Knockdown of Adenosine 5′-Triphosphate-Dependent Caseinolytic Protease Proteolytic Subunit 6 Enhances Aluminum Tolerance in Peanut Plants (Arachis hypogea L.)
Abstract
1. Introduction
2. Results
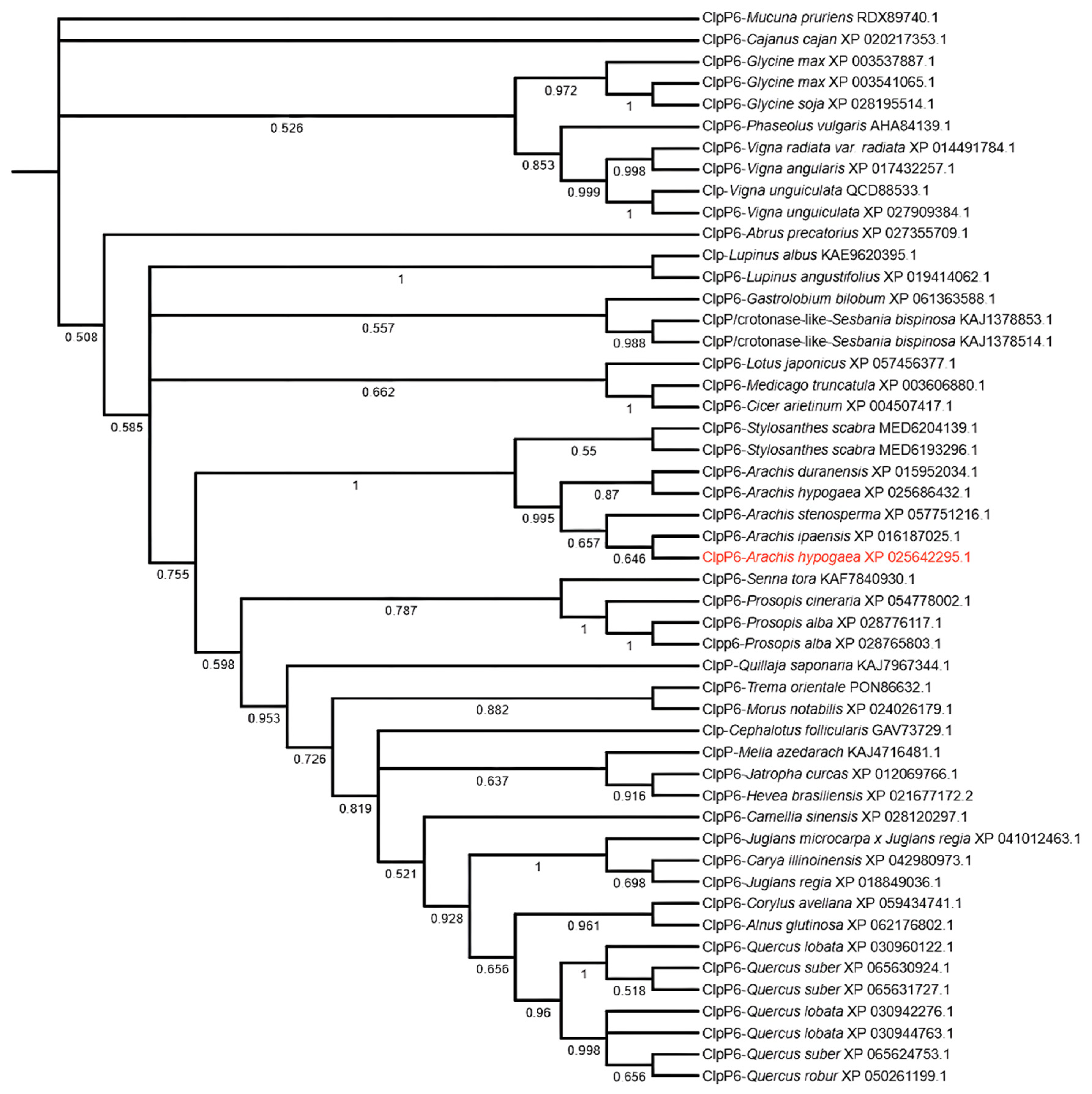
2.1. Phylogenetic Analysis of AhClpP6
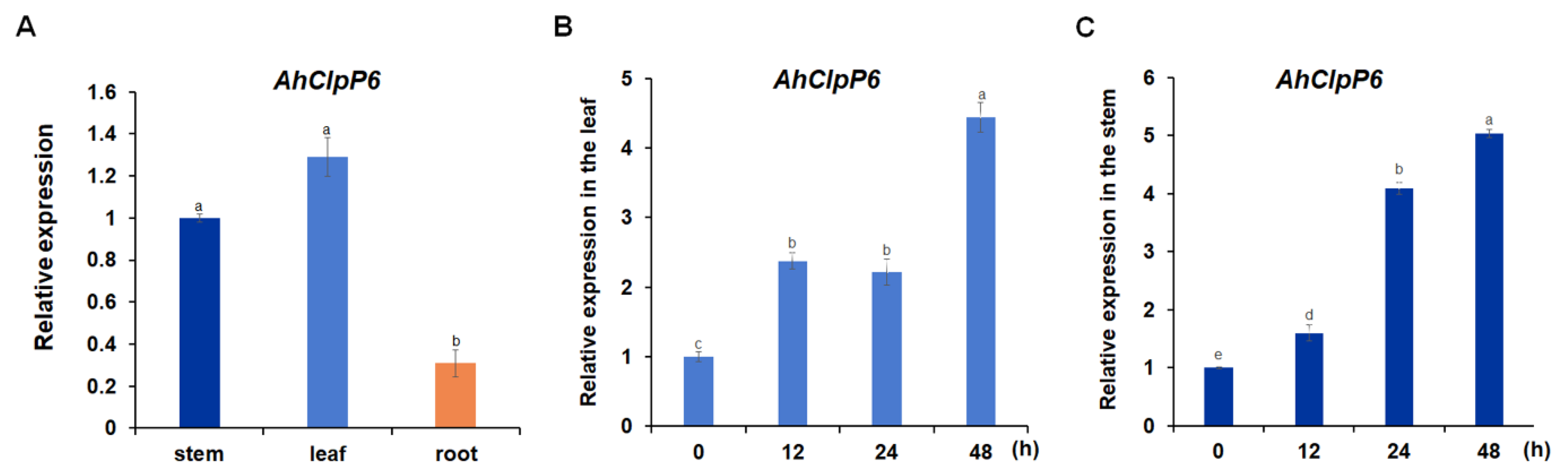
2.2. AhClpP6 Expression Increased with Al3+ Stress in Peanut Plants
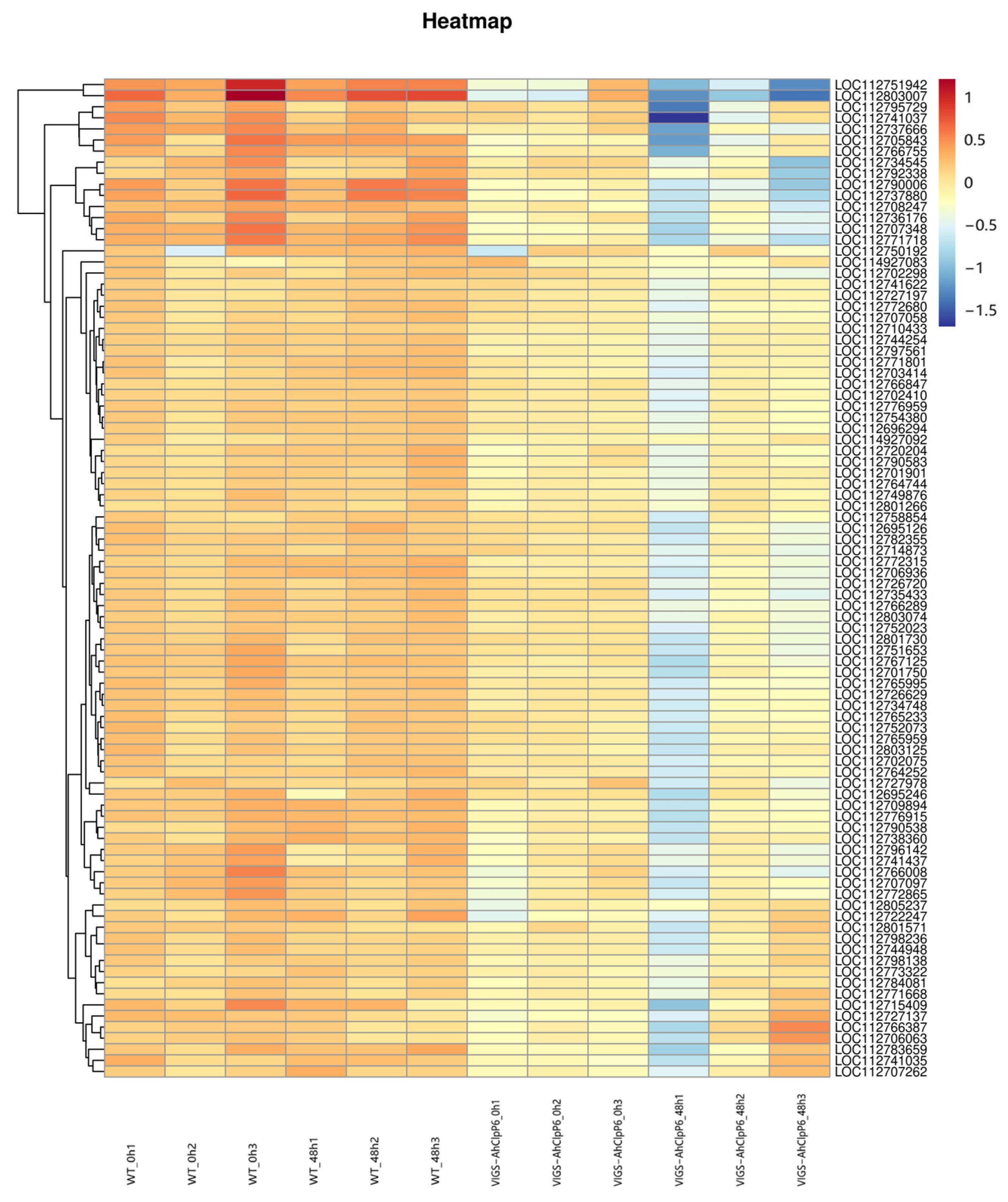
2.3. Sequencing and Identification of Differentially Expressed Genes
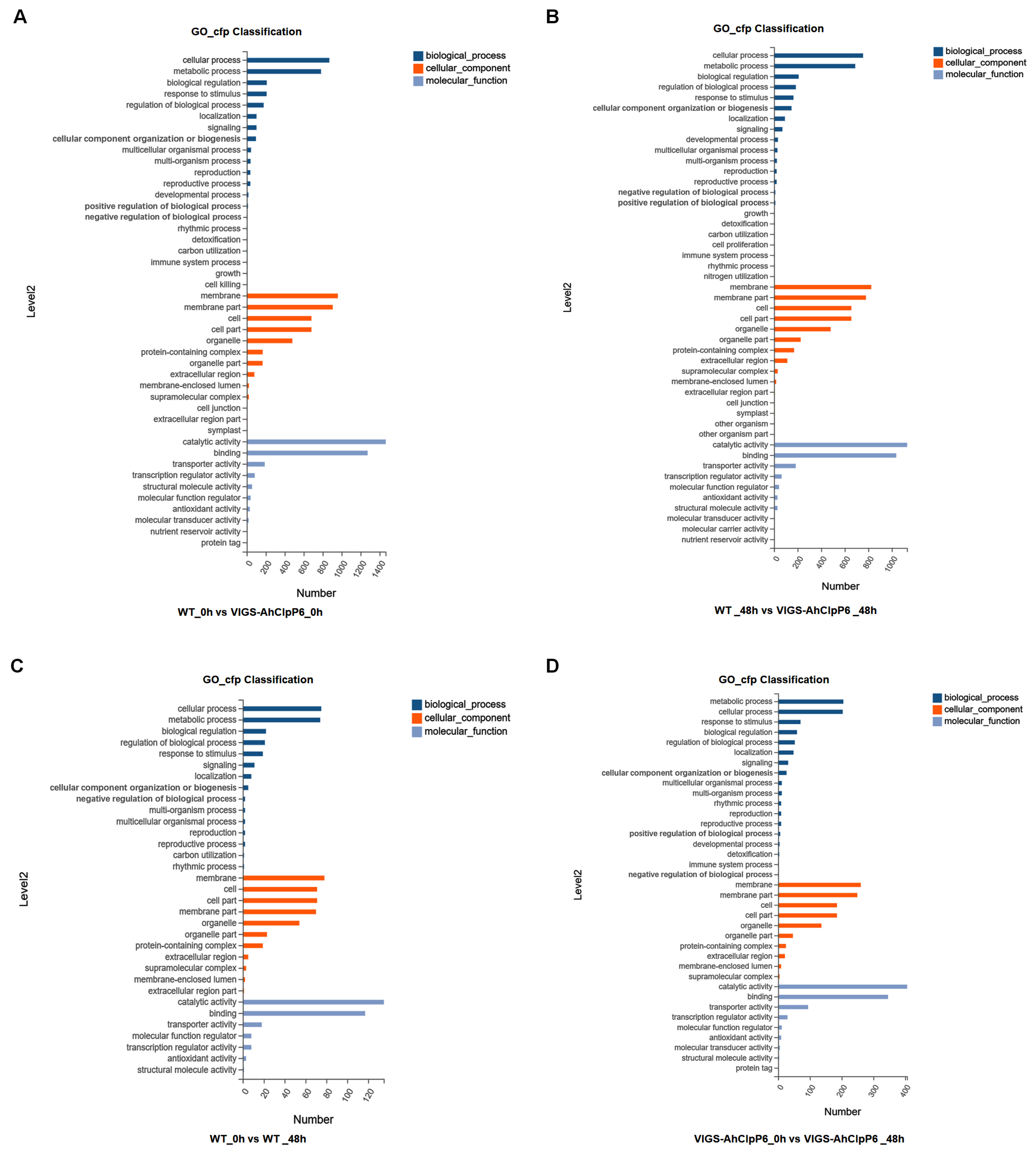
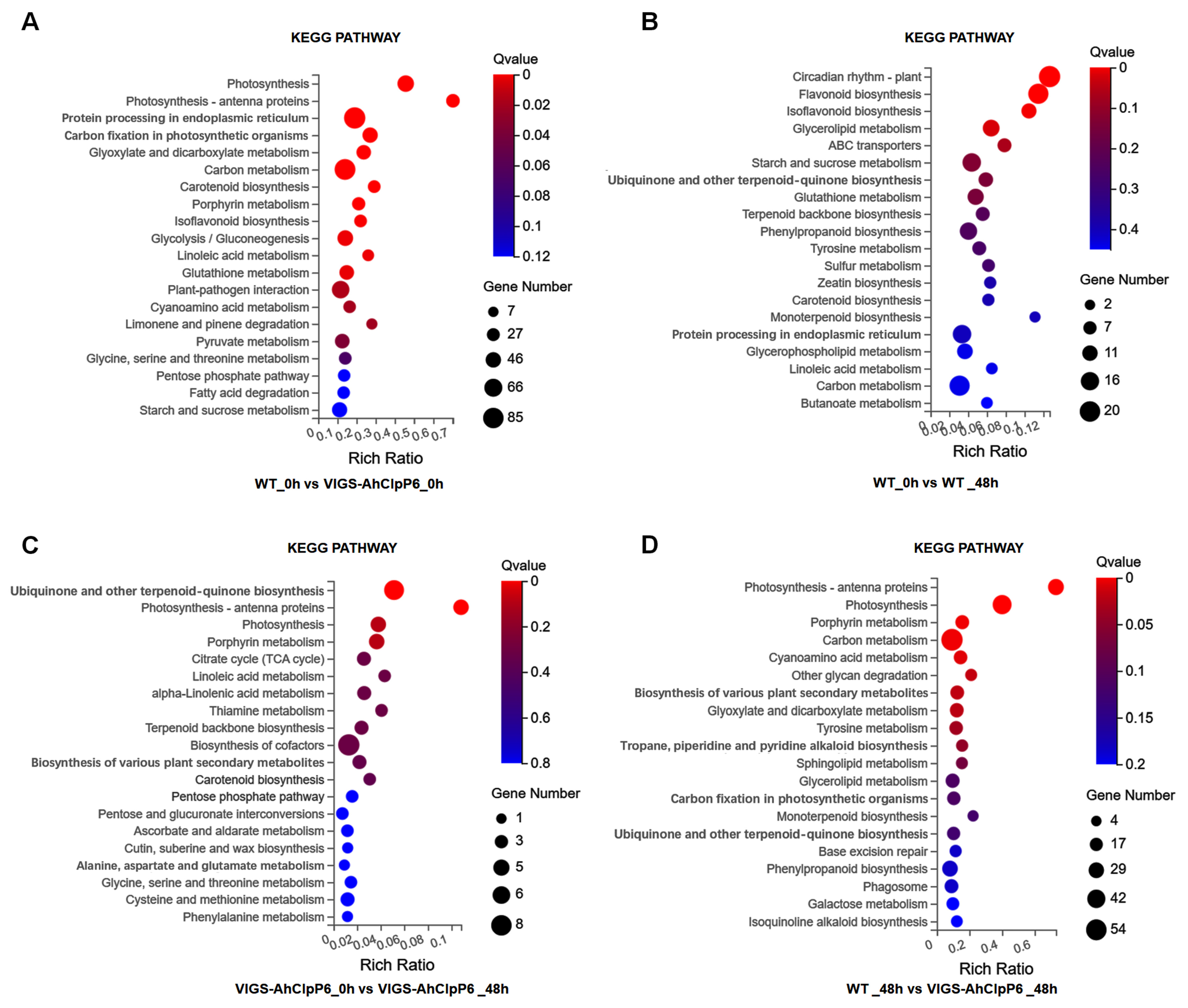
2.4. GO and KEGG Annotation of DEGs
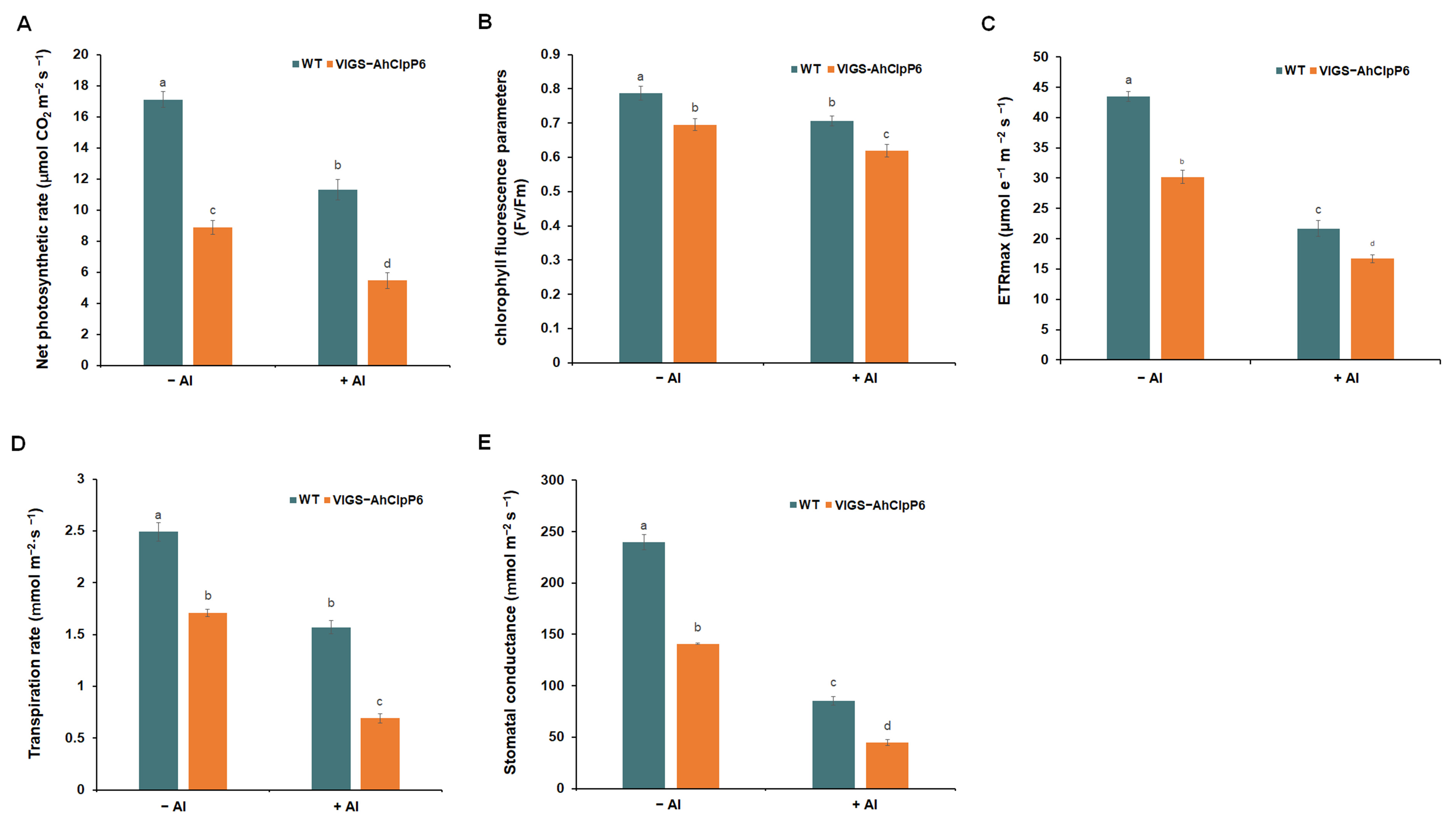
2.5. AhClpP6 Positively Regulates Al3+ Tolerance by Modulating Photosynthesis
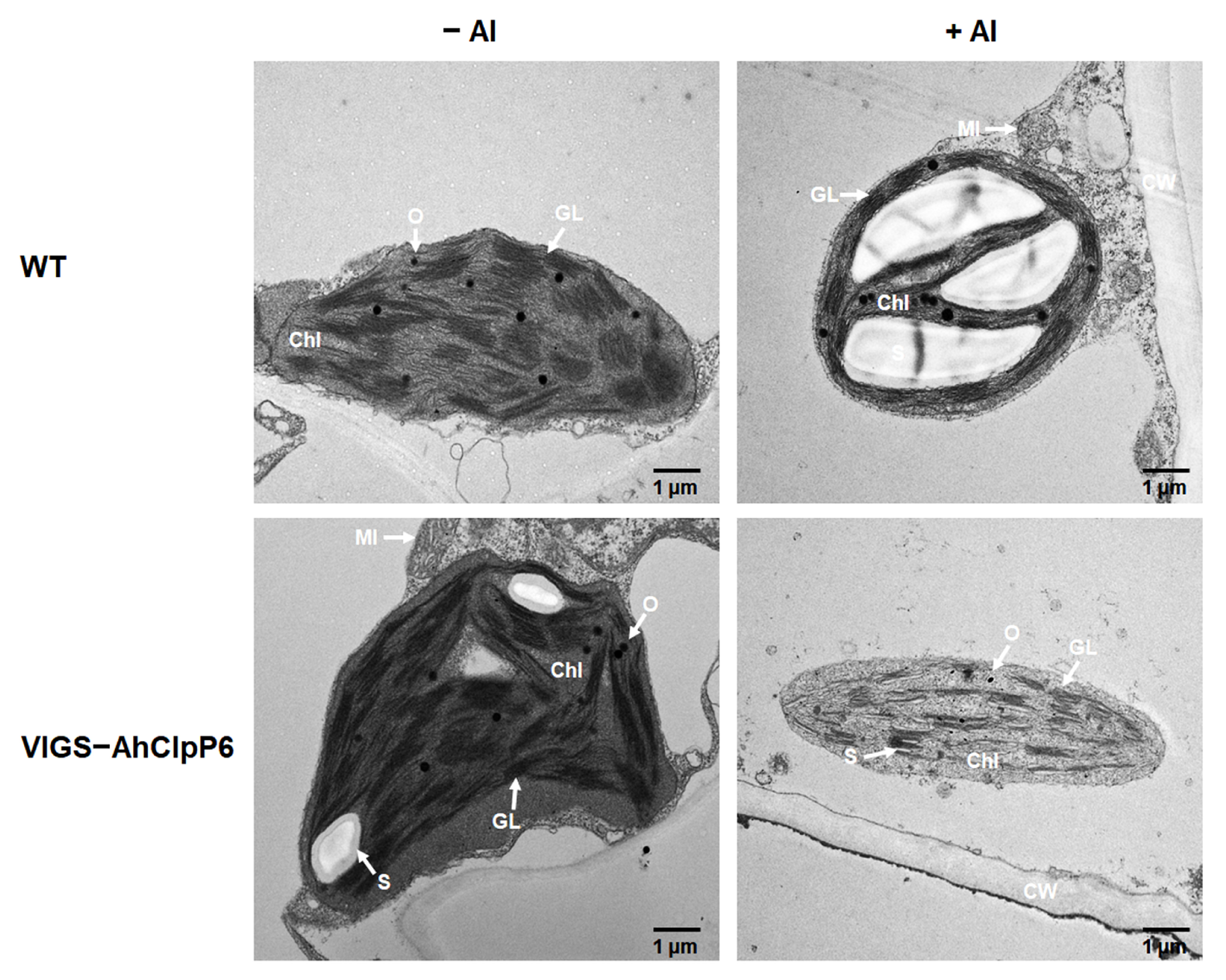
2.6. AhClpP6 Positively Regulates Chloroplast Integrity
3. Discussion
4. Materials and Methods
4.1. Peanut (Arachis hypogea L.) Cultivation and Al3+ Treatment
4.2. Multiple Sequence Alignments and Phylogenetic Analysis
4.3. RT-qPCR Analysis
4.4. Virus-Induced Gene Silencing (VIGS)
4.5. RNA-Seq and Transcriptome Analysis
4.6. Al3+ Tolerance Assay
4.7. Photosynthesis and Chl Fluorescence Measurements
4.8. Transmission Electron Microscopy Observation of Cell Ultrastructure
