Oral Chronic Graft-Versus-Host Disease: Pathogenesis, Diagnosis, Current Treatment, and Emerging Therapies
Abstract
1. Introduction
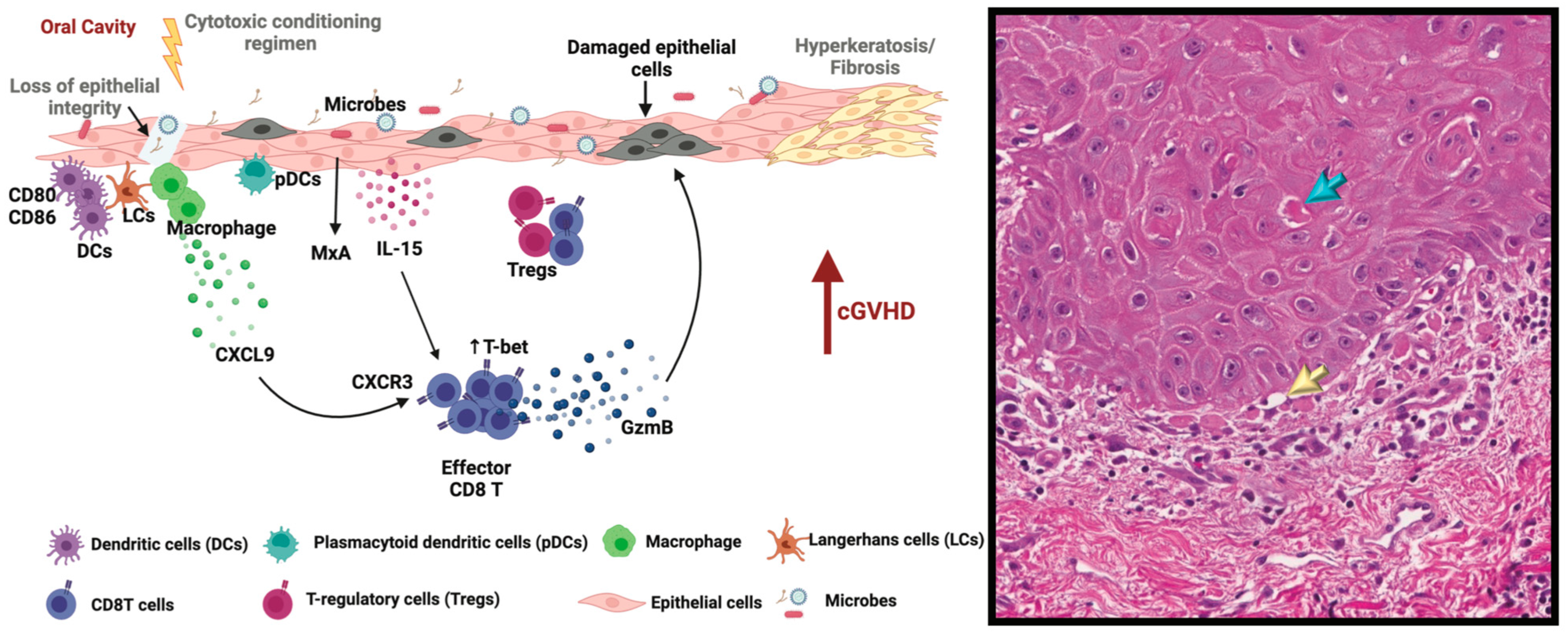
2. Pathogenesis of Oral cGVHD
2.1. Initial Events
2.2. Loss of Tolerance Leads to Dysregulated Immunity
2.3. Inflammation and Tissue Damage/Dysfunction
2.4. Fibrosis
2.5. Oral cGVHD—Evidence from the Literature
2.6. Microbiome in Oral cGVHD
3. Diagnosis and Management of Oral Graft-Versus-Host Disease
3.1. Oral Mucosal cGVHD
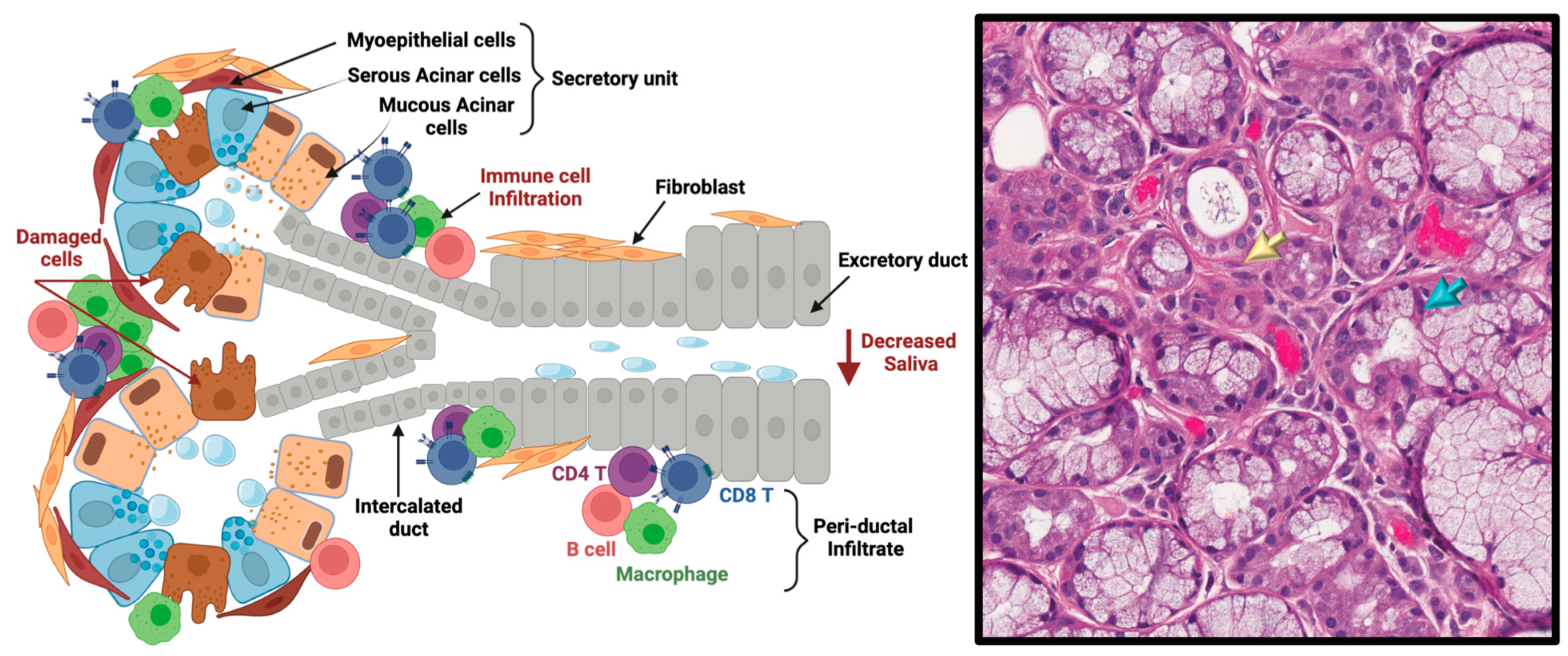
3.2. cGVHD Salivary Gland Dysfunction in Oral cGVHD
3.3. Fibrotic Oral cGVHD
3.4. Diagnosis of Oral cGVHD
3.5. Clinical Scoring of Oral cGVHD
3.6. Oral Health Quality of Life and Oral cGVHD
4. Management of Oral Chronic Graft-Versus-Host Disease
4.1. General Considerations
4.2. Mucosal Disease
4.3. Salivary Gland Hypofunction and Dental Considerations
4.4. Sclerodermatous Disease
4.5. Oral Cancer Risk
5. Emerging Therapies and Future Directions
5.1. Ibrutinib
5.2. Ruxolitinib
5.3. Belumosudil
5.4. Novel Immunosuppressive Agents
5.5. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.J.; Vogelsang, G.; Flowers, M.E. Chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2003, 9, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Klein, J.P.; Weisdorf, D.J.; Hassebroek, A.; Flowers, M.E.D.; Cutler, C.S.; Urbano-Ispizua, A.; Antin, J.H.; Bolwell, B.J.; Boyiadzis, M.; et al. Chronic GVHD risk score: A Center for International Blood and Marrow Transplant Research analysis. Blood 2011, 117, 6714–6720. [Google Scholar] [CrossRef] [PubMed]
- Bachier, C.R.; Aggarwal, S.K.; Hennegan, K.; Milgroom, A.; Francis, K.; Dehipawala, S.; Rotta, M. Epidemiology and Treatment of Chronic Graft-versus-Host Disease Post-Allogeneic Hematopoietic Cell Transplantation: A US Claims Analysis. Biol. Blood Marrow Transplant. 2020, 27, 504.e1–504.e6. [Google Scholar] [CrossRef]
- Przepiorka, D.; Anderlini, P.; Saliba, R.; Cleary, K.; Mehra, R.; Khouri, I.; Huh, Y.O.; Giralt, S.; Braunschweig, I.; van Besien, K.; et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood 2001, 98, 1695–1700. [Google Scholar] [CrossRef]
- Kitko, C.L.; Pidala, J.; Schoemans, H.M.; Lawitschka, A.; Flowers, M.E.; Cowen, E.W.; Tkaczyk, E.; Farhadfar, N.; Jain, S.; Steven, P.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Biol. Blood Marrow Transplant. 2021, 27, 545–557. [Google Scholar] [CrossRef]
- Bajonaid, A.; Guntaka, P.K.; Harper, M.; Cutler, C.; Duncan, C.; Villa, A.; Sroussi, H.Y.; Woo, S.; Treister, N.S. Characterization of orofacial features in sclerodermatous chronic graft-versus-host disease. Oral Dis. 2024. [Google Scholar] [CrossRef]
- Gandelman, J.S.; Byrne, M.T.; Mistry, A.M.; Polikowsky, H.G.; Diggins, K.E.; Chen, H.; Lee, S.J.; Arora, M.; Cutler, C.; Flowers, M.; et al. Machine learning reveals chronic graft-versus-host disease phenotypes and stratifies survival after stem cell transplant for hematologic malignancies. Haematologica 2018, 104, 189–196. [Google Scholar] [CrossRef]
- Baumrin, E.; Loren, A.W.; Falk, S.J.; Mays, J.W.; Cowen, E.W. Chronic graft-versus-host disease. Part I: Epidemiology, pathogenesis, and clinical manifestations. J. Am. Acad. Dermatol. 2022, 90, 1–16. [Google Scholar] [CrossRef]
- Gandelman, J.S.; Zic, J.; Dewan, A.K.; Lee, S.J.; Flowers, M.; Cutler, C.; Pidala, J.; Chen, H.; Jagasia, M.H.; Tkaczyk, E.R. The Anatomic Distribution of Skin Involvement in Patients with Incident Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2018, 25, 279–286. [Google Scholar] [CrossRef]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; Brink, M.R.v.D.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2016, 23, 211–234. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Xun, C.Q.; Thompson, J.S.; Jennings, C.D.; A Brown, S.; Widmer, M.B. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood 1994, 83, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Jethava, Y.S.; Sica, S.; Savani, B.; Socola, F.; Jagasia, M.; Mohty, M.; Nagler, A.; Bacigalupo, A. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transplant. 2017, 52, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Crișan, T.O.; Netea, M.G.; Joosten, L.A.B. Innate immune memory: Implications for host responses to damage-associated molecular patterns. Eur. J. Immunol. 2016, 46, 817–828. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Di Genaro, M.S.; Kalergis, A.M. Trained Immunity Contribution to Autoimmune and Inflammatory Disorders. Front. Immunol. 2022, 13, 868343. [Google Scholar] [CrossRef]
