Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy
Abstract
:1. Introduction
1.1. Epidemiological Data on DM
1.2. Role of Inflammation in DM
1.3. The Importance of Studying the Inflammatory Process in PDN
1.4. The Purpose and Significance of This Review
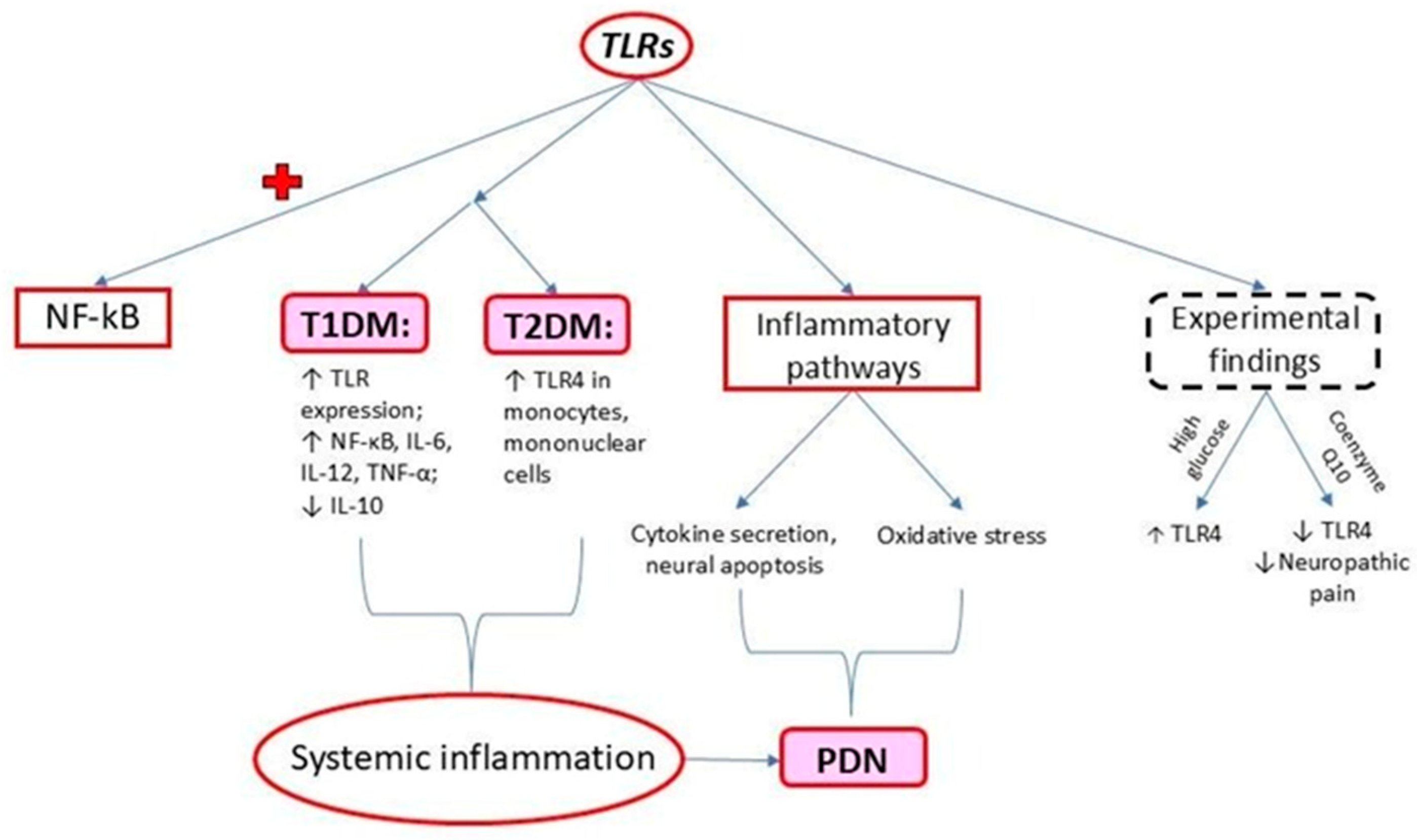
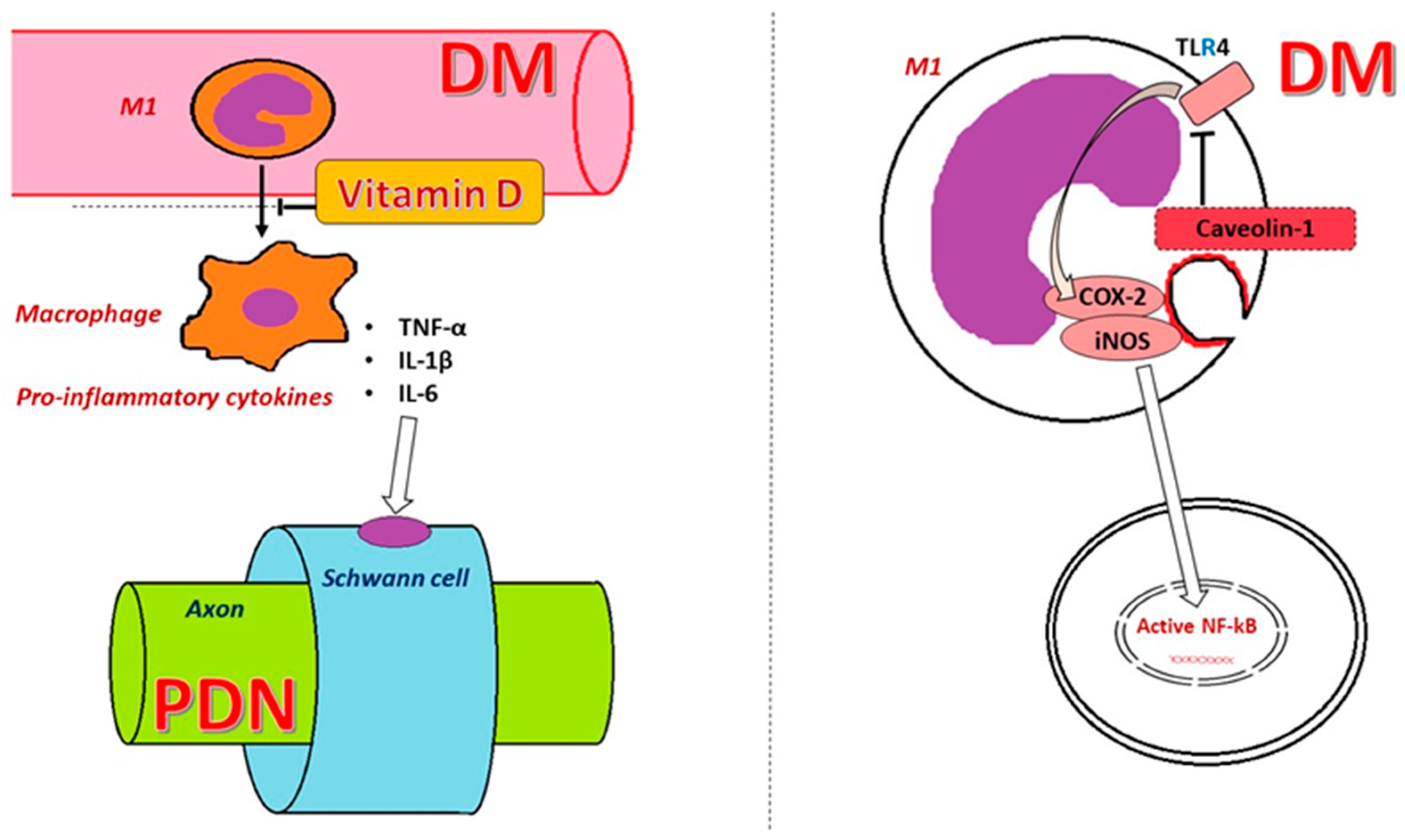
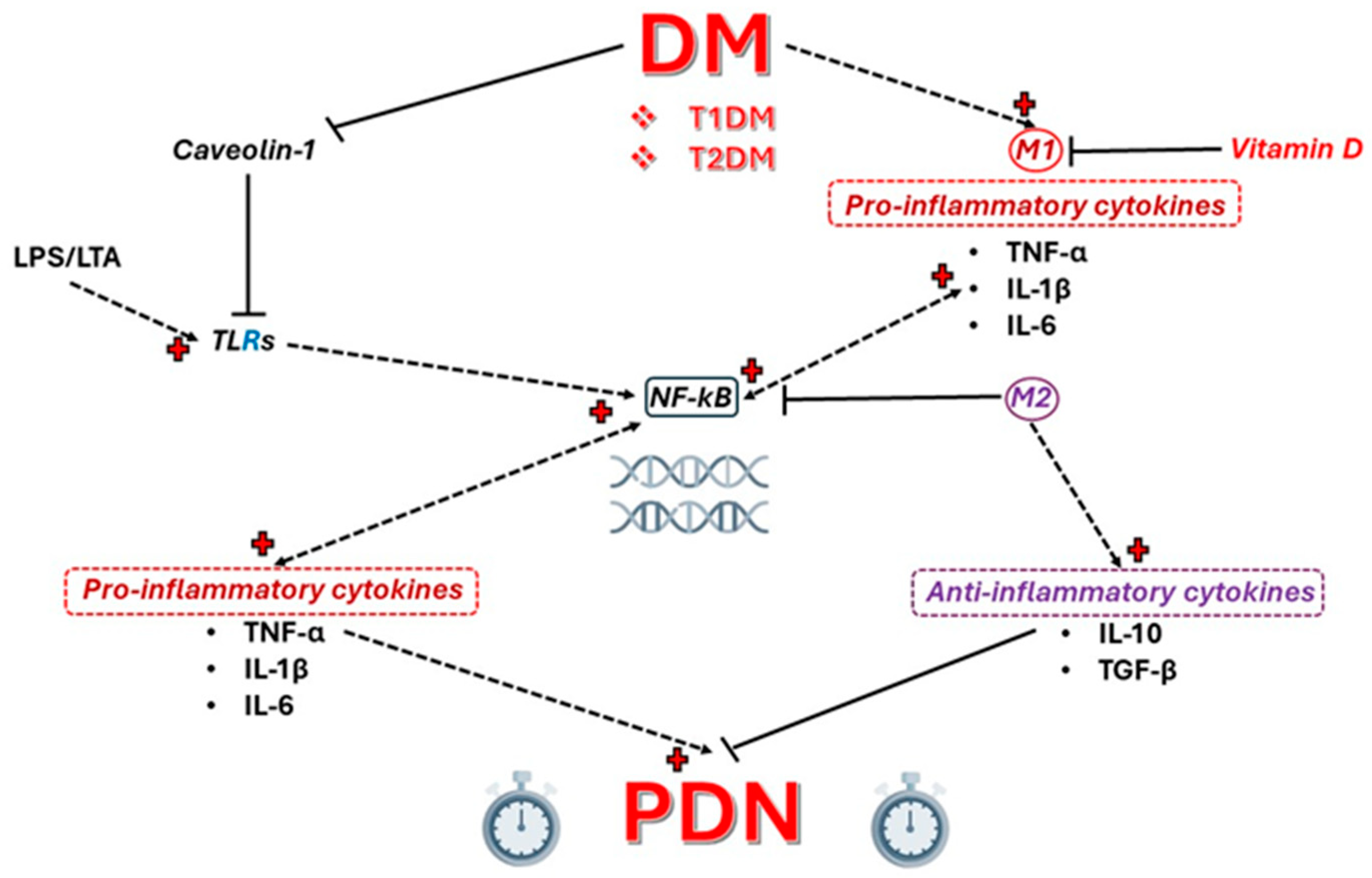
2. Toll-like Receptors (TLRs)
3. Caveolin 1 (CAV1)
4. NF-κB
5. Immune-Related Events
5.1. Immune Cells
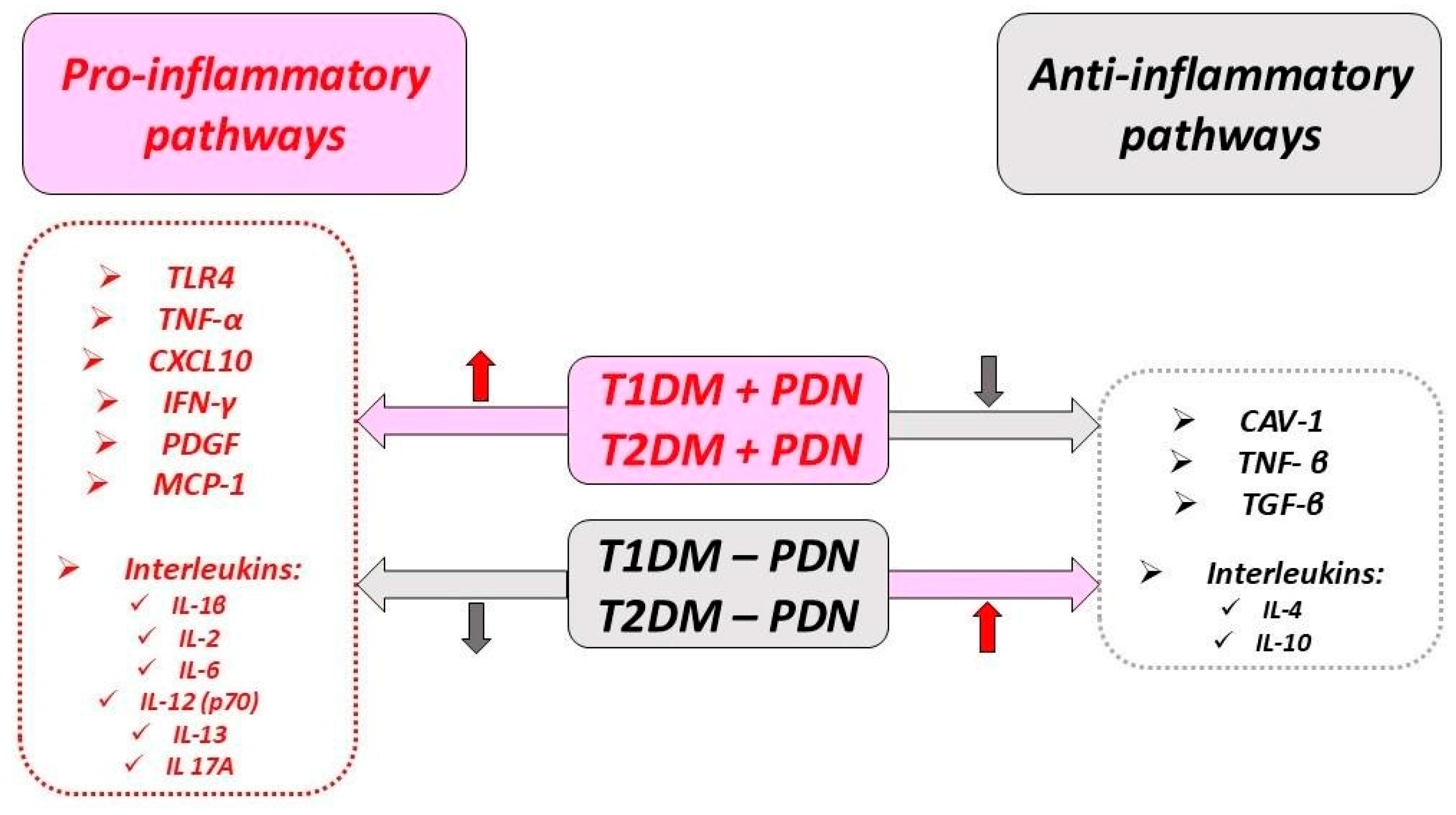
5.2. Cytokines and Chemokines
5.3. Monocyte Chemoattractant Protein 1
6. Vitamin D Deficiency and PDN
7. Potential Treatment Alternatives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | The American Diabetes Association |
| ADIPOQ | Adiponectin C1Q |
| AGEs | Advanced glycation end products |
| ATP | Adenosine triphosphate |
| AS | Ankylosing spondylitis |
| CAV1 | Caveolin 1 |
| CD | Cluster of differentiation |
| CNS | Central nervous system |
| COX2 | Cyclooxygenase 2 |
| CoQ10 | Coenzyme Q10 |
| CRP | C reactive protein |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| Cyt C | Cytochrome C |
| DAN | Diabetic autonomic neuropathy |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| DRG | Dorsal root ganglion |
| 25(OH)D | 25-hydroxyvitamin D |
| GSH | Glutathione |
| HbA1c | Hemoglobin A1c |
| ICAM1 | Intercellular adhesion molecule 1 |
| IFN | Interferon |
| IGT | Impaired glucose tolerance |
| IκB | Inhibitor of kappa B |
| IL | Interleukin |
| IL-R | Interleukin receptor |
| iNOS | Inducible nitric oxide |
| IRS | Insulin receptor substrate |
| JAK | Janus kinase |
| JNK | Jun-N-terminal kinase |
| LEP | Leptin |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| M1 | Monocyte type 1 |
| M2 | Monocyte type 2 |
| MBP | Myelin basic protein |
| MCP1 | Monocyte chemoattractant protein 1 |
| MCV | Motor conduction velocity |
| MMP12 | Matrix metallopeptidase 12 |
| mRNA | Messenger ribonucleic acid |
| MS4A1 | Membrane-spanning 4-domains A1 |
| NGF | Nerve growth factor |
| NAC | N-acetylcysteine |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor-2 erythroid-related factor-2 |
| PDGF | Platelet-derived grown factor |
| PDN | Peripheral diabetic neuropathy |
| RA | Rheumatoid arthritis |
| REGARDS | Reasons for Geographic and Racial Difference in Stroke |
| rER | Rough endoplasmic reticulum |
| SCV | Sensory conduction velocity |
| SMAD | Signal transducer for receptors of the transforming growth factor beta family |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozocin |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| Th1 | T helper 1 |
