Adaptive Differences in Cellular and Behavioral Responses to Circadian Disruption between C57BL/6 and BALB/c Strains
Abstract
:1. Introduction
2. Results
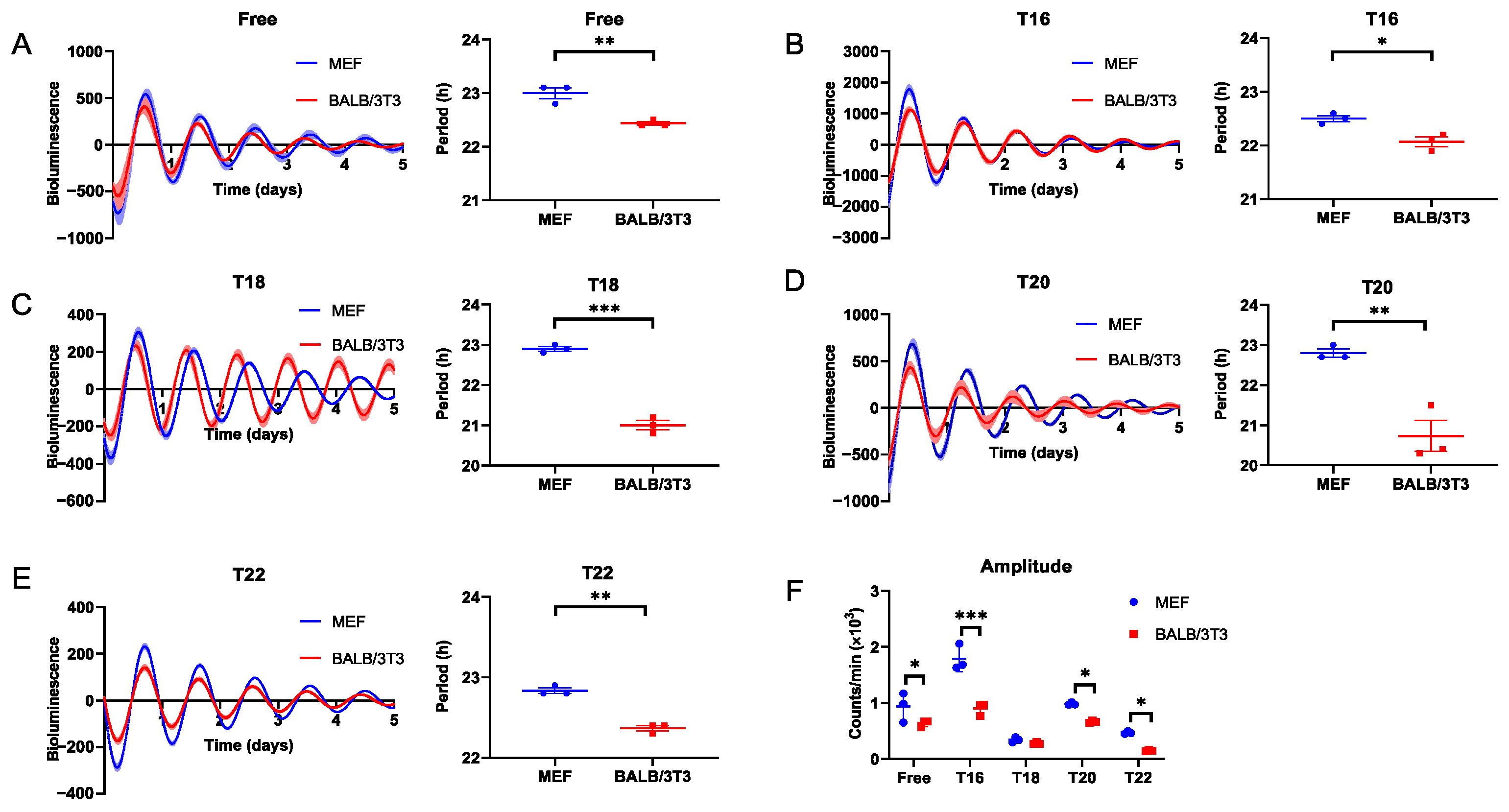
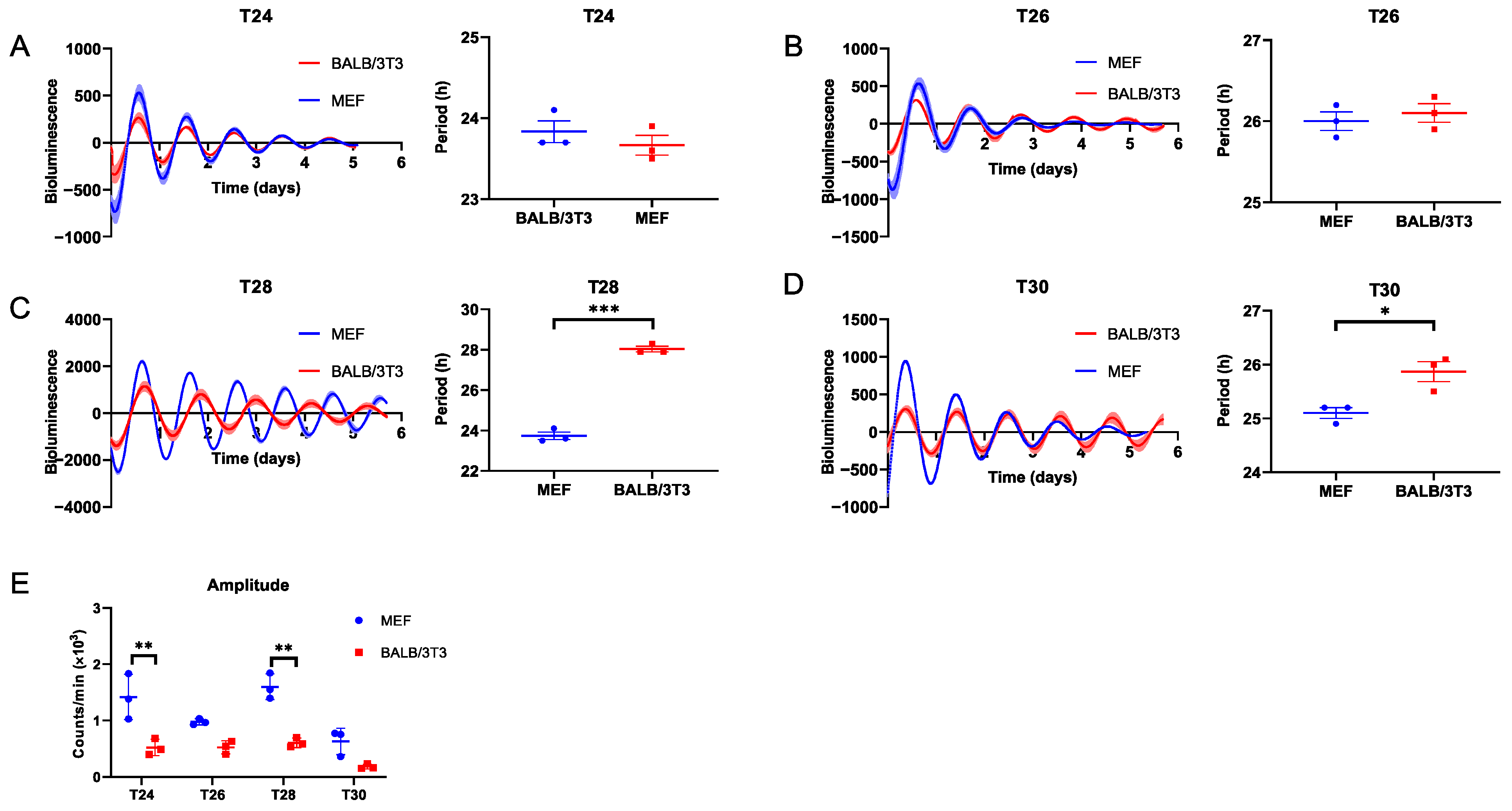
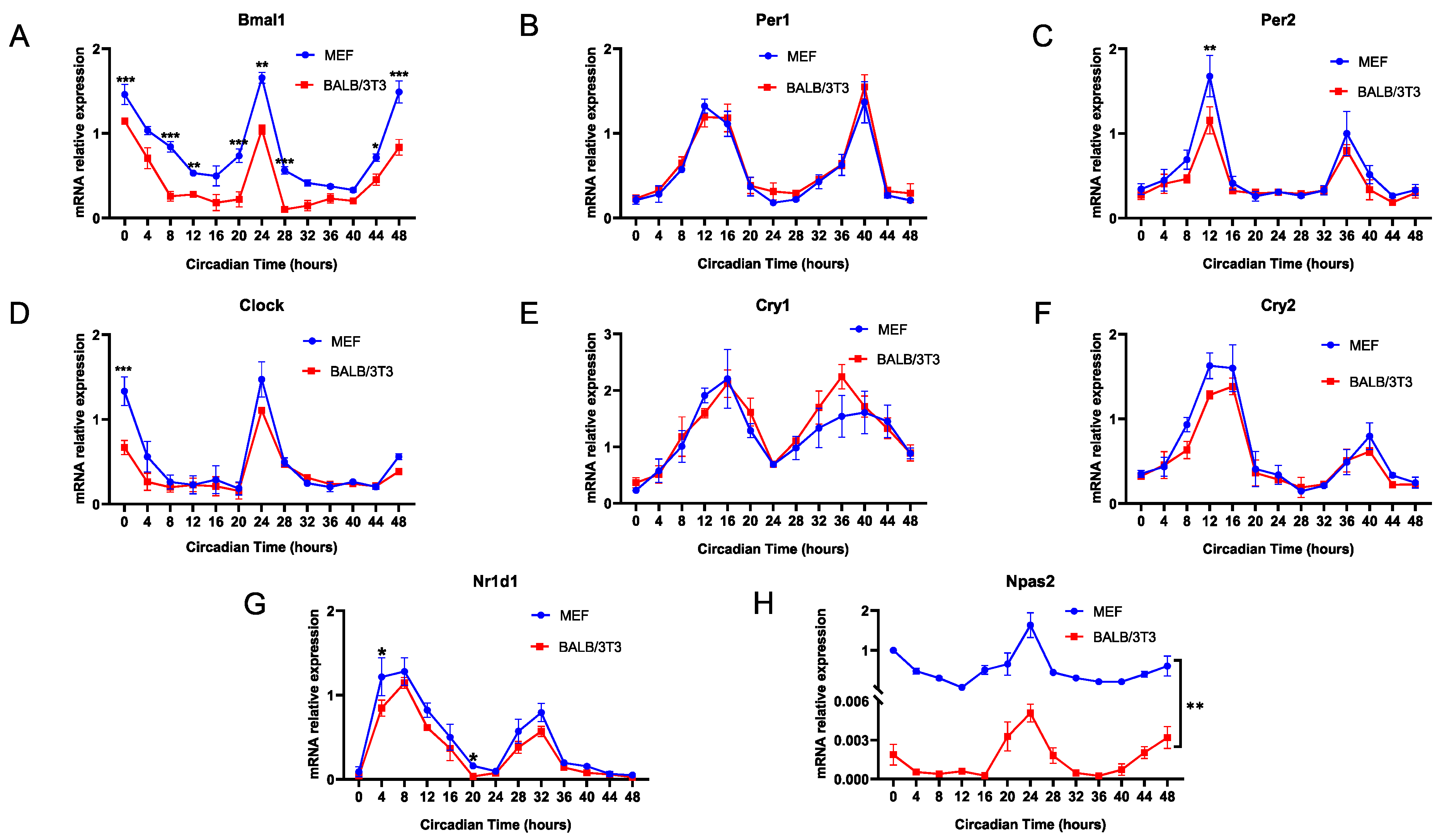
2.1. Entrainment Range of MEF and BALB/3T3 to DEX Stimulation T Cycles
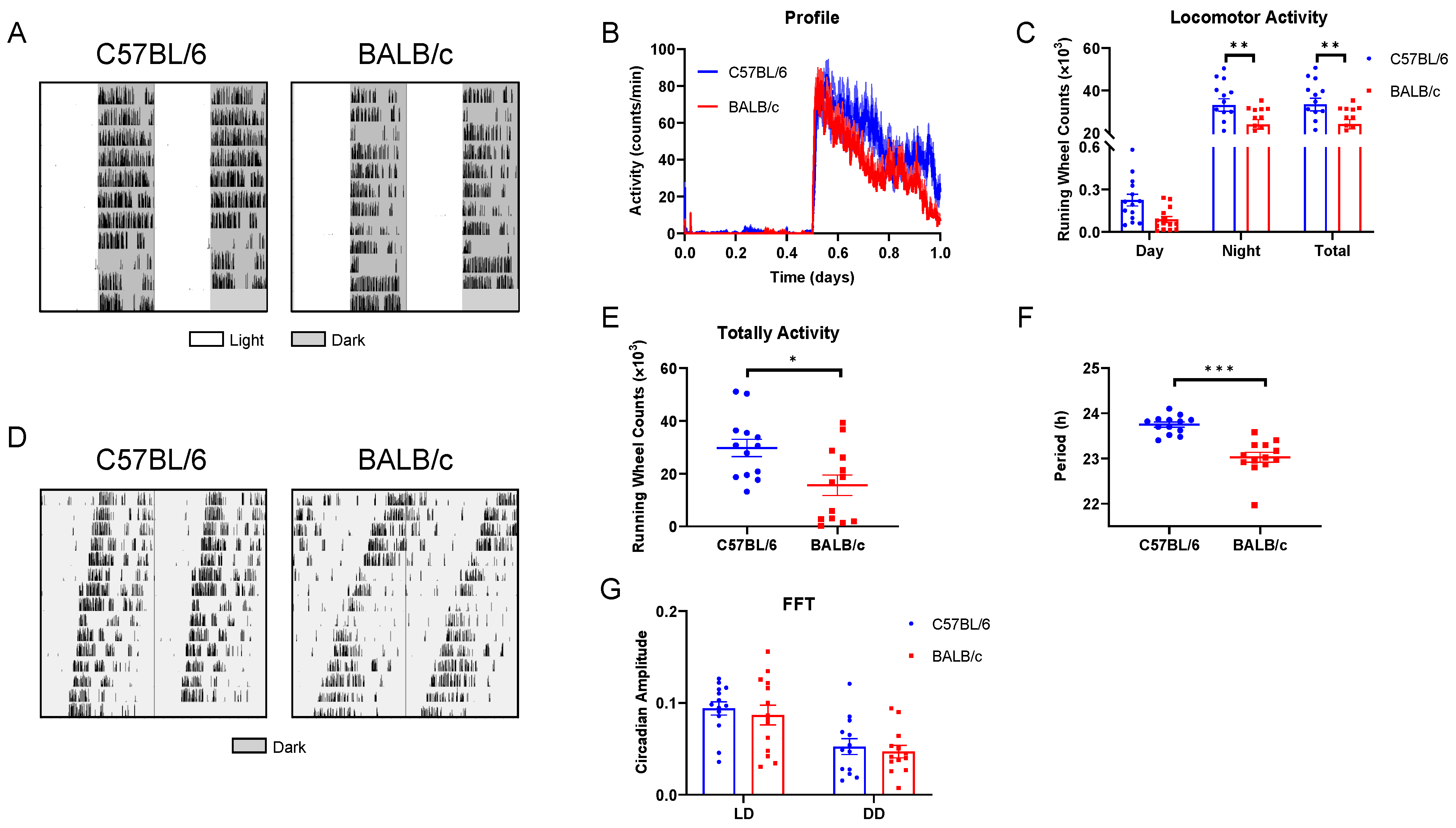
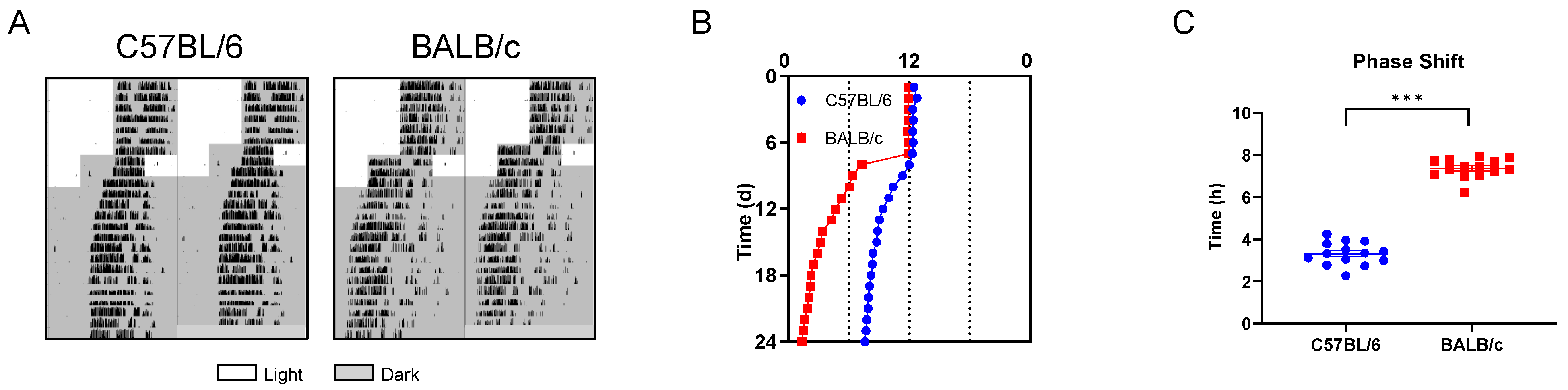
2.2. Behavioral Rhythms of C57BL/6 and BALB/c Mice in LD and DD Condition
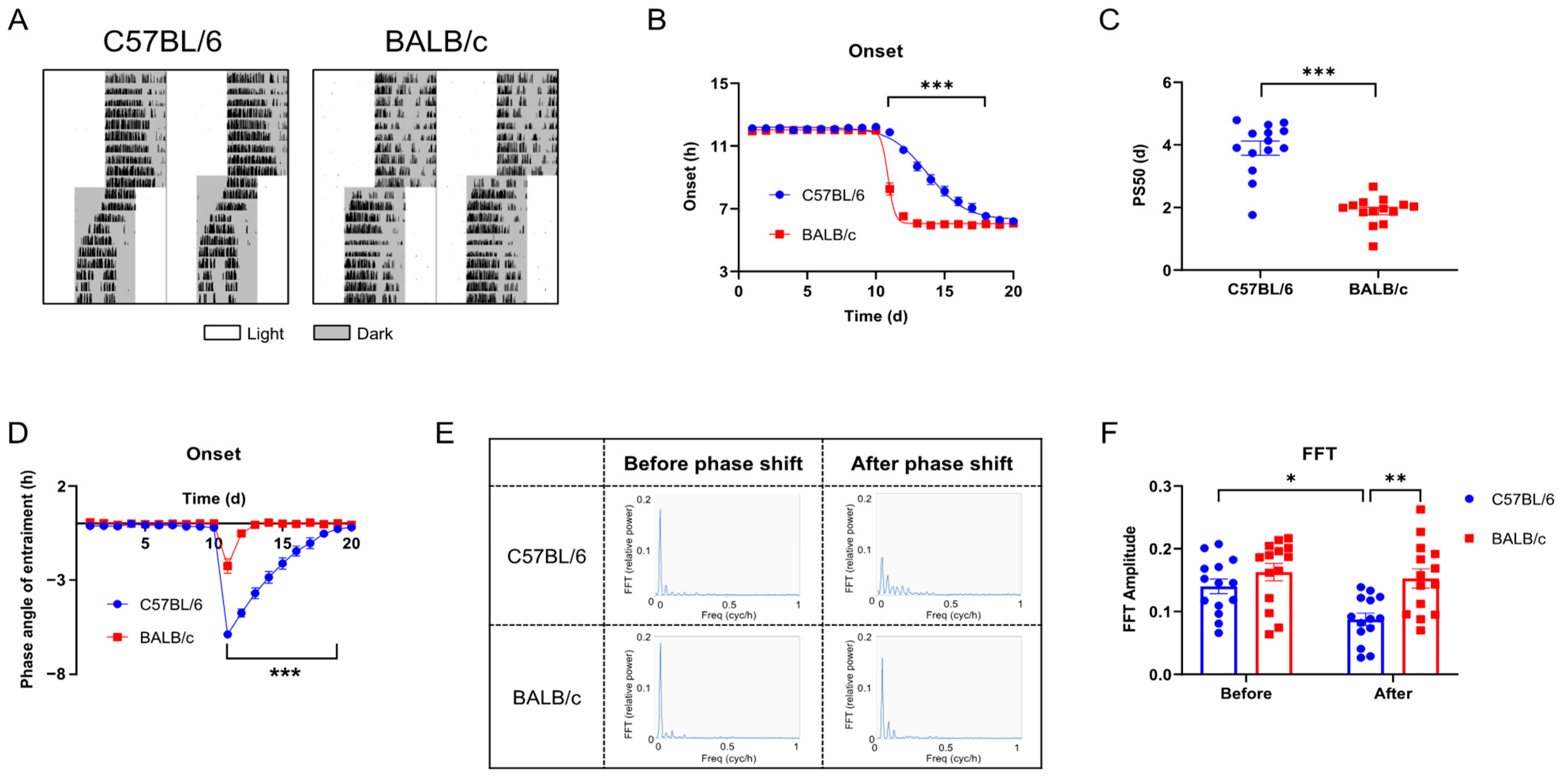
2.3. Behavioral Rhythms of C57BL/6 and BALB/c Mice in Phase Shift
2.4. Expression of Npas2 in C57BL/6 and BALB/c Mice Was Compared
2.5. DEGs in Various Tissues of C57BL/6 and BALB/c Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture and Stable Transfection with the Bmal1-Luc Reporter
4.3. Dexamethasone Periodic Treatment and Bioluminescence Analysis
4.4. Wheel Running Activity Recording and Analysis
4.5. Data Visualization and Identification of DEGs
4.6. RNA Extraction and qRT-PCR
4.7. Western Blot
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.; Paschos, G.; Curtis, A.M.; Musiek, E.S.; McLoughlin, S.C.; FitzGerald, G.A. Knitting up the raveled sleave of care. Sci. Transl. Med. 2013, 5, 212rv3. [Google Scholar] [CrossRef]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. In Circadian Clocks; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 217, pp. 3–27. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3–22. [Google Scholar] [CrossRef]
- Dickmeis, T.; Weger, B.D.; Weger, M. The circadian clock and glucocorticoids--interactions across many time scales. Mol. Cell. Endocrinol. 2013, 380, 2–15. [Google Scholar] [CrossRef]
- Pezuk, P.; Mohawk, J.A.; Wang, L.A.; Menaker, M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology 2012, 153, 4775–4783. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef]
- Lee, R.; Tapia, A.; Kaladchibachi, S.; Grandner, M.A.; Fernandez, F.X. Meta-analysis of light and circadian timekeeping in rodents. Neurosci. Biobehav. Rev. 2021, 123, 215–229. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Zimmerman, P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 1990, 10, 3685–3694. [Google Scholar] [CrossRef]
- Ma, C.; Ren, B.; Chen, L.; Yang, G. Strain and Age Dependent Entrainable Range of Circadian Behavior in C57BL/6 and BALB/c Mice. Physiol. Behav. 2022, 255, 113917. [Google Scholar] [CrossRef]
- Vajtay, T.J.; St Thomas, J.J.; Takacs, T.E.; McGann, E.G.; Weber, E.T. Duration and timing of daily light exposure influence the rapid shifting of BALB/cJ mouse circadian locomotor rhythms. Physiol. Behav. 2017, 179, 200–207. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.D.; Barnard, M.; Tian, H.; Li, X.; Ring, H.Z.; Francke, U.; Shelton, J.; Richardson, J.; Russell, D.W.; McKnight, S.L. Molecular characterization of two mammalian bHLH-PAS domain proteins selectively expressed in the central nervous system. Proc. Natl. Acad. Sci. USA 1997, 94, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, D.; Wang, L.L.; Diemer, T.; Welsh, D.K. NPAS2 Compensates for Loss of CLOCK in Peripheral Circadian Oscillators. PLoS Genet. 2016, 12, e1005882. [Google Scholar] [CrossRef]
- Peng, L.U.; Bai, G.; Pang, Y. Roles of NPAS2 in circadian rhythm and disease. Acta Biochim. Biophys. Sin. 2021, 53, 1257–1265. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Suzuki, T.; Mizoro, Y.; Kori, H.; Okada, K.; Chen, Y.; Fustin, J.M.; Yamazaki, F.; Mizuguchi, N.; Zhang, J.; et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 2013, 342, 85–90. [Google Scholar] [CrossRef]
- Fifel, K.; Yanagisawa, M.; Deboer, T. Mechanisms of Sleep/Wake Regulation under Hypodopaminergic State: Insights from MitoPark Mouse Model of Parkinson’s Disease. Adv. Sci. 2023, 10, e2203170. [Google Scholar] [CrossRef]