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Prakash, N.B. Effect of different biochars on acid soil and growth parameters of rice plants under aluminium toxicity. Sci. Rep. 2020, 10, 12249. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; He, H.; Wang, T.; Wang, A.; Li, C.; He, L.F. Aluminum-induced programmed cell death promoted by AhSAG, a senescence-associated gene in Arachis hypoganea L. Plant Sci. 2013, 210, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y. Aluminum toxicity in plant cells: Mechanisms of cell death and inhibition of cell elongation. Soil Sci. Plant Nutr. 2019, 65, 41–55. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do cropplants tolerate acid soils? Mechanisms of aluminum tolerance andphosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Shandilya, Z.M.; Sarma, P.; Bora, R.K.; Regon, P.; Vemireddy, L.N.R.; Tanti, B. Concurrent effect of aluminum toxicity and phosphorus deficiency in the root growth of aluminum tolerant and sensitive rice cultivars. Acta Physiol. Plant. 2023, 45, 33. [Google Scholar] [CrossRef]
- Yu, H.N.; Liu, P.; Wang, Z.Y.; Chen, W.R.; Xu, G.D. The effect of aluminum treatments on the root growth and cell ultrastructure of two soybean genotypes. Crop Prot. 2011, 30, 323–328. [Google Scholar] [CrossRef]