| TLR | Tool-like receptor |
| TNF-R | Tumor necrosis factor receptor |
| TNF-α | Tumor necrosis factor alpha |
| TNFRSF1A | TNF receptor superfamily member 1A |
| TNFRSF1B | TNF receptor superfamily member 1B |
| TNF-β | Tumor necrosis factor beta |
| TGF-β | Transforming growth factor-β |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of Diabetes Recorded in Mainland China Using 2018 Diagnostic Criteria from the American Diabetes Association: National Cross Sectional Study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Liu, A. Chemokines in Prediabetes and Type 2 Diabetes: A Meta-Analysis. Front. Immunol. 2021, 12, 622438. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Li, M.-Y.; Wang, Y.-Y.; Cao, R.; Hou, X.-H.; Zhang, L.; Yang, R.-H.; Wang, F. Dietary Fish Oil Inhibits Mechanical Allodynia and Thermal Hyperalgesia in Diabetic Rats by Blocking Nuclear Factor-κB-Mediated Inflammatory Pathways. J. Nutr. Biochem. 2015, 26, 1147–1155. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Yousuf, A.; Ibrahim, W.; Greening, N.J.; Brightling, C.E. T2 Biologics for Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. Pract. 2019, 7, 1405–1416. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Gupta, S.; Maratha, A.; Siednienko, J.; Natarajan, A.; Gajanayake, T.; Hoashi, S.; Miggin, S. Analysis of Inflammatory Cytokine and TLR Expression Levels in Type 2 Diabetes with Complications. Sci. Rep. 2017, 7, 7633. [Google Scholar] [CrossRef]
- Rossor, A.M.; Reilly, M.M. Blood Biomarkers of Peripheral Neuropathy. Acta Neuro Scand. 2022, 146, 325–331. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, Y.; Shi, Y.; Jiang, C. Inflammation Role in Sensory Neuropathy in Chinese Patients with Diabetes/Prediabetes. Clin. Neurol. Neurosurg. 2018, 166, 136–140. [Google Scholar] [CrossRef]
- Giulietti, A.; van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from Type 2 Diabetic Patients Have a Pro-Inflammatory Profile. 1,25-Dihydroxyvitamin D(3) Works as Anti-Inflammatory. Diabetes Res. Clin. Pract. 2007, 77, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Menkes, D.L.; Souayah, N. Targeting Neuroinflammation in Distal Symmetrical Polyneuropathy in Diabetes. Drug Discov. Today 2024, 29, 104087. [Google Scholar] [CrossRef] [PubMed]
