Mass Spectrometry Analysis of Neurotransmitter Shifting during Neurogenesis and Neurodegeneration of PC12 Cells
Abstract
1. Introduction
2. Results
2.1. Effects of Rotenone on the Morphology of Cells That Underwent Neurodifferentiation and Degeneration
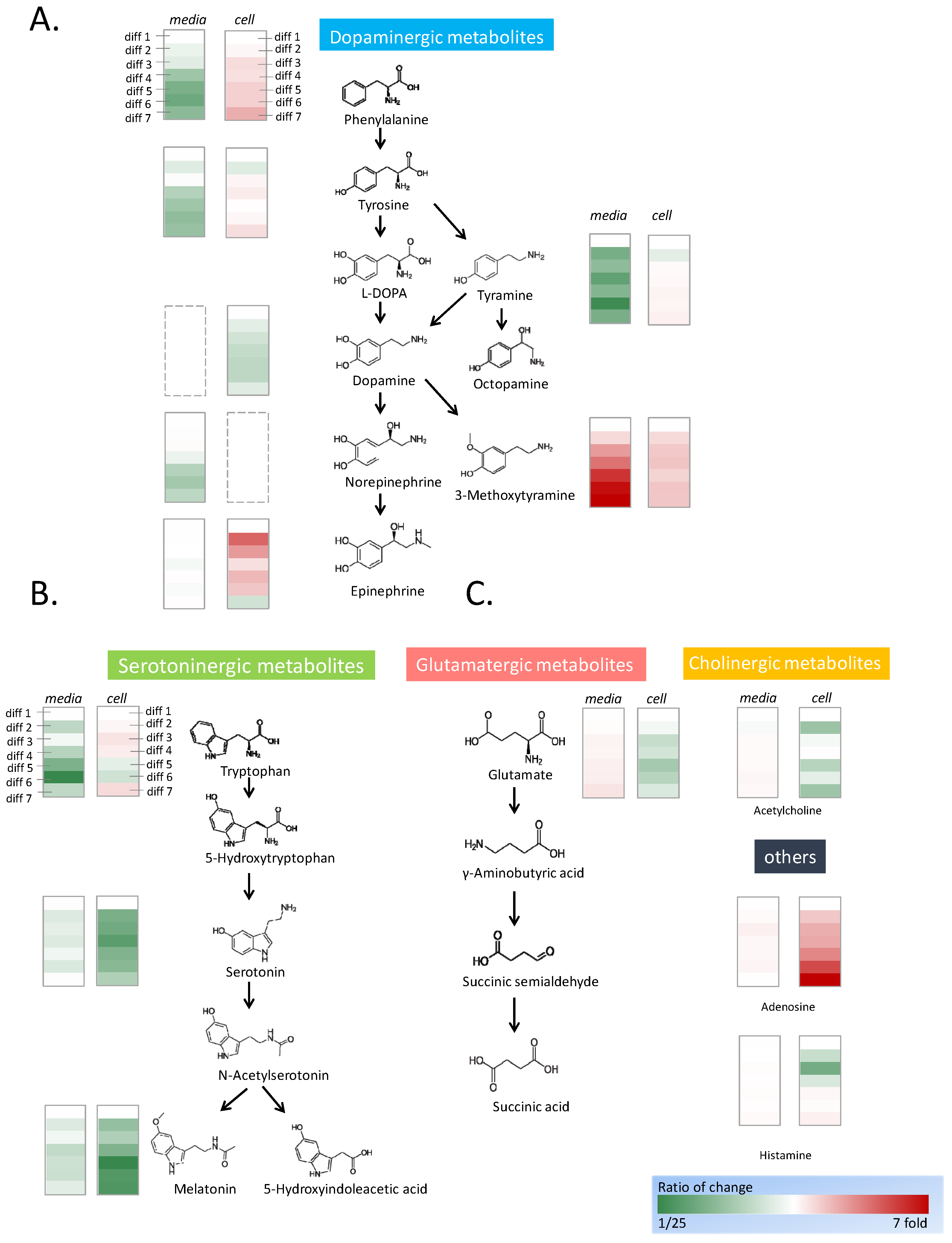
2.2. Neurotransmitter Analysis
2.3. Activation of Dopaminergic Metabolism through PC12 Differentiation
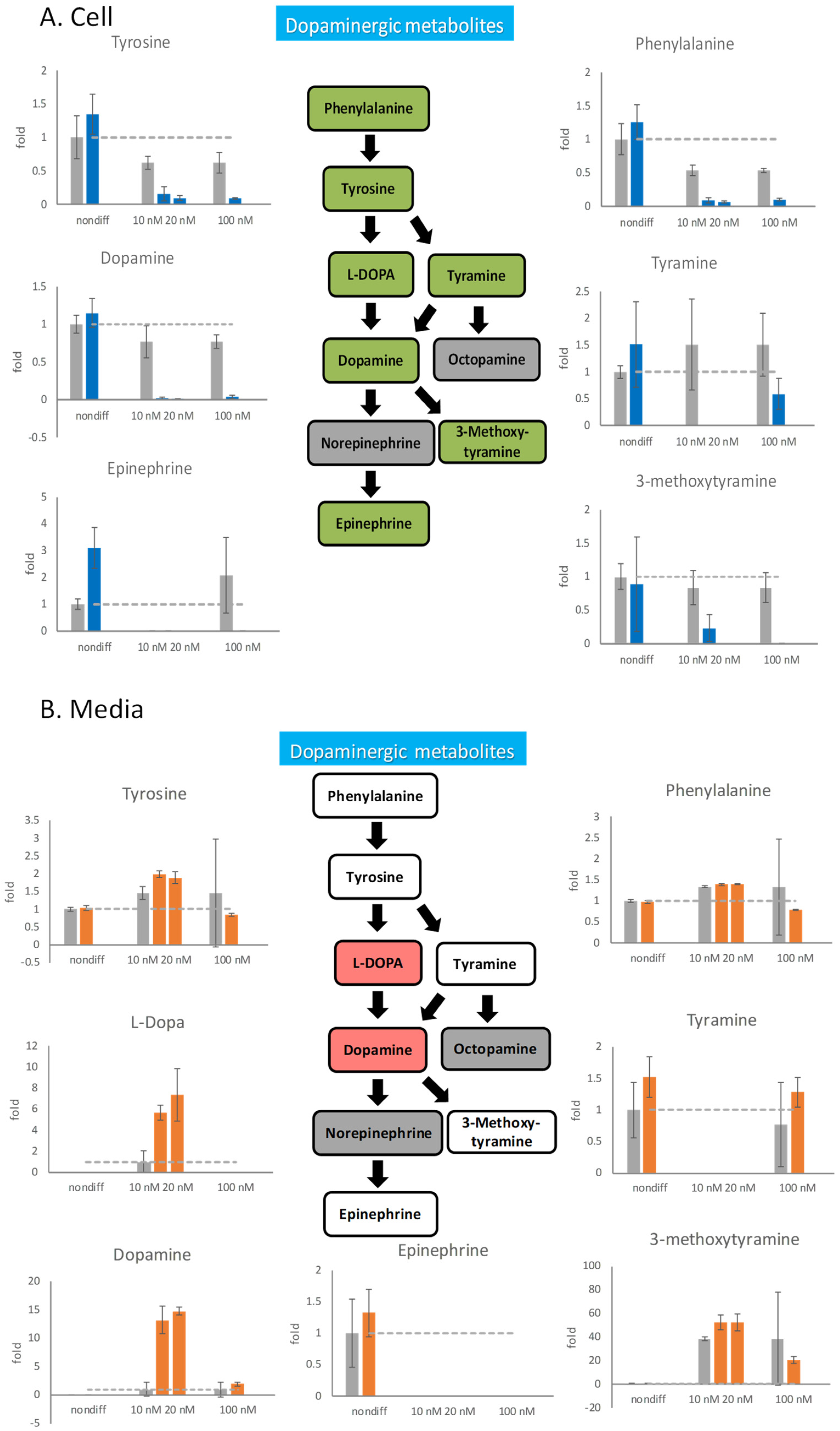
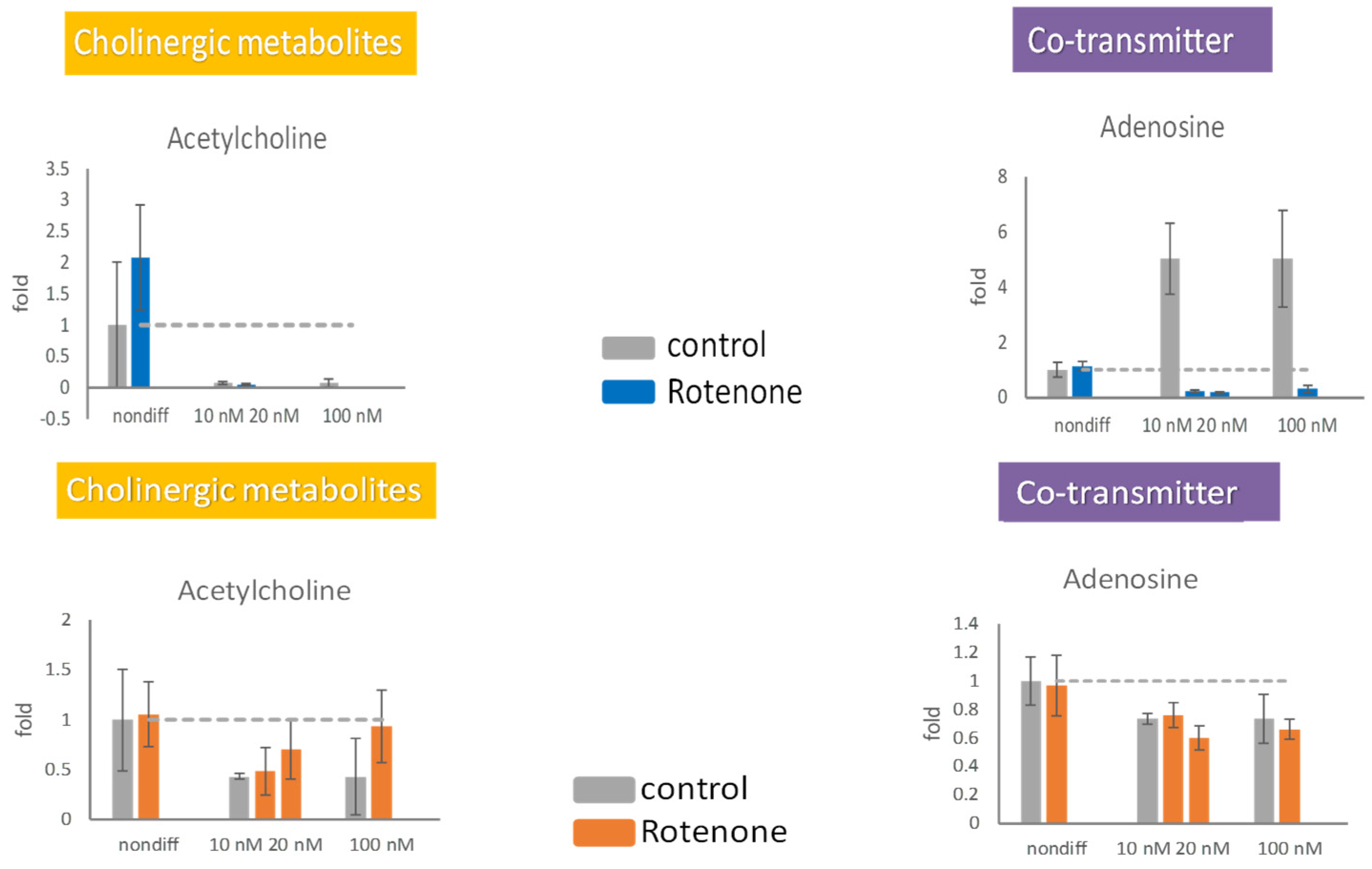
2.4. Effects of Rotenone Pretreatment on PC12 Differentiation
2.5. Effects of Rotenone Pretreatment on Neurotransmitter Release
2.6. Effects of Rotenone on Neurotransmitters during Neurodegeneration
3. Discussion
3.1. Activation of Dopaminergic Metabolites during Neuron Differentiation
3.2. Rotenone Treatment Shifts Dopamine Metabolism and Activates Serotoninergic Pathway
3.3. Detrimental Effect of Rotenone on Differentiated PC12 Cells
4. Materials and Methods
4.1. Materials
4.2. PC12 Cell Culture and Differentiation
4.3. Inhibition of Cell Differentiation by Rotenone
4.4. Effects of Rotenone on Neurodegeneration
4.5. Extraction of Neurotransmitters in Cells and Medium
4.6. Liquid Chromatography–Mass Spectrometry Analysis
4.7. Quantification and Semiquantification of Neurotransmitters
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agid, Y.; Marquez, J.; Vyas, S.; Michel, P.; Mouatt-Prigent, A.; Javoy-Agid, F.; Anglade, P.; Ruberg, M.; Herrero, M.; Hirsch, E. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 1997, 12, 25–31. [Google Scholar]
- Fahn, S.; Cohen, G. The oxidant stress hypothesis in Parkinson’s disease: Evidence supporting it. Ann. Neurol. 1992, 32, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef] [PubMed]