- Phukunkamkaew, S.; Tisarum, R.; Pipatsitee, P.; Samphumphuang, T.; Maksup, S.; Cha-Um, S. Morpho-physiological responses of indica rice (Oryza sativa sub. indica) to Aluminum toxicity at seedling stage. Environ. Sci. Pollut. Res. 2021, 28, 29321–29331. [Google Scholar]
- Cheng, X.; Fang, T.; Zhao, E.; Zheng, B.; Huang, B.; An, Y.; Zhou, P. Protective roles of salicylic acid in maintaining integrity and functions of photosynthetic photosystems for alfalfa (Medicago sativa L.) tolerance to aluminum toxicity. Plant Physiol. Biochem. 2020, 155, 570–578. [Google Scholar] [CrossRef]
- Lin, Q.; Huai, Z.; Riaz, L.; Peng, X.; Wang, S.; Liu, B.; Yu, F.; Ma, J. Aluminum phytotoxicity induced structural and ultrastructural changes in submerged plant Vallisneria natans. Ecotoxicol. Environ. Saf. 2023, 250, 114484. [Google Scholar] [CrossRef]
- Pereira, W.E.; de Siqueira, D.L.; Martínez, C.A.; Puiatti, M. Gas exchange and chlorophyll fluorescence in four Citrus root stocks under aluminium stress. J. Plant Physiol. 2000, 157, 513–520. [Google Scholar] [CrossRef]