- Vozarova, B.; Weyer, C.; Lindsay, R.S.; Pratley, R.E.; Bogardus, C.; Tataranni, P.A. High White Blood Cell Count Is Associated with a Worsening of Insulin Sensitivity and Predicts the Development of Type 2 Diabetes. Diabetes 2002, 51, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Fasshauer, M.; Tönjes, A.; Kratzsch, J.; Schön, M.; Paschke, R. Association of Interleukin-6, C-Reactive Protein, Interleukin-10 and Adiponectin Plasma Concentrations with Measures of Obesity, Insulin Sensitivity and Glucose Metabolism. Exp. Clin. Endocrinol. Diabetes 2005, 113, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Mussa, B.M.; Srivastava, A.; Al-Habshi, A.; Mohammed, A.K.; Halwani, R.; Abusnana, S. Inflammatory Biomarkers Levels in T2DM Emirati Patients with Diabetic Neuropathy. DMSO 2021, 14, 3389–3397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, H.; Zhang, L.; Liu, Z.; Huang, Y.; Liu, Q.; Jin, L.; Zhu, M.; Zhang, L. Inflammation in diabetes complications: Molecular mechanisms and therapeutic interventions. MedComm 2024, 5, e516. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Bacârea, A.; Moţăţăianu, A.; Stoian, M.; Gliga, F.; Bacârea, V.; Duicu, C.; Bănescu, C. Vascular Endothelial Growth Factor Insertion/Deletion Gene Polymorphism in Patients with Type 2 Diabetes and Diabetic Peripheral Polyneuropathy. Rom. Rev. Lab. Med. 2014, 22, 165–172. [Google Scholar] [CrossRef]
- Stoian, A.; Earar, K.; Budacu, C.; Voidazan, S.; Crauciuc, A.; Stoian, M.; Bica, C.I.; Banescu, C. No Association Between Antioxidant Enzyme Gene Polymorphism and Albuminuria in Type 2 Diabetes Mellitus Cases. Rev. Chim. 2016, 67, 2355. [Google Scholar]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Hagen, K.M.; Ousman, S.S. Aging and the Immune Response in Diabetic Peripheral Neuropathy. J. Neuroimmunol. 2021, 355, 577574. [Google Scholar] [CrossRef]
- Vujčić, S.; Kotur-Stevuljević, J.; Vekić, J.; Perović-Blagojević, I.; Stefanović, T.; Ilić-Mijailović, S.; Koprivica Uzelac, B.; Bosić, S.; Antonić, T.; Guzonjić, A.; et al. Oxidative Stress and Inflammatory Biomarkers in Patients with Diabetic Foot. Medicina 2022, 58, 1866. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Elmotaleb Hussein, M.A.; Nabil Hanna, I.; Japer Nashwan, A.J.; Saleh, M.; Abdel Wahed, W.Y.; Mohamed Mansour, A.M.; Ezz Al Arab, M.R.; Fawzy, N.; Sakr, Y.; et al. The Potential Impact and Diagnostic Value of Inflammatory Markers on Diabetic Foot Progression in Type II Diabetes Mellitus: A Case–Control Study. Med. Clín. 2024, 162, e33–e39. [Google Scholar] [CrossRef]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic Neuropathy: Mechanisms to Management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Neuropathy: Practice Essentials, Background, Anatomy. 2024. Available online: https://emedicine.medscape.com/article/1170337-overview?form=fpf (accessed on 23 September 2024).