- Munhall, A.C.; Wu, Y.-N.; Belknap, J.K.; Meshul, C.K.; Johnson, S.W. NMDA alters rotenone toxicity in rat substantia nigra zona compacta and ventral tegmental area dopamine neurons. Neurotoxicology 2012, 33, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Long, X.; Xiong, J.; Jia, M.; Chen, C.; Huang, J.; Ghoorah, D.; Kong, X.; Lin, Z.; Wang, T. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit. Rev. Toxicol. 2012, 42, 613–632. [Google Scholar] [CrossRef]
- Marsden, C.; Parkes, J. Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet 1977, 309, 345–349. [Google Scholar] [CrossRef]
- Friedman, J.H.; Lannon, M.C. An open trial of controlled release carbidopa/L-dopa (sinemet CR) for the treatment of mild-to-moderate Parkinson’s disease. Clin. Neuropharmacol. 1989, 12, 220–223. [Google Scholar] [CrossRef]
- Muller, T. Pharmacokinetics and pharmacodynamics of levodopa/carbidopa cotherapies for Parkinson’s disease. Expert Opin. Drug Metab. Toxicol. 2020, 16, 403–414. [Google Scholar] [CrossRef]
- da Silva, T.M.; Munhoz, R.P.; Alvarez, C.; Naliwaiko, K.; Kiss, Á.; Andreatini, R.; Ferraz, A.C. Depression in Parkinson’s disease: A double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J. Affect. Disord. 2008, 111, 351–359. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H.; National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Francis, P.T.; Perry, E.K. Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Mov. Disord. 2007, 22, S351–S357. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.A.; Baker, K.A.; Hagg, T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004, 20, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Goto, T.; Waeber, C.; Bhide, P.G. Dopamine modulates cell cycle in the lateral ganglionic eminence. J. Neurosci. 2003, 23, 2840–2850. [Google Scholar] [CrossRef]
