 148, 911–925. [Google Scholar] [CrossRef]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Pang, X.; Baeg, G.H. Targeting the JAK/STAT Signaling Pathway for Breast Cancer. Curr. Med. Chem. 2021, 28, 5137–5151. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]





| Antibody | Species | Dilution | Dilution Buffer | Manufacture |
|---|---|---|---|---|
| AKT (40D4) #2920 | Mouse IgG1 | 1:2000 | 5% MP in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| Phospho-Akt (Ser473) (D9E) #4060 | Rabbit IgG | 1:1000 | 5% BSA in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| CREB (48H2) #9197 | Rabbit IgG | 1:1000 | 5% BSA in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| Phospho-CREB (Ser133) (87G3) #9198 | Rabbit IgG | 1:1000 | 5% MP in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| ERK 1/2 (T202/ Y204) #9102 | Rabbit IgG | 1:1000 | 5% BSA in TBS- | Cell Signaling Technology (Danvers, MA, USA) |
| Phospho-ERK 1/2 (Thr202/Tyr204) #9101 | Rabbit IgG | 1:1000 | 5% BSA in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| LMO4 (D6V4Z) #81428 | Rabbit IgG | 1:1000 | 5% BSA in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| GAPDH (14C10) #2118 | Rabbit IgG | 1:1000 | 5% BSA in TBS-T | Abcam (Cambridge, UK) |
| STAT3 (124H6) #9139 | Mouse IgG2a | 1:1000 | 5% MP in TBS-T | Cell Signaling Technology (Danvers, MA, USA) |
| Phospho-STAT3 (Tyr705) (3E2) #9138 | Mouse IgG1 | 1:1000 | 5% MP in TBS-T | Cell Signaling Technology Inc. (Danvers, MA, USA) |
| Anti-Rabbit IgG, HRP-linked Antibody #7074 | Goat | 1:1000 | 2% MP in TBS-T | Cell Signaling Technology Inc. (Danvers, MA, USA) |
| Anti-Mouse IgG, HRP-linked Antibody #7076 | Horse | 1:1000 | 2% MP in TBS-T | Cell Signaling Technology Inc. (Danvers, MA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheer, M.; Polak, M.; Fritzsche, S.; Strauss, C.; Scheller, C.; Leisz, S. Nimodipine Used with Vincristine: Protects Schwann Cells and Neuronal Cells from Vincristine-Induced Cell Death but Increases Tumor Cell Susceptibility. Int. J. Mol. Sci. 2024, 25, 10389. https://doi.org/10.3390/ijms251910389
Scheer M, Polak M, Fritzsche S, Strauss C, Scheller C, Leisz S. Nimodipine Used with Vincristine: Protects Schwann Cells and Neuronal Cells from Vincristine-Induced Cell Death but Increases Tumor Cell Susceptibility. International Journal of Molecular Sciences. 2024; 25(19):10389. https://doi.org/10.3390/ijms251910389
Chicago/Turabian StyleScheer, Maximilian, Mateusz Polak, Saskia Fritzsche, Christian Strauss, Christian Scheller, and Sandra Leisz. 2024. "Nimodipine Used with Vincristine: Protects Schwann Cells and Neuronal Cells from Vincristine-Induced Cell Death but Increases Tumor Cell Susceptibility" International Journal of Molecular Sciences 25, no. 19: 10389. https://doi.org/10.3390/ijms251910389
APA StyleScheer, M., Polak, M., Fritzsche, S., Strauss, C., Scheller, C., & Leisz, S. (2024). Nimodipine Used with Vincristine: Protects Schwann Cells and Neuronal Cells from Vincristine-Induced Cell Death but Increases Tumor Cell Susceptibility. International Journal of Molecular Sciences, 25(19), 10389. https://doi.org/10.3390/ijms251910389