- Rowland, E.; Kim, J.; Friso, G.; Poliakov, A.; Ponnala, L.; Sun, Q.; van Wijk, K.J. The CLP and PREP protease systems coordinate maturation and degradation of the chloroplast proteome in Arabidopsis thaliana. New Phytol. 2022, 236, 1339–1357. [Google Scholar] [CrossRef]
- Welsch, R.; Zhou, X.; Yuan, H.; Álvarez, D.; Sun, T.; Schlossarek, D.; Yang, Y.; Shen, G.; Zhang, H.; Rodriguez-Concepcion, M.; et al. Clp protease and OR directly control the proteostasis of phytoene synthase, the crucial enzyme for carotenoid biosynthesis in Arabidopsis. Mol. Plant 2018, 11, 149–162. [Google Scholar] [CrossRef]
- Adam, Z.; Clarke, A.K. Cutting edge of chloroplast proteolysis. Trends Plant Sci. 2002, 7, 451–456. [Google Scholar] [CrossRef]
- Adam, Z.; Rudella, A.; van Wijk, K.J. Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 2006, 9, 234–240. [Google Scholar] [CrossRef]
- Halperin, T.; Ostersetzer, O.; Adam, Z. ATP-dependent association between subunits of Clp protease in pea chloroplasts. Planta 2001, 213, 614–619. [Google Scholar] [CrossRef]
- López, B.; Izquierdo, Y.; Cascón, T.; Zamarreño, Á.M.; García-Mina, J.M.; Pulido, P.; Castresana, C. Mutant noxy8 exposes functional specificities between the chloroplast chaperones CLPC1 and CLPC2 in the response to organelle stress and plant defence. Plant Cell Environ. 2024, 47, 2336–2350. [Google Scholar] [CrossRef]