- Brezun, J.; Daszuta, A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 1999, 89, 999–1002. [Google Scholar] [CrossRef]
- Encinas, J.M.; Vaahtokari, A.; Enikolopov, G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 2006, 103, 8233–8238. [Google Scholar] [CrossRef]
- Banasr, M.; Hery, M.; Printemps, R.; Daszuta, A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 2004, 29, 450. [Google Scholar] [CrossRef]
- Kulkarni, V.A.; Jha, S.; Vaidya, V.A. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur. J. Neurosci. 2002, 16, 2008–2012. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Eckman, C.B. Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology 2010, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-H.; Shin, M.-C.; Jung, S.-B.; Lee, T.-H.; Bahn, G.-H.; Kwon, Y.K.; Kim, E.-H.; Kim, C.-J. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport 2002, 13, 1509–1513. [Google Scholar] [CrossRef]
- LoTurco, J.J.; Owens, D.F.; Heath, M.J.; Davis, M.B.; Kriegstein, A.R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 1995, 15, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.N.; Eleuteri, B.; Abdelhady, S.; Nussenzweig, A.; Andäng, M.; Ernfors, P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5837–5842. [Google Scholar] [CrossRef] [PubMed]
- Andäng, M.; Hjerling-Leffler, J.; Moliner, A.; Lundgren, T.K.; Castelo-Branco, G.; Nanou, E.; Pozas, E.; Bryja, V.; Halliez, S.; Nishimaru, H.; et al. Histone H2AX-dependent GABA A receptor regulation of stem cell proliferation. Nature 2008, 451, 460. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Recent Advances in the Detection of Neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef]
- Sugiura, Y.; Zaima, N.; Setou, M.; Ito, S.; Yao, I. Visualization of acetylcholine distribution in central nervous system tissue sections by tandem imaging mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1851–1861. [Google Scholar] [CrossRef]