- Dudley, C.A.; Erbel-Sieler, C.; Estill, S.J.; Reick, M.; Franken, P.; Pitts, S.; McKnight, S.L. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 2003, 301, 379–383. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Zhang, J.; Ren, B.; FitzGerald, G.A. Bmal1 deletion in mice facilitates adaptation to disrupted light/dark conditions. JCI Insight 2019, 5, e125133. [Google Scholar] [CrossRef] [PubMed]
- Pilz, L.K.; Quiles, C.L.; Dallegrave, E.; Levandovski, R.; Hidalgo, M.P.; Elisabetsky, E. Differential susceptibility of BALB/c, C57BL/6N, and CF1 mice to photoperiod changes. Braz. J. Psychiatry 2015, 37, 185–190. [Google Scholar] [CrossRef]
- DeBruyne, J.P.; Weaver, D.R.; Reppert, S.M. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat. Neurosci. 2007, 10, 543–545. [Google Scholar] [CrossRef]
- Ahmed, R.; Ashimori, A.; Iwamoto, S.; Matsui, T.; Nakahata, Y.; Bessho, Y. Replicative senescent human cells possess altered circadian clocks with a prolonged period and delayed peak-time. Aging 2019, 11, 950–973. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.K.; Yoo, S.H.; Liu, A.C.; Takahashi, J.S.; Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004, 14, 2289–2295. [Google Scholar] [CrossRef]