- Maeda, Y. Pathogenesis of graft-versus-host disease: Innate immunity amplifying acute alloimmune responses. Int. J. Hematol. 2013, 98, 293–299. [Google Scholar] [CrossRef][Green Version]
- Wilhelm, K.; Ganesan, J.; Müller, T.; Dürr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Jüttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef]
- Koyama, M.; Hill, G.R. Alloantigen presentation and graft-versus-host disease: Fuel for the fire. Blood 2016, 127, 2963–2970. [Google Scholar] [CrossRef]
- Alexander, K.A.; Flynn, R.; Lineburg, K.E.; Kuns, R.D.; Teal, B.E.; Olver, S.D.; Lor, M.; Raffelt, N.C.; Koyama, M.; Leveque, L.; et al. CSF-1–dependant donor-derived macrophages mediate chronic graft-versus-host disease. J. Clin. Investig. 2014, 124, 4266–4280. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Watanabe, T.; Kawakami, E.; Iwasaki, M.; Tomizawa-Murasawa, M.; Matsuda, M.; Najima, Y.; Takagi, S.; Fujiki, S.; Sato, R.; et al. Co-activation of macrophages and T cells contribute to chronic GVHD in human IL-6 transgenic humanised mouse model. EBioMedicine 2019, 41, 584–596. [Google Scholar] [CrossRef]
- Mouttie, L.L.-E.; Koyama, M.; Le Texier, L.; Markey, K.A.; Cheong, M.; Kuns, R.D.; Lineburg, K.E.; Teal, B.E.; Alexander, K.A.; Clouston, A.D.; et al. Corruption of dendritic cell antigen presentation during acute GVHD leads to regulatory T-cell failure and chronic GVHD. Blood 2016, 128, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Y.; Li, J.-X.; Das, P.K.; Zhang, H.; Passang, T.; Li, J.M.; Nagy, T.; Gandhi, K.; Ravindranathan, S.; et al. Donor plasmacytoid dendritic cells limit graft-versus-host disease through vasoactive intestinal polypeptide expression. Blood 2022, 140, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Meng, L.; Wang, Y.; Li, B.; Yu, H.; Zhou, Y.; Bui, T.; Abraham, C.; Li, A.; Zhang, Y.; et al. Graft-versus-host disease depletes plasmacytoid dendritic cell progenitors to impair tolerance induction. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, J.; Cao, B.; Le Texier, L.; Xiong, L.Y.; Hunter, C.R.; Llanes, G.; Aguliar, E.G.; Schroder, W.A.; Phipps, S.; Lynch, J.P.; et al. Bone Marrow Regulatory T Cells Are a Unique Population, Supported by Niche-Specific Cytokines and Plasmacytoid Dendritic Cells, and Required for Chronic Graft-Versus-Host Disease Control. Front. Cell Dev. Biol. 2021, 9, 737880. [Google Scholar] [CrossRef]
- Le Huu, D.; Kimura, H.; Date, M.; Hamaguchi, Y.; Hasegawa, M.; Hau, K.T.; Fujimoto, M.; Takehara, K.; Matsushita, T. Blockade of Syk ameliorates the development of murine sclerodermatous chronic graft-versus-host disease. J. Dermatol. Sci. 2014, 74, 214–221. [Google Scholar] [CrossRef]
- Dubovsky, J.A.; Flynn, R.; Du, J.; Harrington, B.K.; Zhong, Y.; Kaffenberger, B.; Yang, C.; Towns, W.H.; Lehman, A.; Johnson, A.J.; et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J. Clin. Investig. 2014, 124, 4867–4876. [Google Scholar] [CrossRef]
- Brink, M.R.M.v.D.; Moore, E.; Ferrara, J.L.M.; Burakoff, S.J. Graft-versus-host-disease-associated thymic damage results in the appearance of T cell clones with anti-host reactivity1. Transplantation 2000, 69, 446–450. [Google Scholar] [CrossRef]
- Sakoda, Y.; Hashimoto, D.; Asakura, S.; Takeuchi, K.; Harada, M.; Tanimoto, M.; Teshima, T. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood 2006, 109, 1756–1764. [Google Scholar] [CrossRef]
- Teshima, T.; Reddy, P.; Liu, C.; Williams, D.; Cooke, K.R.; Ferrara, J.L.M. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood 2003, 102, 429–435. [Google Scholar] [CrossRef]
- Wu, T.; Young, J.S.; Johnston, H.; Ni, X.; Deng, R.; Racine, J.; Wang, M.; Wang, A.; Todorov, I.; Wang, J.; et al. Thymic Damage, Impaired Negative Selection, and Development of Chronic Graft-versus-Host Disease Caused by Donor CD4+ and CD8+ T Cells. J. Immunol. 2013, 191, 488–499. [Google Scholar] [CrossRef]
- Zhang, Y.; Hexner, E.; Frank, D.; Emerson, S.G. CD4+ T Cells Generated De Novo from Donor Hemopoietic Stem Cells Mediate the Evolution from Acute to Chronic Graft-versus-Host Disease. J. Immunol. 2007, 179, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Alawam, A.S.; Cosway, E.J.; James, K.D.; Lucas, B.; Bacon, A.; Parnell, S.M.; White, A.J.; Jenkinson, W.E.; Anderson, G. Failures in thymus medulla regeneration during immune recovery cause tolerance loss and prime recipients for auto-GVHD. J. Exp. Med. 2021, 219, e20211239. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-R.; Reddy, P. Tissue tolerance: A distinct concept to control acute GVHD severity. Blood 2017, 129, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.C.; Kim, H.T.; Chammas, M.J.; Reynolds, C.G.; Matos, T.R.; Forcade, E.; Whangbo, J.; Nikiforow, S.; Cutler, C.S.; Koreth, J.; et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood 2016, 127, 646–657. [Google Scholar] [CrossRef]