- American Diabetes Association Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin. Diabetes 2022, 41, 4–31. [CrossRef]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic Neuropathy in Diabetes Mellitus. Front. Endocrinol. 2014, 5, 205. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Al-Maatouq, M.A.; Al-Tuwaijri, A.S. Subclinical Autonomic Neuropathy in Saudi Type 2 Diabetic Patients. Neurosciences 2007, 12, 46–49. [Google Scholar]
- Mohammad, M.K.; Morran, M.; Slotterbeck, B.; Leaman, D.W.; Sun, Y.; Grafenstein, H.V.; Hong, S.-C.; McInerney, M.F. Dysregulated Toll-like Receptor Expression and Signaling in Bone Marrow-Derived Macrophages at the Onset of Diabetes in the Non-Obese Diabetic Mouse. Int. Immunol. 2006, 18, 1101–1113. [Google Scholar] [CrossRef]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Mashhadi, M.A.; Javadian, F.; Haghighi, A.; Kohan, F.; Bahari, A.; Sargazi, A. Human Toll like Receptor 4 Gene Expression of PBMCs in Diabetes Mellitus Type 2 Patients. Cell. Mol. Biol. 2015, 61, 92–95. [Google Scholar]
- Dasu, M.R.; Devaraj, S.; Zhao, L.; Hwang, D.H.; Jialal, I. High Glucose Induces Toll-Like Receptor Expression in Human Monocytes. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef]
- Elzinga, S.; Murdock, B.J.; Guo, K.; Hayes, J.M.; Tabbey, M.A.; Hur, J.; Feldman, E.L. Toll-like Receptors and Inflammation in Metabolic Neuropathy; a Role in Early versus Late Disease? Exp. Neurol. 2019, 320, 112967. [Google Scholar] [CrossRef]
- Sood, B.; Patel, P.; Keenaghan, M. Coenzyme Q10. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zhang, Y.P.; Song, C.Y.; Yuan, Y.; Eber, A.; Rodriguez, Y.; Levitt, R.C.; Takacs, P.; Yang, Z.; Goldberg, R.; Candiotti, K.A. Diabetic Neuropathic Pain Development in Type 2 Diabetic Mouse Model and the Prophylactic and Therapeutic Effects of Coenzyme Q10. Neurobiol. Dis. 2013, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Meng, Q.; Ji, J.; Zhang, L.; Lou, X. TLR4 and Caveolin-1 in Monocytes Are Associated With Inflammatory Conditions in Diabetic Neuropathy. Clin. Transl. Sci. 2017, 10, 178–184. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, Stress, and Diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, X.; Liu, H.; Lv, Q.; Zou, J.; Shi, Y.; Liu, Z. Expression of Nrf2 Promotes Schwann Cell-Mediated Sciatic Nerve Recovery in Diabetic Peripheral Neuropathy. Cell. Physiol. Biochem. 2018, 46, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yuan, W.; Lou, X.; Zhu, T. Streptozotocin-Induced Diabetic Hyperalgesia in Rats Is Associated with Upregulation of Toll-like Receptor 4 Expression. Neurosci. Lett. 2012, 526, 54–58. [Google Scholar] [CrossRef]
- Rudofsky, G.; Reismann, P.; Witte, S.; Humpert, P.M.; Isermann, B.; Chavakis, T.; Tafel, J.; Nosikov, V.V.; Hamann, A.; Nawroth, P.; et al. Asp299Gly and Thr399Ile Genotypes of the TLR4 Gene Are Associated with a Reduced Prevalence of Diabetic Neuropathy in Patients With Type 2 Diabetes. Diabetes Care 2004, 27, 179–183. [Google Scholar] [CrossRef]
- Haddad, D.; Al Madhoun, A.; Nizam, R.; Al-Mulla, F. Role of Caveolin-1 in Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Wang, X.M.; Kim, H.P.; Song, R.; Choi, A.M.K. Caveolin-1 Confers Antiinflammatory Effects in Murine Macrophages via the MKK3/P38 MAPK Pathway. Am. J. Respir. Cell Mol. Biol. 2006, 34, 434–442. [Google Scholar] [CrossRef]
- Wang, X.M.; Kim, H.P.; Nakahira, K.; Ryter, S.W.; Choi, A.M.K. The Heme Oxygenase-1/Carbon Monoxide Pathway Suppresses TLR4 Signaling by Regulating the Interaction of TLR4 with Caveolin-1. J. Immunol. 2009, 182, 3809–3818. [Google Scholar] [CrossRef]
- Jia, G.; Huang, Q.; Cao, Y.; Xie, C.; Shen, Y.; Chen, J.; Lu, J.; Zhang, M.; Li, J.; Tao, Y.; et al. Cav-1 Participates in the Development of Diabetic Neuropathy Pain through the TLR4 Signaling Pathway. J. Cell. Physiol. 2020, 235, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Juliet, P.A.R.; Miyazaki, A.; Ignarro, L.J.; Iguchi, A. High Glucose Downregulates the Number of Caveolae in Monocytes through Oxidative Stress from NADPH Oxidase: Implications for Atherosclerosis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2007, 1772, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Rouen, S.; Dobrowsky, R.T. Hyperglycemia and Downregulation of Caveolin-1 Enhance Neuregulin-induced Demyelination. Glia 2008, 56, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Young, G.S.; Conquer, J.A.; Thomas, R. Effect of Randomized Supplementation with High Dose Olive, Flax or Fish Oil on Serum Phospholipid Fatty Acid Levels in Adults with Attention Deficit Hyperactivity Disorder. Reprod. Nutr. Dev. 2005, 45, 549–558. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Ang, L.; Holmes, C.; Gallagher, K.; Feldman, E.L. Inflammation as a Therapeutic Target for Diabetic Neuropathies. Curr. Diabetes Rep. 2016, 16, 29. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Adki, K.M.; Kulkarni, Y.A. Biomarkers in Diabetic Neuropathy. Arch. Physiol. Biochem. 2023, 129, 460–475. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-Κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Goldfine, A.B. Getting Away from Glucose: Fanning the Flames of Obesity-Induced Inflammation. Nat. Med. 2009, 15, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schmeichel, A.M.; Iida, H.; Schmelzer, J.D.; Low, P.A. Enhanced Inflammatory Response via Activation of NF-κB in Acute Experimental Diabetic Neuropathy Subjected to Ischemia–Reperfusion Injury. J. Neurol. Sci. 2006, 247, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential Therapeutic Effects of the Simultaneous Targeting of the Nrf2 and NF-κB Pathways in Diabetic Neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-Antioxidant Signaling Attenuates NFκB-Inflammatory Response and Elicits Apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Lampiasi, N.; Russo, R.; Zito, F. The Alternative Faces of Macrophage Generate Osteoclasts. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Stoian, A.; Bălașa, R.; Grigorescu, B.; Maier, S.; Andone, S.; Cocuz, I.; Bajko, Z.; Filep, C.; Stoian, M. Guillain-Barré Syndrome Associated with Covid-19: A Close Relationship or Just a Coincidence? (Review). Exp. Ther. Med. 2021, 22, 916. [Google Scholar] [CrossRef]