- Johnsen, E.; Leknes, S.; Wilson, S.R.; Lundanes, E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci. Rep. 2015, 5, 9308. [Google Scholar] [CrossRef]
- Marcos, J.; Renau, N.; Valverde, O.; Aznar-Lain, G.; Gracia-Rubio, I.; Gonzalez-Sepulveda, M.; Perez-Jurado, L.A.; Ventura, R.; Segura, J.; Pozo, O.J. Targeting tryptophan and tyrosine metabolism by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1434, 91–101. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Oprea, D.; Sanz, C.G.; Barsan, M.M.; Enache, T.A. PC-12 Cell Line as a Neuronal Cell Model for Biosensing Applications. Biosensors 2022, 12, 500. [Google Scholar] [CrossRef]
- Westerink, R.H.S.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef]
- Timmers, E.R.; van Faassen, M.; Smit, M.; Kuiper, A.; Hof, I.H.; Kema, I.P.; Tijssen, M.A.J.; Niezen-Koning, K.E.; de Koning, T.J. Dopaminergic and serotonergic alterations in plasma in three groups of dystonia patients. Park. Relat. Disord. 2021, 91, 48–54. [Google Scholar] [CrossRef]
- Beaujean, D.; Rosenbaum, C.; Muller, H.W.; Willemsen, J.J.; Lenders, J.; Bornstein, S.R. Combinatorial code of growth factors and neuropeptides define neuroendocrine differentiation in PC12 cells. Exp. Neurol. 2003, 184, 348–358. [Google Scholar] [CrossRef]
- Olah, M.E.; Stiles, G.L. The role of receptor structure in determining adenosine receptor activity. Pharmacol. Therapeut. 2000, 85, 55–75. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Burke, W.J.; Schmitt, C.A.; Gillespie, K.N.; Li, S.W. Norepinephrine transmitter metabolite is a selective cell death messenger in differentiated rat pheochromocytoma cells. Brain Res. 1996, 722, 232–235. [Google Scholar] [CrossRef]