- Chen, X.; Vodanovic-Jankovic, S.; Johnson, B.; Keller, M.; Komorowski, R.; Drobyski, W.R. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood 2007, 110, 3804–3813. [Google Scholar] [CrossRef]
- Miura, Y.; Thoburn, C.J.; Bright, E.C.; Phelps, M.L.; Shin, T.; Matsui, E.C.; Matsui, W.H.; Arai, S.; Fuchs, E.J.; Vogelsang, G.B.; et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 2004, 104, 2187–2193. [Google Scholar] [CrossRef]
- Rieger, K.; Loddenkemper, C.; Maul, J.; Fietz, T.; Wolff, D.; Terpe, H.; Steiner, B.; Berg, E.; Miehlke, S.; Bornhäuser, M.; et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 2006, 107, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Clark, F.J.; Gregg, R.; Piper, K.; Dunnion, D.; Freeman, L.; Griffiths, M.; Begum, G.; Mahendra, P.; Craddock, C.; Moss, P.; et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood 2004, 103, 2410–2416. [Google Scholar] [CrossRef]
- Imanguli, M.M.; Cowen, E.W.; Rose, J.; Dhamala, S.; Swaim, W.; Lafond, S.; Yagi, B.; E Gress, R.; Pavletic, S.Z.; Hakim, F.T. Comparative analysis of FoxP3+ regulatory T cells in the target tissues and blood in chronic graft versus host disease. Leukemia 2014, 28, 2016–2027. [Google Scholar] [CrossRef]
- Kielsen, K.; Ryder, L.P.; Lennox-Hvenekilde, D.; Gad, M.; Nielsen, C.H.; Heilmann, C.; Ifversen, M.; Pedersen, A.E.; Müller, K. Reconstitution of Th17, Tc17 and Treg cells after paediatric haematopoietic stem cell transplantation: Impact of interleukin-7. Immunobiology 2018, 223, 220–226. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, P.; Chen, X.; He, C.; Huang, X.; Geng, S.; Luo, C.; Wu, S.; Ling, W.; Zhong, L.; et al. Attenuation of cGVHD by C5a/C5aR blockade is associated with increased frequency of Treg. Sci. Rep. 2017, 7, 3603. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Yoshimura, H.; Satake, A.; Tsubokura, Y.; Ito, T.; Nomura, S. GM-CSF therapy inhibits chronic graft-versus-host disease via expansion of regulatory T cells. Eur. J. Immunol. 2018, 49, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Fondi, C.; Nozzoli, C.; Benemei, S.; Baroni, G.; Saccardi, R.; Guidi, S.; Nicoletti, P.; Bartolozzi, B.; Pimpinelli, N.; Santucci, M.; et al. Increase in FOXP3+ Regulatory T Cells in GVHD Skin Biopsies Is Associated with Lower Disease Severity and Treatment Response. Biol. Blood Marrow Transplant. 2009, 15, 938–947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gail, L.M.; Schell, K.J.; Łacina, P.; Strobl, J.; Bolton, S.J.; Ulriksen, E.S.; Bogunia-Kubik, K.; Greinix, H.; Crossland, R.E.; Inngjerdingen, M.; et al. Complex interactions of cellular players in chronic Graft-versus-Host Disease. Front. Immunol. 2023, 14, 1199422. [Google Scholar] [CrossRef]
- Jiang, H.; Fu, D.; Bidgoli, A.; Paczesny, S. T Cell Subsets in Graft Versus Host Disease and Graft Versus Tumor. Front. Immunol. 2021, 12, 761448. [Google Scholar] [CrossRef]
- Dander, E.; Balduzzi, A.; Zappa, G.; Lucchini, G.; Perseghin, P.; Andrè, V.; Todisco, E.; Rahal, D.; Migliavacca, M.; Longoni, D.; et al. Interleukin-17–Producing T-Helper Cells as New Potential Player Mediating Graft-Versus-Host Disease in Patients Undergoing Allogeneic Stem-Cell Transplantation. Transplantation 2009, 88, 1261–1272. [Google Scholar] [CrossRef]
- Klimczak, A.; Suchnicki, K.; Sedzimirska, M.; Lange, A. Diverse Activity of IL-17+ Cells in Chronic Skin and Mucosa Graft-Versus-Host Disease. Arch. Immunol. Ther. Exp. 2019, 67, 311–323. [Google Scholar] [CrossRef]
- Brüggen, M.-C.; Klein, I.; Greinix, H.; Bauer, W.; Kuzmina, Z.; Rabitsch, W.; Kalhs, P.; Petzelbauer, P.; Knobler, R.; Stingl, G.; et al. Diverse T-cell responses characterize the different manifestations of cutaneous graft-versus-host disease. Blood 2014, 123, 290–299. [Google Scholar] [CrossRef]
- Nishimori, H.; Maeda, Y.; Teshima, T.; Sugiyama, H.; Kobayashi, K.; Yamasuji, Y.; Kadohisa, S.; Uryu, H.; Takeuchi, K.; Tanaka, T.; et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood 2012, 119, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.T.; Memon, S.; Jin, P.; Imanguli, M.M.; Wang, H.; Rehman, N.; Yan, X.-Y.; Rose, J.; Mays, J.W.; Dhamala, S.; et al. Upregulation of IFN-Inducible and Damage-Response Pathways in Chronic Graft-versus-Host Disease. J. Immunol. 2016, 197, 3490–3503. [Google Scholar] [CrossRef]