- Stoian, A.; Bajko, Z.; Stoian, M.; Cioflinc, R.A.; Niculescu, R.; Arbănași, E.M.; Russu, E.; Botoncea, M.; Bălașa, R. The Occurrence of Acute Disseminated Encephalomyelitis in SARS-CoV-2 Infection/Vaccination: Our Experience and a Systematic Review of the Literature. Vaccines 2023, 11, 1225. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular Mechanisms of Diabetic Cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Bene, N.C.; Alcaide, P.; Wortis, H.H.; Jaffe, I.Z. Mineralocorticoid Receptors in Immune Cells: Emerging Role in Cardiovascular Disease. Steroids 2014, 91, 38–45. [Google Scholar] [CrossRef]
- Alvarado-Vázquez, P.; Grosick, R.; Moracho-Vilrriales, C.; Ward, E.; Threatt, T.; Romero-Sandoval, E.A. Cytokine Production Capabilities of Human Primary Monocyte-Derived Macrophages from Patients with Diabetes Mellitus Type 2 with and without Diabetic Peripheral Neuropathy. JPR 2018, 12, 69–81. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.S.; Cheng, C.; Martinez, J.A.; Zochodne, D.W. Regenerative Arrest of Inflamed Peripheral Nerves: Role of Nitric Oxide. NeuroReport 2007, 18, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.; Scarpini, E.; Baron, P.; Livraghi, S.; Tiriticco, M.; Bianchi, R.; Vedeler, C.; Scarlato, G. Macrophage Infiltration and Death in the Nerve during the Early Phases of Experimental Diabetic Neuropathy: A Process Concomitant with Endoneurial Induction of IL-1β and p75NTR. J. Neurol. Sci. 2002, 195, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Nagai, R. Islet Inflammation in Type 2 Diabetes and Physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal Stromal Cell-Derived Exosomes Ameliorate Peripheral Neuropathy in a Mouse Model of Diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Baum, P.; Kosacka, J.; Estrela-Lopis, I.; Woidt, K.; Serke, H.; Paeschke, S.; Stockinger, M.; Klöting, N.; Blüher, M.; Dorn, M.; et al. The Role of Nerve Inflammation and Exogenous Iron Load in Experimental Peripheral Diabetic Neuropathy (PDN). Metabolism 2016, 65, 391–405. [Google Scholar] [CrossRef]
- Kosacka, J.; Nowicki, M.; Klöting, N.; Kern, M.; Stumvoll, M.; Bechmann, I.; Serke, H.; Blüher, M. COMP-Angiopoietin-1 Recovers Molecular Biomarkers of Neuropathy and Improves Vascularisation in Sciatic Nerve of Ob/Ob Mice. PLoS ONE 2012, 7, e32881. [Google Scholar] [CrossRef]
- Anand, P.; Terenghi, G.; Warner, G.; Kopelman, P.; Williams-Chestnut, R.E.; Sinicropi, D.V. The Role of Endogenous Nerve Growth Factor in Human Diabetic Neuropathy. Nat. Med. 1996, 2, 703–707. [Google Scholar] [CrossRef]
- You, H.; Gao, T.; Cooper, T.K.; Brian Reeves, W.; Awad, A.S. Macrophages Directly Mediate Diabetic Renal Injury. Am. J. Physiol. -Ren. Physiol. 2013, 305, F1719–F1727. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Sheng, H.; Liang, C.; Liu, H.; Moran Guerrero, J.A.; Lu, Z.; Mao, W.; Dai, Z.; Liu, X.; et al. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice with Type 2 Diabetes Mellitus. Front. Immunol. 2021, 12, 733808. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, Y.; Wu, W.; Yan, B.; Meng, Y.; Liang, Y.; Yao, X.; Luo, J. Exploring the Immune Infiltration Landscape and M2 Macrophage-Related Biomarkers of Proliferative Diabetic Retinopathy. Front. Endocrinol. 2022, 13, 841813. [Google Scholar] [CrossRef] [PubMed]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.; Romero-Sandoval, E.A. High Glucose Induces a Priming Effect in Macrophages and Exacerbates the Production of Pro-Inflammatory Cytokines after a Challenge. JPR 2018, 11, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Zwadlo, G.; Voegeíi, R.; Osthoff, K.S.; Sorg, C. A Monoclonal Antibody to a Novel Differentiation Antigen on Human Macrophages Associated with the Down-Regulatory Phase of the Inflammatory Process. Pathobiology 1987, 55, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Philippidis, P.; Mason, J.C.; Evans, B.J.; Nadra, I.; Taylor, K.M.; Haskard, D.O.; Landis, R.C. Hemoglobin Scavenger Receptor CD163 Mediates Interleukin-10 Release and Heme Oxygenase-1 Synthesis: Antiinflammatory Monocyte-Macrophage Responses In Vitro, in Resolving Skin Blisters In Vivo, and after Cardiopulmonary Bypass Surgery. Circ. Res. 2004, 94, 119–126. [Google Scholar] [CrossRef]
- Min, D.; Brooks, B.; Wong, J.; Aamidor, S.; Seehoo, R.; Sutanto, S.; Harrisberg, B.; Yue, D.K.; Twigg, S.M.; McLennan, S.V. Monocyte CD163 Is Altered in Association with Diabetic Complications: Possible Protective Role. J. Leukoc. Biol. 2016, 100, 1375–1383. [Google Scholar] [CrossRef]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-Inflammatory Chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef]
- Siqueira Mietto, B.; Kroner, A.; Girolami, E.I.; Santos-Nogueira, E.; Zhang, J.; David, S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J. Neurosci. 2015, 35, 16431–16442. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Vela, S.A.; Quebedeaux, T.L.; Fleischli, J.G. Practical Criteria for Screening Patients at High Risk for Diabetic Foot Ulceration. Arch. Intern. Med. 1998, 158, 157. [Google Scholar] [CrossRef]
- Younger, D.S.; Rosoklija, G.; Hays, A.P.; Trojaborg, W.; Latov, N. Diabetic Peripheral Neuropathy: A Clinicopathologic and Immunohistochemical Analysis of Sural Nerve Biopsies. Muscle Nerve 1996, 19, 722–727. [Google Scholar] [CrossRef]