- Kreis, E.; Niemeyer, J.; Merz, M.; Scheuring, D.; Schroda, M. CLPB3 is required for the removal of chloroplast protein aggregates and thermotolerance in Chlamydomonas. J. Exp. Bot. 2023, 74, 3714–3728. [Google Scholar] [CrossRef] [PubMed]
- Panzade, K.P.; Vishwakarma, H.; Padaria, J.C. Heat stress inducible cytoplasmic isoform of ClpB1 from Z. nummularia exhibits enhanced thermotolerance in transgenic tobacco. Mol. Biol. Rep. 2020, 47, 3821–3831. [Google Scholar] [CrossRef]
- Chen, S.; Ye, M.; Kuai, P.; Chen, L.; Lou, Y. Silencing an ATP-Dependent caseinolytic protease proteolytic subunit gene enhances the resistance of rice to Nilaparvata lugens. Int. J. Mol Sci. 2024, 25, 3699. [Google Scholar] [CrossRef]
- Killi, F.; Beycioğlu, T. Genetic and environmental variability, heritability and genetic advance in pod yield, yield components, oil and protein content of peanut varieties. Turk. J. Field Crops 2022, 27, 71–77. [Google Scholar] [CrossRef]
- Xu, R.K.; Li, J.Y.; Zhou, S.W.; Xu, M.G.; Shen, R.F. Scientific issues and controlling strategies of soil acidification of croplands in China. Bull. Chin. Acad. Sci. 2018, 33, 160–167. [Google Scholar]