- Okamoto, S.; Fujiwara, H.; Nishimori, H.; Matsuoka, K.-I.; Fujii, N.; Kondo, E.; Tanaka, T.; Yoshimura, A.; Tanimoto, M.; Maeda, Y. Anti–IL-12/23 p40 Antibody Attenuates Experimental Chronic Graft-versus-Host Disease via Suppression of IFN-γ/IL-17–Producing Cells. J. Immunol. 2015, 194, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Zouali, H.; Lemasson, J.; Calugareanu, A.; Battail, C.; Michonneau, D.; le Buanec, H.; Grolleau, C.; Cassius, C.; Robin, M.; Merandet, M.M.; et al. RNA sequencing of chronic GVHD skin lesions defines shared and unique inflammatory pathways characterizing lichen planus and morphea. Blood Adv. 2022, 6, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Hashino, S.; Kobayashi, H.; Kubayashi, S.; Hirano, S.; Minagawa, T.; Tanaka, J.; Fujii, Y.; Kobayashi, M.; Kasai, M. Serum cytokine levels in bone marrow transplantation: Synergistic interaction of interleukin-6, interferon-gamma, and tumor necrosis factor-alpha in graft-versus-host disease. Bone Marrow Transplant. 1994, 13, 745–751. [Google Scholar] [PubMed]
- Hayashida, J.-N.; Nakamura, S.; Toyoshima, T.; Moriyama, M.; Sasaki, M.; Kawamura, E.; Ohyama, Y.; Kumamaru, W.; Shirasuna, K. Possible involvement of cytokines, chemokines and chemokine receptors in the initiation and progression of chronic GVHD. Bone Marrow Transplant. 2012, 48, 115–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ritchie, D.; Seconi, J.; Wood, C.; Walton, J.; Watt, V. Prospective Monitoring of Tumor Necrosis Factor α and Interferon γ to Predict the Onset of Acute and Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2005, 11, 706–712. [Google Scholar] [CrossRef]
- Körholz, D.; Kunst, D.; Hempel, L.; Söhngen, D.; Heyll, A.; Bönig, H.; Göbel, U.; Zintl, F.; Burdach, S. Decreased interleukin 10 and increased interferon-γ production in patients with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997, 19, 691–695. [Google Scholar] [CrossRef]
- Hill, G.R.; Olver, S.D.; Kuns, R.D.; Varelias, A.; Raffelt, N.C.; Don, A.L.; Markey, K.A.; Wilson, Y.A.; Smyth, M.J.; Iwakura, Y.; et al. Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood 2010, 116, 819–828. [Google Scholar] [CrossRef]
- Parker, M.H.; Stone, D.B.; Abrams, K.B.; Johnson, M.B.; Granot, N.; Storb, R. Anti-ICOS mAb Targets Pathogenic IL-17A-expressing Cells in Canine Model of Chronic GVHD. Transplantation 2021, 105, 1008–1016. [Google Scholar] [CrossRef]
- Malard, F.; Bossard, C.; Brissot, E.; Chevallier, P.; Guillaume, T.; Delaunay, J.; Mosnier, J.-F.; Moreau, P.; Grégoire, M.; Gaugler, B.; et al. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant. 2014, 49, 539–544. [Google Scholar] [CrossRef]
- Koh, S.; Koh, H.; Nakashima, Y.; Katayama, T.; Sakabe, M.; Okamura, H.; Yoshimura, T.; Nanno, S.; Nishimoto, M.; Hayashi, Y.; et al. Plasma Kinetics of Th1, Th2 and Th17 Cytokines in Polymyositis Related to Chronic Graft-versus-Host Disease. Intern. Med. 2016, 55, 2265–2270. [Google Scholar] [CrossRef][Green Version]
- Forcade, E.; Paz, K.; Flynn, R.; Griesenauer, B.; Amet, T.; Li, W.; Liu, L.; Bakoyannis, G.; Jiang, D.; Chu, H.W.; et al. An activated Th17-prone T cell subset involved in chronic graft-versus-host disease sensitive to pharmacological inhibition. J. Clin. Investig. 2017, 2, e92111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, X.-Z. IL-17A ≠ Th17 in GvHD. Cell. Mol. Immunol. 2016, 15, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Debureaux, P.; Masson, A.; Battistella, M.; Fontbrune, F.; Socié, G.; Bouaziz, J.D.; Michonneau, D. Chronic graft-versus-host disease and inhibition of interleukin-17: Proof of concept in humans. Br. J. Dermatol. 2019, 182, 1038–1041. [Google Scholar] [CrossRef]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef]
- Palaniyandi, S.; Strattan, E.; Kumari, R.; Mysinger, M.; Hakim, N.; Kesler, M.V.; Apatira, M.; Bittencourt, F.; Wang, L.; Jia, Z.; et al. Combinatorial inhibition of Tec kinases BTK and ITK is beneficial in ameliorating murine sclerodermatous chronic graft versus host disease. Bone Marrow Transplant. 2023, 58, 924–935. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, R.; Chen, G.; Wang, J.; Feng, J.; Li, Y.; Yu, Z.; Xiao, H. Rapamycin Treatment Alleviates Chronic GVHD-Induced Lupus Nephritis in Mice by Recovering IL-2 Production and Regulatory T Cells While Inhibiting Effector T Cells Activation. Biomedicines 2023, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.-B.; Lim, J.-Y.; Kim, T.-W.; Shin, S.; Lee, S.-E.; Park, G.; Min, C.-K. Preclinical evaluation of JAK1/2 inhibition by ruxolitinib in a murine model of chronic graft-versus-host disease. Exp. Hematol. 2021, 98, 36–46.e2. [Google Scholar] [CrossRef]