- Xue, T.; Zhang, X.; Xing, Y.; Liu, S.; Zhang, L.; Wang, X.; Yu, M. Advances About Immunoinflammatory Pathogenesis and Treatment in Diabetic Peripheral Neuropathy. Front. Pharmacol. 2021, 12, 748193. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Xue, R.; Fan, S.-Y.; Fan, Q.-Y.; An, L.; Li, J.; Zhu, L.; Ran, Y.-H.; Zhang, L.-M.; Zhong, B.-H.; et al. Ammoxetine Attenuates Diabetic Neuropathic Pain through Inhibiting Microglial Activation and Neuroinflammation in the Spinal Cord. J. Neuroinflammation 2018, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, T.; Wang, J.; Huang, X.; Deng, X.; Cao, Y.; Zhou, M.; Zhao, C. An Update on Potential Biomarkers for Diagnosing Diabetic Foot Ulcer at Early Stage. Biomed. Pharmacother. 2021, 133, 110991. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of Growth Factors and Cytokines in Diabetic Foot Ulcer Healing: A Detailed Review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hinder, L.M.; Murdock, B.J.; Park, M.; Bender, D.E.; O’Brien, P.D.; Rumora, A.E.; Hur, J.; Feldman, E.L. Transcriptional Networks of Progressive Diabetic Peripheral Neuropathy in the Db/Db Mouse Model of Type 2 Diabetes: An Inflammatory Story. Experimental Neurology 2018, 305, 33–43. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Eid, S.; Rumora, A.E.; Murdock, B.; Guo, K.; de Anda-Jáuregui, G.; Porter, J.E.; Feldman, E.L.; Hur, J. Conserved Transcriptional Signatures in Human and Murine Diabetic Peripheral Neuropathy. Sci. Rep. 2018, 8, 17678. [Google Scholar] [CrossRef] [PubMed]
- Yanik, B.M.; Dauch, J.R.; Cheng, H.T. Interleukin-10 Reduces Neurogenic Inflammation and Pain Behavior in a Mouse Model of Type 2 Diabetes. JPR 2020, 13, 3499–3512. [Google Scholar] [CrossRef]
- Lampropoulou, I.-T.; Stangou, M.; Papagianni, A.; Didangelos, T.; Iliadis, F.; Efstratiadis, G. TNF- α and Microalbuminuria in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Action LADA Study Group; Pham, M.N.; Hawa, M.I.; Pfleger, C.; Roden, M.; Schernthaner, G.; Pozzilli, P.; Buzzetti, R.; Scherbaum, W.; Seissler, J.; et al. Pro- and Anti-Inflammatory Cytokines in Latent Autoimmune Diabetes in Adults, Type 1 and Type 2 Diabetes Patients: Action LADA 4. Diabetologia 2011, 54, 1630–1638. [Google Scholar] [CrossRef]
- Herder, C.; Lankisch, M.; Ziegler, D.; Rathmann, W.; Koenig, W.; Illig, T.; Döring, A.; Thorand, B.; Holle, R.; Giani, G.; et al. Subclinical Inflammation and Diabetic Polyneuropathy. Diabetes Care 2009, 32, 680–682. [Google Scholar] [CrossRef]
- Doupis, J.; Lyons, T.E.; Wu, S.; Gnardellis, C.; Dinh, T.; Veves, A. Microvascular Reactivity and Inflammatory Cytokines in Painful and Painless Peripheral Diabetic Neuropathy. J. Clin. Endocrinol. Metab. 2009, 94, 2157–2163. [Google Scholar] [CrossRef]
- Wagner, R.; Myers, R.R. Schwann Cells Produce Tumor Necrosis Factor Alpha: Expression in Injured and Non-Injured Nerves. Neuroscience 1996, 73, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Mázala-de-Oliveira, T.; Jannini De Sá, Y.A.P.; Carvalho, V.D.F. Impact of Gut-Peripheral Nervous System Axis on the Development of Diabetic Neuropathy. Mem. Inst. Oswaldo Cruz 2023, 118, e220197. [Google Scholar] [CrossRef] [PubMed]
- Ehses, J.A.; Perren, A.; Eppler, E.; Ribaux, P.; Pospisilik, J.A.; Maor-Cahn, R.; Gueripel, X.; Ellingsgaard, H.; Schneider, M.K.J.; Biollaz, G.; et al. Increased Number of Islet-Associated Macrophages in Type 2 Diabetes. Diabetes 2007, 56, 2356–2370. [Google Scholar] [CrossRef] [PubMed]
- Purwata, T. High TNF-Alpha Plasma Levels and Macrophages iNOS and TNF-Alpha Expression as Risk Factors for Painful Diabetic Neuropathy. JPR 2011, 169, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Q.; Wang, Q.; Che, X.Q.; Liu, X.; Zhao, S.; Wang, S. Clinical Significance of Apelin in the Treatment of Type 2 Diabetic Peripheral Neuropathy. Medicine 2021, 100, e25710. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.; Sampaio, E.P.; Aarestrup, F.; Teles, R.M.B.; Silva, T.P.; Oliveira, A.L.; Antas, P.R.Z.; Sarno, E.N. Cytokines and Mycobacterium leprae Induce Apoptosis in Human Schwann Cells. J. Neuropathol. Exp. Neurol. 2005, 64, 882–890. [Google Scholar] [CrossRef]
- Stoian, A.; Bajko, Z.; Maier, S.; Cioflinc, R.; Grigorescu, B.; Moțățăianu, A.; Bărcuțean, L.; Balașa, R.; Stoian, M. High-dose Intravenous Immunoglobulins as a Therapeutic Option in Critical Illness Polyneuropathy Accompanying SARS-CoV-2 Infection: A Case-based Review of the Literature (Review). Exp. Ther. Med. 2021, 22, 1182. [Google Scholar] [CrossRef]
- He, X.-H.; Zang, Y.; Chen, X.; Pang, R.-P.; Xu, J.-T.; Zhou, X.; Wei, X.-H.; Li, Y.-Y.; Xin, W.-J.; Qin, Z.-H.; et al. TNF-α Contributes to up-Regulation of Nav1.3 and Nav1.8 in DRG Neurons Following Motor Fiber Injury. Pain 2010, 151, 266–279. [Google Scholar] [CrossRef]
- Nadi, M.; Bambaeichi, E.; Marandi, S.M. Comparison of the Effect of Two Therapeutic Exercises on the Inflammatory and Physiological Conditions and Complications of Diabetic Neuropathy in Female Patients. DMSO 2019, 12, 1493–1501. [Google Scholar] [CrossRef]
- Matsuda, M.; Kawasaki, F.; Inoue, H.; Kanda, Y.; Yamada, K.; Harada, Y.; Saito, M.; Eto, M.; Matsuki, M.; Kaku, K. Possible Contribution of Adipocytokines on Diabetic Neuropathy. Diabetes Res. Clin. Pract. 2004, 66, S121–S123. [Google Scholar] [CrossRef]
- Duksal, T.; Tiftikcioglu, B.I.; Bilgin, S.; Kose, S.; Zorlu, Y. Role of Inflammation in Sensory Neuropathy in Prediabetes or Diabetes. Acta Neurol. Scand. 2016, 133, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rizvi, S.A.A.; Singhal, S.; Zubair, M.; Ahmad, J. Serum Levels of TNF-α in Peripheral Neuropathy Patients and Its Correlation with Nerve Conduction Velocity in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2013, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.G.; Vlaicu, R.; Niculescu, F. Interleukin-6 and Interleukin-8 Protein and Gene Expression in Human Arterial Atherosclerotic Wall. Atherosclerosis 1996, 127, 263–271. [Google Scholar] [CrossRef]