- Yang, D.; Oike, H.; Furuse, M.; Yasuo, S. Effect of regular and irregular stimulation cycles of dexamethasone on circadian clock in NIH3T3 cells. Chronobiol. Int. 2022, 39, 97–105. [Google Scholar] [CrossRef]
- Lee, Y.; Lahens, N.F.; Zhang, S.; Bedont, J.; Field, J.M.; Sehgal, A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 2019, 17, e3000228. [Google Scholar] [CrossRef] [PubMed]
- Legates, T.A.; Dunn, D.; Weber, E.T. Accelerated re-entrainment to advanced light cycles in BALB/cJ mice. Physiol. Behav. 2009, 98, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M. Circadian activity rhythms in BALB/c mice: A weakly-coupled circadian system? Biol. Rhythm. Res. 1990, 21, 91–96. [Google Scholar] [CrossRef]
- Hamnett, R.; Crosby, P.; Chesham, J.E.; Hastings, M.H. Vasoactive intestinal peptide controls the suprachiasmatic circadian clock network via ERK1/2 and DUSP4 signalling. Nat. Commun. 2019, 10, 542. [Google Scholar] [CrossRef]
- An, S.; Harang, R.; Meeker, K.; Granados-Fuentes, D.; Tsai, C.A.; Mazuski, C.; Kim, J.; Doyle, F.J., 3rd; Petzold, L.R.; Herzog, E.D. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc. Natl. Acad. Sci. USA 2013, 110, E4355–E4361. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; King, D.P.; Chang, A.M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Lippa, C.F.; Koh, E.T.; Schwartz, W.J. Architecture of the suprachiasmatic nuclei in BALB/c and C57BL/6 inbred mouse strains. Brain Res. Bull. 1992, 28, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Low-Zeddies, S.S.; King, D.P.; Steeves, T.D.; Whiteley, A.; Kushla, J.; Zemenides, P.D.; Lin, A.; Vitaterna, M.H.; Churchill, G.A.; et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001, 11, 959–980. [Google Scholar] [CrossRef] [PubMed]
- Flint, J. Analysis of quantitative trait loci that influence animal behavior. J. Neurobiol. 2003, 54, 46–77. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Botstein, D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 1989, 121, 185–199. [Google Scholar] [CrossRef]
- Kim, S.M.; Vadnie, C.A.; Philip, V.M.; Gagnon, L.H.; Chowdari, K.V.; Chesler, E.J.; McClung, C.A.; Logan, R.W. High-throughput measurement of fibroblast rhythms reveals genetic heritability of circadian phenotypes in diversity outbred mice and their founder strains. Sci. Rep. 2021, 11, 2573. [Google Scholar] [CrossRef]
- Ono, D.; Wang, H.; Hung, C.J.; Wang, H.T.; Kon, N.; Yamanaka, A.; Li, Y.; Sugiyama, T. Network-driven intracellular cAMP coordinates circadian rhythm in the suprachiasmatic nucleus. Sci. Adv. 2023, 9, eabq7032. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, P.G.; Bushell, W.N. The chi square periodogram: Its utility for analysis of circadian rhythms. J. Theor. Biol. 1978, 72, 131–160. [Google Scholar] [CrossRef]
- Pfeffer, M.; Korf, H.W.; von Gall, C. Chronotype and stability of spontaneous locomotor activity rhythm in BMAL1-deficient mice. Chronobiol. Int. 2015, 32, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, S.; Eichele, G.; Oster, H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J. Clin. Investig. 2010, 120, 2600–2609. [Google Scholar] [CrossRef]
- Kumar, D.; Soni, S.K.; Kronfeld-Schor, N.; Singaravel, M. Wheel-running activity rhythms and masking responses in the diurnal palm squirrel, Funambulus pennantii. Chronobiol. Int. 2020, 37, 1693–1708. [Google Scholar] [CrossRef]
- Li, J.; Miao, B.; Wang, S.; Dong, W.; Xu, H.; Si, C.; Wang, W.; Duan, S.; Lou, J.; Bao, Z.; et al. Hiplot: A comprehensive and easy-to-use web service for boosting publication-ready biomedical data visualization. Brief. Bioinform. 2022, 23, bbac261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Li, S.; Wang, H.; Ren, B.; Li, J.; Bao, Z.; Liu, J.; Guo, M.; Yang, G.; et al. Circadian control of ConA-induced acute liver injury and inflammatory response via Bmal1 regulation of Junb. JHEP Rep. 2023, 5, 100856. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Li, H.; Li, W.; Yang, G.; Chen, L. Adaptive Differences in Cellular and Behavioral Responses to Circadian Disruption between C57BL/6 and BALB/c Strains. Int. J. Mol. Sci. 2024, 25, 10404. https://doi.org/10.3390/ijms251910404
Ma C, Li H, Li W, Yang G, Chen L. Adaptive Differences in Cellular and Behavioral Responses to Circadian Disruption between C57BL/6 and BALB/c Strains. International Journal of Molecular Sciences. 2024; 25(19):10404. https://doi.org/10.3390/ijms251910404
Chicago/Turabian StyleMa, Changxiao, Haonan Li, Wenyu Li, Guangrui Yang, and Lihong Chen. 2024. "Adaptive Differences in Cellular and Behavioral Responses to Circadian Disruption between C57BL/6 and BALB/c Strains" International Journal of Molecular Sciences 25, no. 19: 10404. https://doi.org/10.3390/ijms251910404
APA StyleMa, C., Li, H., Li, W., Yang, G., & Chen, L. (2024). Adaptive Differences in Cellular and Behavioral Responses to Circadian Disruption between C57BL/6 and BALB/c Strains. International Journal of Molecular Sciences, 25(19), 10404. https://doi.org/10.3390/ijms251910404