- Saito, A.; Ichimura, Y.; Kubota, N.; Tanaka, R.; Nakamura, Y.; Fujisawa, Y.; Watanabe, R.; Ishitsuka, Y.; Fujimoto, M.; Okiyama, N. IFN-γ–Stimulated Apoptotic Keratinocytes Promote Sclerodermatous Changes in Chronic Graft-Versus-Host Disease. J. Investig. Dermatol. 2020, 141, 1473–1481.e4. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Aspects Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Meier, J.K.-H.; Wolff, D.; Pavletic, S.; Greinix, H.; Gosau, M.; Bertz, H.; Lee, S.J.; Lawitschka, Á.; Elad, S. Oral chronic graft-versus-host disease: Report from the International Consensus Conference on clinical practice in cGVHD. Clin. Oral Investig. 2010, 15, 127–139. [Google Scholar] [CrossRef]
- Huang, E.; Peng, N.; Xiao, F.; Hu, D.; Wang, X.; Lu, L. The Roles of Immune Cells in the Pathogenesis of Fibrosis. Int. J. Mol. Sci. 2020, 21, 5203. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Paz, K.; Flynn, R.; Vulic, A.; Robinson, T.M.; Lineburg, K.E.; Alexander, K.A.; Meng, J.; Roy, S.; Panoskaltsis-Mortari, A.; et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood 2017, 129, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Sun, Y.; Huang, J.; Qi, H.; Shao, R.; Wu, Q.; Jiang, Q.; Fu, R.; Liu, Q.; et al. Nestin+ Mesenchymal Stromal Cells Fibrotic Transition Mediated by CD169+ Macrophages in Bone Marrow Chronic Graft-versus-Host Disease. J. Immunol. 2023, 211, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.; Distler, J.H.W.; Beyer, C. Deciphering the pro-fibrotic phenotype of fibroblasts in systemic sclerosis. Exp. Dermatol. 2014, 23, 99–100. [Google Scholar] [CrossRef]
- Park, M.-J.; Moon, S.-J.; Lee, E.-J.; Jung, K.-A.; Kim, E.-K.; Kim, D.-S.; Lee, J.-H.; Kwok, S.-K.; Min, J.-K.; Park, S.-H.; et al. IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front. Immunol. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Murata, M.; Fujimoto, M.; Matsushita, T.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Komura, K.; Sato, S. Clinical association of serum interleukin-17 levels in systemic sclerosis: Is systemic sclerosis a Th17 disease? J. Dermatol. Sci. 2008, 50, 240–242. [Google Scholar] [CrossRef]
- Hinz, B. Formation and Function of the Myofibroblast during Tissue Repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages during the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef]
- Wolff, D.; Radojcic, V.; Lafyatis, R.; Cinar, R.; Rosenstein, R.K.; Cowen, E.W.; Cheng, G.-S.; Sheshadri, A.; Bergeron, A.; Williams, K.M.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Biol. Blood Marrow Transplant. 2021, 27, 817–835. [Google Scholar] [CrossRef]
- Nalkurthi, C.; Schroder, W.A.; Melino, M.; Irvine, K.M.; Nyuydzefe, M.; Chen, W.; Liu, J.; Teng, M.W.; Hill, G.R.; Bertolino, P.; et al. ROCK2 inhibition attenuates profibrogenic immune cell function to reverse thioacetamide-induced liver fibrosis. JHEP Rep. 2021, 4, 100386. [Google Scholar] [CrossRef]
- Socié, G. Treating chronic GVHD-induced fibrosis? Blood 2018, 131, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.; Nagler, A. The Molecular Basis of Salivary Gland Involvement in Graft-vs.-Host Disease. J. Dent. Res. 2004, 83, 98–103. [Google Scholar] [CrossRef]
- Soares, A.B.; Faria, P.R.; Magna, L.A.; Correa, M.E.P.; De Sousa, C.A.; Almeida, O.P.; Cintra, M.L. Chronic GVHD in minor salivary glands and oral mucosa: Histopathological and immunohistochemical evaluation of 25 patients. J. Oral Pathol. Med. 2005, 34, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Shulman, H.M.; Kleiner, D.; Lee, S.J.; Morton, T.; Pavletic, S.Z.; Farmer, E.; Moresi, J.M.; Greenson, J.; Janin, A.; Martin, P.J.; et al. Histopathologic Diagnosis of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol. Blood Marrow Transplant. 2006, 12, 31–47. [Google Scholar] [CrossRef]
- Costa-Da-Silva, A.C.; Aure, M.H.; Dodge, J.; Martin, D.; Dhamala, S.; Cho, M.; Rose, J.J.; Bassim, C.W.; Ambatipudi, K.; Hakim, F.T.; et al. Salivary ZG16B expression loss follows exocrine gland dysfunction related to oral chronic graft-versus-host disease. iScience 2021, 25, 103592. [Google Scholar] [CrossRef]