- Mamaeva, A.; Taliansky, M.; Filippova, A.; Love, A.J.; Golub, N.; Fesenko, I. The role of chloroplast protein remodeling in stress responses and shaping of the plant peptidome. New Phytol. 2020, 227, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, L.L.; Stanne, T.M.; Zheng, B.; Sutinen, S.; Clarke, A.K. Structural and functional insights into the chloroplast ATP-dependent Clp protease in Arabidopsis. Plant Cell 2006, 18, 2635–2649. [Google Scholar] [CrossRef]
- Adamiec, M.; Dobrogojski, J.; Wojtyla, Ł.; Luciński, R. Stress-related expression of the chloroplast EGY3 pseudoprotease and its possible impact on chloroplasts’ proteome composition. Front. Plant Sci. 2022, 13, 965143. [Google Scholar] [CrossRef]
- Siqueira, J.A.; Barros, J.A.S.; Dal-Bianco, M.; Martins, S.C.V.; Magalhães, P.C.; Ribeiro, D.M.; DaMatta, F.M.; Araújo, W.L.; Ribeiro, C. Metabolic and physiological adjustments of maize leaves in response to aluminum stress. Theor. Exp. Plant Physiol. 2020, 32, 133–145. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernández, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, X.; Lu, C. Improving drought tolerance by altering the photosynthetic rate and stomatal aperture via green light in tomato (Solanum lycopersicum L.) seedlings under drought conditions. Environ. Exp. Bot. 2019, 167, 103844. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yao, S.; Zhan, J.; Pan, C.; Xiong, W.; Xiao, D.; Wang, Y.; Shen, H.; Wang, A.; He, L.F. Identification and validation of reference genes for real-time qPCR normalization during Al-induced programmed cell death in peanut. Biol. Plant. 2019, 63, 237–246. [Google Scholar] [CrossRef]
- Liao, G.; Luo, S.; Li, X.; Li, A.; Mo, Y.; Wang, A.; Xiao, D.; He, L.F.; Zhan, J. Identification and functional characterization of REGULATORY PARTICLE NONATPASE 1a-like (AhRPN1a-like) in peanuts during aluminum-induced programmed cell death. J. Plant Physiol. 2023, 289, 154079. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Huang, W.; Pan, C.; Zhan, J.; He, L.F. Caspase-like proteases regulate aluminum-induced programmed cell death in peanut. Plant Cell Tissue Organ Cult. 2016, 127, 691–703. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of Vitis vinifera L. cv. ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, D.; Liang, R.; Xiao, D.; Wang, A.; He, L.; Zhan, J. Knockdown of Adenosine 5′-Triphosphate-Dependent Caseinolytic Protease Proteolytic Subunit 6 Enhances Aluminum Tolerance in Peanut Plants (Arachis hypogea L.). Int. J. Mol. Sci. 2024, 25, 10416. https://doi.org/10.3390/ijms251910416