- El Sheikh, W.M.; Alahmar, I.E.; Salem, G.M.; El-Sheikh, M.A. Tumor Necrosis Factor Alpha in Peripheral Neuropathy in Type 2 Diabetes Mellitus. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 37. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Giugliano, F.; Giugliano, G.; Marfella, R.; Nicoletti, G.; Giugliano, D. Association of Low Interleukin-10 Levels with the Metabolic Syndrome in Obese Women. J. Clin. Endocrinol. Metab. 2003, 88, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Magrinelli, F.; Briani, C.; Romano, M.; Ruggero, S.; Toffanin, E.; Triolo, G.; Peter, G.C.; Praitano, M.; Lauriola, M.F.; Zanette, G.; et al. The Association between Serum Cytokines and Damage to Large and Small Nerve Fibers in Diabetic Peripheral Neuropathy. J. Diabetes Res. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bäckryd, E.; Ghafouri, B.; Larsson, B.; Gerdle, B. Plasma Pro-Inflammatory Markers in Chronic Neuropathic Pain: A Multivariate, Comparative, Cross-Sectional Pilot Study. Scand. J. Pain 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Angst, D.B.M.; Pinheiro, R.O.; Vieira, J.S.D.S.; Cobas, R.A.; Hacker, M.D.A.V.-B.; Pitta, I.J.R.; Giesel, L.M.; Sarno, E.N.; Jardim, M.R. Cytokine Levels in Neural Pain in Leprosy. Front. Immunol. 2020, 11, 23. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S. Inflammation: Therapeutic Targets for Diabetic Neuropathy. Mol. Neurobiol. 2014, 49, 536–546. [Google Scholar] [CrossRef]
- Cameron, N.; Cotter, M. Pro-Inflammatory Mechanisms in Diabetic Neuropathy: Focus on the Nuclear Factor Kappa B Pathway. CDT 2008, 9, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Andriambeloson, E.; Baillet, C.; Vitte, P.; Garotta, G.; Dreano, M.; Callizot, N. Interleukin-6 Attenuates the Development of Experimental Diabetes-related Neuropathy. Neuropathology 2006, 26, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Cotter, M.A.; Gibson, T.M.; Nangle, M.R.; Cameron, N.E. Effects of Interleukin-6 Treatment on Neurovascular Function, Nerve Perfusion and Vascular Endothelium in Diabetic Rats. Diabetes Obes. Metab. 2010, 12, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Van Exel, E.; Gussekloo, J.; De Craen, A.J.M.; Frölich, M.; Bootsma-van Der Wiel, A.; Westendorp, R.G.J. Low Production Capacity of Interleukin-10 Associates With the Metabolic Syndrome and Type 2 Diabetes. Diabetes 2002, 51, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Rogausch, J.P.; Toyka, K.V.; Sommer, C. Differential Expression of Cytokines in Painful and Painless Neuropathies. Neurology 2007, 69, 42–49. [Google Scholar] [CrossRef]
- Xiaohua, G.; Dongdong, L.; Xiaoting, N.; Shuoping, C.; Feixia, S.; Huajun, Y.; Qi, Z.; Zimiao, C. Severe Vitamin D Deficiency Is Associated With Increased Expression of Inflammatory Cytokines in Painful Diabetic Peripheral Neuropathy. Front. Nutr. 2021, 8, 612068. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Kiefer, J.; Braig, D.; Loseff-Silver, J.; Potempa, L.A.; Eisenhardt, S.U.; Peter, K. Dissociation of C-Reactive Protein Localizes and Amplifies Inflammation: Evidence for a Direct Biological Role of C-Reactive Protein and Its Conformational Changes. Front. Immunol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte Chemoattractant Protein 1 Acts as a T-Lymphocyte Chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.; Park, J.-Y.; Lind, A.-L.; Ma, Q.; Ji, R.-R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, H.-S.; Kawada, T.; Kim, J.-H.; Lim, D.; Hubbard, N.E.; Kwon, B.-S.; Erickson, K.L.; Yu, R. Circulating Levels of MCP-1 and IL-8 Are Elevated in Human Obese Subjects and Associated with Obesity-Related Parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- White, F.A.; Jung, H.; Miller, R.J. Chemokines and the Pathophysiology of Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 20151–20158. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and Immune Function: An Overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, B.; Han, W.; Zhu, Z.; Wang, X.; Jin, X.; Antony, B.; Cicuttini, F.; Wluka, A.; Winzenberg, T.; et al. Vitamin D Supplementation and Inflammatory and Metabolic Biomarkers in Patients with Knee Osteoarthritis: Post Hoc Analysis of a Randomised Controlled Trial—Corrigendum. Br. J. Nutr. 2019, 121, 118–119. [Google Scholar] [CrossRef]
- Nyulas, K.-I.; Simon-Szabó, Z.; Preg, Z.; Pál, S.; Sharma, A.; Pál, T.; Germán-Salló, M.; Nemes-Nagy, E. Assessment of Vitamin D Levels in Relation to Statin Therapy in Elderly Hypertensive Patients with Comorbidities. J. Interdiscip. Med. 2022, 7, 88–91. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients 2021, 13, 3491. [Google Scholar] [CrossRef]
- Upreti, V.; Maitri, V.; Dhull, P.; Handa, A.; Prakash, M.S.; Behl, A. Effect of Oral Vitamin D Supplementation on Glycemic Control in Patients with Type 2 Diabetes Mellitus with Coexisting Hypovitaminosis D: A Parellel Group Placebo Controlled Randomized Controlled Pilot Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 509–512. [Google Scholar] [CrossRef]
- Leung, P. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of Vitamin D on Immune Function: Lessons Learned from Genome-Wide Analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Christakos, S.; Liu, Y. Biological Actions and Mechanism of Action of Calbindin in the Process of Apoptosis. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 401–404. [Google Scholar] [CrossRef]
- Ozuguz, U.; Oruc, S.; Ulu, M.S.; Demirbas, H.; Acay, A.; Coker, B.; Beyazıt, B.; Yaman, M.; Koken, T. Does Vitamin D Have Any Role in the Improvement of Diabetic Peripheral Neuropathy in Type 1 Diabetic Patients? J. Endocrinol. Investig. 2016, 39, 1411–1417. [Google Scholar] [CrossRef]
- Ou, Y.; Liang, Z.; Yang, Y.; Zhou, Y. Association of Diabetic Peripheral Neuropathy with Vitamin D Levels Depends on Vitamin D Status. Med. Sci. Monit. 2021, 27, e931244-1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-K.; Liang, Z.; Guo, Y.; Zhang, H.-T.; Wang, K.-H. High Glucose Upregulates CYP24A1 Expression Which Attenuates the Ability of 1,25(OH)2D3 to Increase NGF Secretion in a Rat Schwann Cell Line RSC96. Mol. Cell. Endocrinol. 2015, 404, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shillo, P.; Selvarajah, D.; Greig, M.; Gandhi, R.; Rao, G.; Wilkinson, I.D.; Anand, P.; Tesfaye, S. Reduced Vitamin D Levels in Painful Diabetic Peripheral Neuropathy. Diabet. Med. 2019, 36, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Adela, R.; Borkar, R.M.; Mishra, N.; Bhandi, M.M.; Vishwakarma, G.; Varma, B.A.; Ragampeta, S.; Banerjee, S.K. Lower Serum Vitamin D Metabolite Levels in Relation to Circulating Cytokines/Chemokines and Metabolic Hormones in Pregnant Women with Hypertensive Disorders. Front. Immunol. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Pratyush, D.D.; Gupta, S.K.; Singh, S.K. Vitamin D Deficiency Is Associated with Inflammatory Cytokine Concentrations in Patients with Diabetic Foot Infection. Br. J. Nutr. 2014, 112, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Basit, K.A.; Fawwad, A.; Shaheen, F.; Fatima, N.; Petropoulos, I.N.; Alam, U.; Malik, R.A. Vitamin D for the Treatment of Painful Diabetic Neuropathy. BMJ Open Diabetes Res. Care 2016, 4, e000148. [Google Scholar] [CrossRef]