- Pérez, C.A.; Rabanales, R.; Rojas-Alcayaga, G.; Larrondo, M.; Escobar, A.F.; López, M.N.; Salazar-Onfray, F.; Alfaro, J.I.; González, F.E. Dendritic cell chimerism in oral mucosa of transplanted patients affected by graft-versus-host disease. J. Oral Pathol. Med. 2015, 45, 127–135. [Google Scholar] [CrossRef]
- Hiroki, A.; Nakamura, S.; Shinohara, M.; Oka, M. Significance of oral examination in chronic graft-versus-host disease. J. Oral Pathol. Med. 1994, 23, 209–215. [Google Scholar] [CrossRef]
- Alborghetti, M.R.; Corrêa, M.E.P.; Adam, R.L.; Metze, K.; Coracin, F.L.; de Souza, C.A.; Cintra, M.L. Late effects of chronic graft-vs.-host disease in minor salivary glands. J. Oral Pathol. Med. 2005, 34, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tokuda, N.; Fukumoto, T.; Mano, T.; Sato, T.; Ueyama, Y. Immunohistopathological study of the oral lichenoid lesions of chronic GVHD. J. Oral Pathol. Med. 2005, 35, 33–36. [Google Scholar] [CrossRef]
- Imanguli, M.M.; Swaim, W.D.; League, S.C.; Gress, R.E.; Pavletic, S.Z.; Hakim, F.T. Increased T-bet+ cytotoxic effectors and type I interferon–mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 2009, 113, 3620–3630. [Google Scholar] [CrossRef]
- Mays, J.; Fassil, H.; Edwards, D.; Pavletic, S.; Bassim, C. Oral chronic graft-versus-host disease: Current pathogenesis, therapy, and research. Oral Dis. 2012, 19, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Tollemar, V.; Arvidsson, H.; Häbel, H.; Tudzarovski, N.; Legert, K.G.; Le Blanc, K.; Warfvinge, G.; Sugars, R. Grading of minor salivary gland immuno-histopathology post-allogenic hematopoietic cell transplantation. Heliyon 2023, 9, e15517. [Google Scholar] [CrossRef] [PubMed]
- Hasséus, B.; Jontell, M.; Brune, M.; Johansson, P.; Dahlgren, U.I. Langerhans Cells and T Cells in Oral Graft versus Host Disease and Oral Lichen Planus. Scand. J. Immunol. 2001, 54, 516–524. [Google Scholar] [CrossRef] [PubMed]
- McManigle, W.; Youssef, A.; Sarantopoulos, S. B cells in chronic graft-versus-host disease. Hum. Immunol. 2019, 80, 393–399. [Google Scholar] [CrossRef]
- Solomon, S.R.; Sizemore, C.A.; Ridgeway, M.; Zhang, X.; Brown, S.; Holland, H.K.; Morris, L.E.; Solh, M.; Bashey, A. Safety and efficacy of rituximab-based first line treatment of chronic GVHD. Bone Marrow Transplant. 2018, 54, 1218–1226. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, X.; Liu, Q.; Zhang, X.; Huang, K.; Liu, L.; Li, H.; Zhou, M.; Huang, F.; Fan, Z.; et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 2014, 29, 636–646. [Google Scholar] [CrossRef]
- Devic, I.; Shi, M.; Schubert, M.M.; Lloid, M.; Izutsu, K.T.; Pan, C.; Missaghi, M.; Morton, T.H.; Mancl, L.A.; Zhang, J.; et al. Proteomic Analysis of Saliva from Patients with Oral Chronic Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; van den Brink, M.R.M.; Kamboj, M.; et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef]
- Ames, N.J.; Sulima, P.; Ngo, T.; Barb, J.; Munson, P.J.; Paster, B.J.; Hart, T.C. A Characterization of the Oral Microbiome in Allogeneic Stem Cell Transplant Patients. PLoS ONE 2012, 7, e47628. [Google Scholar] [CrossRef]
- Laheij, A.M.G.A.; Rozema, F.R.; Brennan, M.T.; von Bültzingslöwen, I.; van Leeuwen, S.J.M.; Potting, C.; Huysmans, M.-C.D.N.J.M.; Hazenberg, M.D.; Brandt, B.W.; Zaura, E.; et al. Long-Term Analysis of Resilience of the Oral Microbiome in Allogeneic Stem Cell Transplant Recipients. Microorganisms 2022, 10, 734. [Google Scholar] [CrossRef]
- Rashidi, A.; Pidala, J.; Hamilton, B.K.; Pavletic, S.Z.; Kim, K.; Zevin, A.; Mays, J.W.; Lee, S.J. Oral and gut microbiome alterations in oral chronic graft-versus-host disease: Results from Close Assessment and Testing for Chronic GVHD (CATCH study). Clin. Cancer Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Eshel, A.; Dubovski, B.; Kuperman, A.A.; Danylesko, I.; Fein, J.A.; Fried, S.; Geva, M.; Kouniavski, E.; Neuman, H.; et al. Patterns of salivary microbiota injury and oral mucositis in recipients of allogeneic hematopoietic stem cell transplantation. Blood Adv. 2020, 4, 2912–2917. [Google Scholar] [CrossRef]
- Malard, F.; Jenq, R.R. The Microbiome and Its Impact on Allogeneic Hematopoietic Cell Transplantation. Cancer J. 2023, 29, 75–83. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Vos, J.; Blom, B.; Hazenberg, M.D. Allogeneic hematopoietic cell transplantation, the microbiome, and graft-versus-host disease. Gut Microbes 2023, 15, 2178805. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2014, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Janowiak-Majeranowska, A.; Osowski, J.; Mikaszewski, B.; Majeranowski, A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers 2022, 14, 2175. [Google Scholar] [CrossRef] [PubMed]