- Park, J.W.; Youn, Y.C.; Kwon, O.S.; Jang, Y.Y.; Han, E.S.; Lee, C.S. Protective effect of serotonin on 6-hydroxydopamine- and dopamine-induced oxidative damage of brain mitochondria and synaptosomes and PC12 cells. Neurochem. Int. 2002, 40, 223–233. [Google Scholar] [CrossRef]
- de Groot, M.; Westerink, R.H.S. Chemically-induced oxidative stress increases the vulnerability of PC12 cells to rotenone-induced toxicity. Neurotoxicology 2014, 43, 102–109. [Google Scholar] [CrossRef]
- Alam, M.; Schmidt, W.J. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 2002, 136, 317–324. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.S.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A.B.F. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef]
- Dukes, A.A.; Korwek, K.M.; Hastings, T.G. The effect of endogenous dopamine in rotenone-induced toxicity in PC12 cells. Antioxid. Redox Sign. 2005, 7, 630–638. [Google Scholar] [CrossRef]



| No. | Observed Mass m/z | Identified Mass m/z | Neurotransmitter | Retention Time (min) | Section (min) | Collision (eV) |
|---|---|---|---|---|---|---|
| 1 | 104.07 | 87 | γ-Aminobutyric acid | 4.0–4.8 | I (0–14) | 30 |
| 2 | 112.08 | 95 | Histamine | 3.7–4.8 | I (0–14) | 30 |
| 3 | 146.11 | 87 | Acetylcholine | 5.9–9.0 | I (0–14) | 20 |
| 4 | 148.06 | 84 | Glutamate | 4.7–5.9 | I (0–14) | 25 |
| 5 | 153.09 | 88 | Glutamate-D5 | 4.7–5.9 | I (0–14) | 25 |
| 6 | 154.08 | 91 | Dopamine | 9.4–12.0 | I (0–14) | 25 |
| 7 | 158.11 | 95 | Dopamine-D4 | 9.4–12.0 | I (0–14) | 25 |
| 8 | 170.08 | 152 | Norepinephrine | 4.8–5.6 | I (0–14) | 25 |
| 9 | 176.11 | 158 | Norepinephrine-D6 | 4.8–5.6 | I (0–14) | 25 |
| 10 | 184.09 | 166 | Epinephrine | 5.9–8.0 | I (0–14) | 25 |
| 11 | 138.05 | 121 | Tyramine | 19.7–20.1 | II (15–25) | 30 |