Shi Y, Zhang D, Liang R, Xiao D, Wang A, He L, Zhan J. Knockdown of Adenosine 5′-Triphosphate-Dependent Caseinolytic Protease Proteolytic Subunit 6 Enhances Aluminum Tolerance in Peanut Plants (Arachis hypogea L.). International Journal of Molecular Sciences. 2024; 25(19):10416. https://doi.org/10.3390/ijms251910416
Chicago/Turabian StyleShi, Yusun, Dayue Zhang, Ronghua Liang, Dong Xiao, Aiqin Wang, Longfei He, and Jie Zhan. 2024. "Knockdown of Adenosine 5′-Triphosphate-Dependent Caseinolytic Protease Proteolytic Subunit 6 Enhances Aluminum Tolerance in Peanut Plants (Arachis hypogea L.)" International Journal of Molecular Sciences 25, no. 19: 10416. https://doi.org/10.3390/ijms251910416
APA StyleShi, Y., Zhang, D., Liang, R., Xiao, D., Wang, A., He, L., & Zhan, J. (2024). Knockdown of Adenosine 5′-Triphosphate-Dependent Caseinolytic Protease Proteolytic Subunit 6 Enhances Aluminum Tolerance in Peanut Plants (Arachis hypogea L.). International Journal of Molecular Sciences, 25(19), 10416. https://doi.org/10.3390/ijms251910416