- Pukhalskaya, T.; Smoller, B.R.; Becker, M.; Maly, A.; Zadik, Y.; Elad, S. Oral white lesion in patients post-hematopoietic stem cell transplantation: A case series demonstrating the diagnostic dilemma. Support. Care Cancer 2021, 29, 7999–8007. [Google Scholar] [CrossRef] [PubMed]
- Imanguli, M.M.; Atkinson, J.C.; Mitchell, S.A.; Avila, D.N.; Bishop, R.J.; Cowen, E.W.; Datiles, M.B.; Hakim, F.T.; Kleiner, D.E.; Krumlauf, M.C.; et al. Salivary Gland Involvement in Chronic Graft-Versus-Host Disease: Prevalence, Clinical Significance, and Recommendations for Evaluation. Biol. Blood Marrow Transplant. 2010, 16, 1362–1369. [Google Scholar] [CrossRef]
- Haverman, T.M.; Raber-Durlacher, J.E.; Raghoebar, I.I.; Rademacher, W.M.; Rozema, F.R.; Hazenberg, M.D.; Epstein, J.B.; Treister, N.S. Oral chronic graft-versus-host disease. J. Am. Dent. Assoc. 2020, 151, 846–856. [Google Scholar] [CrossRef]
- Charters, E.; Dunn, M.; Cheng, K.; Aung, V.; Mukherjee, P.; Froggatt, C.; Dusseldorp, J.R.; Clark, J.R. Trismus therapy devices: A systematic review. Oral Oncol. 2022, 126, 105728. [Google Scholar] [CrossRef]
- Epstein, J.B.; Raber-Durlacher, J.E.; Huysmans, M.-C.; Schoordijk, M.C.; Cheng, J.E.; Bensadoun, R.-J.; Arany, P.R. Photobiomodulation Therapy Alleviates Tissue Fibroses Associated with Chronic Graft-Versus-Host Disease: Two Case Reports and Putative Anti-Fibrotic Roles of TGF-β. Photomed. Laser Surg. 2018, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Raber-Durlacher, J.E.; Lill, M.; Linhares, Y.P.L.; Chang, J.; Barasch, A.; Slief, R.I.C.; Geuke, M.; Zecha, J.A.E.M.; Milstein, D.M.J.; et al. Photobiomodulation therapy in the management of chronic oral graft-versus-host disease. Support. Care Cancer 2016, 25, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.; Duncan, C.; Cutler, C.; Lehmann, L. How we treat oral chronic graft-versus-host disease. Blood 2012, 120, 3407–3418. [Google Scholar] [CrossRef]
- Reed, D.N.; Hall, D.L.; Cottle, J.H.; Frimenko, K.; Horton, C.K.; Abu Sharkh, F.; Beckett, R.; Hernandez, B.; Mabe, H.; Mansour, S.T.; et al. Dental management of scleroderma patients using pentoxifylline plus vitamin E with and without TheraBite® to reduce trismus: Two case reports and brief review of literature. Clin. Case Rep. 2020, 8, 247–253. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Tollemar, V.; Tudzarovski, N.; Warfvinge, G.; Yarom, N.; Remberger, M.; Heymann, R.; Legert, K.G.; Sugars, R.V. Histopathological Grading of Oral Mucosal Chronic Graft-versus-Host Disease: Large Cohort Analysis. Biol. Blood Marrow Transplant. 2020, 26, 1971–1979. [Google Scholar] [CrossRef]
- Shulman, H.M.; Cardona, D.M.; Greenson, J.K.; Hingorani, S.; Horn, T.; Huber, E.; Kreft, A.; Longerich, T.; Morton, T.; Myerson, D.; et al. NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. The 2014 Pathology Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 589–603. [Google Scholar] [CrossRef]
- Nakamura, S.; Hiroki, A.; Shinohara, M.; Gondo, H.; Ohyama, Y.; Mouri, T.; Sasaki, M.; Shirasuna, K.; Harada, M.; Niho, Y. Oral involvement in chronic graft-versus-host disease after allogeneic bone marrow transplantation. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 1996, 82, 556–563. [Google Scholar] [CrossRef]
- Schubert, M.M.; Williams, B.E.; Lloid, M.E.; Donaldson, G.; Chapk, M.K. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation development of an oral mucositis index. Cancer 1992, 69, 2469–2477. [Google Scholar] [CrossRef]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 984–999. [Google Scholar] [CrossRef]
- Lee, S.J.; Cook, E.; Soiffer, R.; Antin, J.H. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2002, 8, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.; Onstad, L.; Lee, S.J. Reliability and Validity of the Modified 7-Day Lee Chronic Graft-versus-Host Disease Symptom Scale. Biol Blood Marrow Transplant. 2020, 26, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Stolze, J.; Boor, M.; Hazenberg, M.D.; Brand, H.S.; Raber-Durlacher, J.E.; Laheij, A.M.G.A. Oral health–related quality of life of patients with oral chronic graft-versus-host disease. Support. Care Cancer 2021, 29, 6353–6360. [Google Scholar] [CrossRef]
- Fall-Dickson, J.M.; Pavletic, S.Z.; Mays, J.W.; Schubert, M.M. Oral Complications of Chronic Graft-Versus-Host Disease. JNCI Monogr. 2019, 2019, lgz007. [Google Scholar] [CrossRef]
- Schubert, M.M.; Correa, M.E.P. Oral Graft-Versus-Host Disease. Dent. Clin. North Am. 2008, 52, 79–109. [Google Scholar] [CrossRef]