| 12 | 166.08 | 120 | Phenylalanine | 19.4–20.3 | II (15–25) | 25 |
| 13 | 168.10 | 91 | 3-Methoxytyramine | 17.7–18.7 | II (15–25) | 30 |
| 14 | 177.09 | 160 | Serotonin | 18.1–19.0 | II (15–25) | 25 |
| 15 | 182.08 | 91 | Tyrosine | 16.1–17.0 | II (15–25) | 30 |
| 16 | 192.05 | 146 | 5-Hydroxyindoleacetic acid | 21.3–21.9 | II (15–25) | 30 |
| 17 | 198.13 | 152 | L-dopa | 12.6–15.0 | II (15–25) | 30 |
| 18 | 205.08 | 188 | Tryptophan | 20.6–21.0 | II (15–25) | 30 |
| 19 | 233.12 | 174 | Melatonin | 22.1–23.5 | II (15–25) | 30 |
| 20 | 268.09 | 136 | Adenosine | 16.5–17.3 | II (15–25) | 20 |
| 21 | 269.10 | 137 | Adenosine-D1 | 16.5–17.3 | II (15–25) | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jao, Y.-N.; Chao, Y.-J.; Chan, J.-F.; Hsu, Y.-H.H. Mass Spectrometry Analysis of Neurotransmitter Shifting during Neurogenesis and Neurodegeneration of PC12 Cells. Int. J. Mol. Sci. 2024, 25, 10399. https://doi.org/10.3390/ijms251910399
Jao Y-N, Chao Y-J, Chan J-F, Hsu Y-HH. Mass Spectrometry Analysis of Neurotransmitter Shifting during Neurogenesis and Neurodegeneration of PC12 Cells. International Journal of Molecular Sciences. 2024; 25(19):10399. https://doi.org/10.3390/ijms251910399
Chicago/Turabian StyleJao, Yu-Ning, Yu-Jen Chao, Jui-Fen Chan, and Yuan-Hao Howard Hsu. 2024. "Mass Spectrometry Analysis of Neurotransmitter Shifting during Neurogenesis and Neurodegeneration of PC12 Cells" International Journal of Molecular Sciences 25, no. 19: 10399. https://doi.org/10.3390/ijms251910399
APA StyleJao, Y.-N., Chao, Y.-J., Chan, J.-F., & Hsu, Y.-H. H. (2024). Mass Spectrometry Analysis of Neurotransmitter Shifting during Neurogenesis and Neurodegeneration of PC12 Cells. International Journal of Molecular Sciences, 25(19), 10399. https://doi.org/10.3390/ijms251910399