- Paul, S.; Judd, S.E.; Howard, V.J.; Safford, M.S.; Gutiérrez, O.M. Association of 25-Hydroxyvitamin D with Incident Coronary Heart Disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am. Heart. J. 2019, 217, 140–147. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Vatanparast, H. Impact of Vitamin D Supplementation on C-Reactive Protein; a Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Nutr. 2018, 4, 1. [Google Scholar] [CrossRef]
- Jackson, J.L.; Judd, S.E.; Panwar, B.; Howard, V.J.; Wadley, V.G.; Jenny, N.S.; Gutiérrez, O.M. Associations of 25-Hydroxyvitamin D with Markers of Inflammation, Insulin Resistance and Obesity in Black and White Community-Dwelling Adults. J. Clin. Transl. Endocrinol. 2016, 5, 21–25. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Subhan, F.; Akbar, A.; Habib, F.; Shahbaz, N.; Ahmad, A.; Wadood, A.; Salman, S. Targeting Anti-Inflammatory Pathways to Treat Diabetes-Induced Neuropathy by 6-Hydroxyflavanone. Nutrients 2023, 15, 2552. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, K.; Arai, T.; Ota, H.; Kato, S.; Natsume, T.; Kurimoto, S.; Yamamoto, M.; Hirata, H. Targeting Anti-Inflammatory Treatment Can Ameliorate Injury-Induced Neuropathic Pain. PLoS ONE 2013, 8, e57721. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; De Rivero Vaccari, J.P.; Keane, R.W.; Lacroix, S. Functional Recovery after Peripheral Nerve Injury Is Dependent on the Pro-Inflammatory Cytokines IL-1β and TNF: Implications for Neuropathic Pain. J. Neurosci. 2011, 31, 12533–12542. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Y.; Nadeem, L.; Xu, G. Beneficial Effect of TNF-α Inhibition on Diabetic Peripheral Neuropathy. J. Neuroinflammation 2013, 10, 836. [Google Scholar] [CrossRef]
- Kiortsis, D.N.; Mavridis, A.K.; Vasakos, S.; Nikas, S.N.; Drosos, A.A. Effects of Infliximab Treatment on Insulin Resistance in Patients with Rheumatoid Arthritis and Ankylosing Spondylitis. Ann. Rheum. Dis. 2005, 64, 765–766. [Google Scholar] [CrossRef]
- Yamakawa, I.; Kojima, H.; Terashima, T.; Katagi, M.; Oi, J.; Urabe, H.; Sanada, M.; Kawai, H.; Chan, L.; Yasuda, H.; et al. Inactivation of TNF-α Ameliorates Diabetic Neuropathy in Mice. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E844–E852. [Google Scholar] [CrossRef]
- Sagara, M.; Satoh, J.; Wada, R.; Yagihashi, S.; Takahashi, K.; Fukuzawa, M.; Muto, G.; Muto, Y.; Toyota, T. Inhibition of Development of Peripheral Neuropathy in Streptozotocin-Induced Diabetic Rats with N-Acetylcysteine. Diabetologia 1996, 39, 263–269. [Google Scholar] [CrossRef]
- Qiang, X.; Satoh, J.; Sagara, M.; Fukuzawa, M.; Masuda, T.; Sakata, Y.; Muto, G.; Muto, Y.; Takahashi, K.; Toyota, T. Inhibitory Effect of Troglitazone on Diabetic Neuropathy in Streptozotocin-Induced Diabetic Rats. Diabetologia 1998, 41, 1321–1326. [Google Scholar] [CrossRef]
- Negi, G.; Kumar, A.; Sharma, S.S. Melatonin Modulates Neuroinflammation and Oxidative Stress in Experimental Diabetic Neuropathy: Effects on NF-κB and Nrf2 Cascades. J. Pineal Res. 2011, 50, 124–131. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Silver, R.; Aldhahi, W.; Cai, D.; Tatro, E.; Lee, J.; Shoelson, S.E. Use of Salsalate to Target Inflammation in the Treatment of Insulin Resistance and Type 2 Diabetes. Clin. Transl. Sci. 2008, 1, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Srivastava, A.K.; Kumar, D.A.; Chakrawarty, A.; Khan, M.A.; Ambashtha, A.K.; Kumar, V.; De Taboada, L.; Dey, A.B. Effect of Deep Tissue Laser Therapy Treatment on Peripheral Neuropathic Pain in Older Adults with Type 2 Diabetes: A Pilot Randomized Clinical Trial. BMC Geriatr. 2019, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. Blockade of CCR4 Diminishes Hypersensitivity and Enhances Opioid Analgesia—Evidence from a Mouse Model of Diabetic Neuropathy. Neuroscience 2020, 441, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Gordon Smith, A. Recent Updates in the Treatment of Diabetic Polyneuropathy. Fac. Rev. 2022, 11, 30. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoian, A.; Muntean, C.; Babă, D.-F.; Manea, A.; Dénes, L.; Simon-Szabó, Z.; Kosovski, I.B.; Nemes-Nagy, E.; Gliga, F.I.; Stoian, M. Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy. Int. J. Mol. Sci. 2024, 25, 10395. https://doi.org/10.3390/ijms251910395
Stoian A, Muntean C, Babă D-F, Manea A, Dénes L, Simon-Szabó Z, Kosovski IB, Nemes-Nagy E, Gliga FI, Stoian M. Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy. International Journal of Molecular Sciences. 2024; 25(19):10395. https://doi.org/10.3390/ijms251910395
Chicago/Turabian StyleStoian, Adina, Carmen Muntean, Dragoș-Florin Babă, Andrei Manea, Lóránd Dénes, Zsuzsánna Simon-Szabó, Irina Bianca Kosovski, Enikő Nemes-Nagy, Florina Ioana Gliga, and Mircea Stoian. 2024. "Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy" International Journal of Molecular Sciences 25, no. 19: 10395. https://doi.org/10.3390/ijms251910395
APA StyleStoian, A., Muntean, C., Babă, D. -F., Manea, A., Dénes, L., Simon-Szabó, Z., Kosovski, I. B., Nemes-Nagy, E., Gliga, F. I., & Stoian, M. (2024). Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy. International Journal of Molecular Sciences, 25(19), 10395. https://doi.org/10.3390/ijms251910395