- Mawardi, H.; Stevenson, K.; Gokani, B.; Soiffer, R.; Treister, N. Combined topical dexamethasone/tacrolimus therapy for management of oral chronic GVHD. Bone Marrow Transplant. 2009, 45, 1062–1067. [Google Scholar] [CrossRef][Green Version]
- Elad, S.; Epstein, J.B.; Yarom, N.; Drucker, S.; Tzach, R.; Bültzingslöwen, I.V. Topical immunomodulators for management of oral mucosal conditions, a systematic review; part I: Calcineurin inhibitors. Expert Opinion on Emerging Drugs 2010, 15, 713–726. [Google Scholar] [CrossRef]
- Elad, S.; Or, R.; Garfunkel, A.A.; Shapira, M.Y. Budesonide: A novel treatment for oral chronic graft versus host disease. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2003, 95, 308–311. [Google Scholar] [CrossRef]
- Treister, N.; Li, S.; Kim, H.; Lerman, M.; Sultan, A.; Alyea, E.P.; Armand, P.; Cutler, C.; Ho, V.; Koreth, J.; et al. An Open-Label Phase II Randomized Trial of Topical Dexamethasone and Tacrolimus Solutions for the Treatment of Oral Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2016, 22, 2084–2091. [Google Scholar] [CrossRef]
- Elad, S.; Jensen, S.B.; Raber-Durlacher, J.E.; Mouradian, N.; Correa, E.M.P.; Schubert, M.M.; Blijlevens, N.M.A.; Epstein, J.B.; Saunders, D.P.; Waltimo, T.; et al. Clinical approach in the management of oral chronic graft-versus-host disease (cGVHD) in a series of specialized medical centers. Support. Care Cancer 2014, 23, 1615–1622. [Google Scholar] [CrossRef]
- Wolff, D.; Gerbitz, A.; Ayuk, F.; Kiani, A.; Hildebrandt, G.C.; Vogelsang, G.B.; Elad, S.; Lawitschka, A.; Socie, G.; Pavletic, S.Z.; et al. Consensus Conference on Clinical Practice in Chronic Graft-versus-Host Disease (GVHD): First-Line and Topical Treatment of Chronic GVHD. Biol. Blood Marrow Transplant. 2010, 16, 1611–1628. [Google Scholar] [CrossRef] [PubMed]
- Garbutcheon-Singh, K.B.; Fernández-Peñas, P. Phototherapy for the treatment of cutaneous graft versus host disease. Australas. J. Dermatol. 2014, 56, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Anders, V.; Corio, R.; Horn, T.; Morison, W.; Farmer, E.; Vogelsang, G. Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs.-host disease. Photodermatol. Photoimmunol. Photomed. 2004, 20, 184–190. [Google Scholar] [CrossRef]
- Treister, N.; Li, S.; A Lerman, M.; Lee, S.; Soiffer, R. Narrow-band UVB phototherapy for management of oral chronic graft-versus-host disease. Photodermatol. Photoimmunol. Photomed. 2014, 31, 75–82. [Google Scholar] [CrossRef]
- Campos, L.; Rezende, S.B.; Simões, A.; Palma, L.F.; Tateno, R.Y.; da Silva, R.L.; Macedo, M.C. Photobiomodulation and photodynamic therapy for the management of oral graft-versus-host disease: A case report. Photodiagnosis Photodyn. Ther. 2020, 30, 101776. [Google Scholar] [CrossRef]
- Epstein, J.B.; Raber-Durlacher, J.E.; Epstein, G.L.; Hazenberg, M.D.; Tzachanis, D.; Spielberger, R.T. Chronic oral graft-versus-host disease: Induction and maintenance therapy with photobiomodulation therapy. Support. Care Cancer 2020, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Sroussi, H. Oral Chronic Graft-Versus-Host Disease. Front. Oral Heal. 2022, 3, 903154. [Google Scholar] [CrossRef]
- Kuten-Shorrer, M.; Woo, S.-B.; Treister, N.S. Oral Graft-Versus-Host Disease. Dent. Clin. North Am. 2014, 58, 351–368. [Google Scholar] [CrossRef]
- Nagler, R.; Nagler, A. Pilocarpine hydrochloride relieves xerostomia in chronic graft-versus-host disease: A sialometrical study. Bone Marrow Transplant. 1999, 23, 1007–1011. [Google Scholar] [CrossRef]
- Chitlange, N.M.; Phansopkar, P. Physiotherapeutic Approach in Oral Submucous Fibrosis: A Systematic Review. Cureus 2023, 15, e48155. [Google Scholar] [CrossRef]
- Kruse, A.L.; Grätz, K.W. Oral carcinoma after hematopoietic stem cell transplantation—A new classification based on a literature review over 30 years. Head Neck Oncol. 2009, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Kofman, E.R.; Shazib, M.A.; Woo, S.-B.; Reardon, B.; Treister, N.S.; Haddad, R.I.; Cutler, C.S.; Antin, J.H.; Van Allen, E.M.; et al. Integrated genomic characterization of oral carcinomas in post-hematopoietic stem cell transplantation survivors. Oral Oncol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Rotz, S.J.; Bhatt, N.S.; Hamilton, B.K.; Duncan, C.; Aljurf, M.; Atsuta, Y.; Beebe, K.; Buchbinder, D.; Burkhard, P.; Carpenter, P.A.; et al. International Recommendations for Screening and Preventative Practices for Long-Term Survivors of Transplantation and Cellular Therapy: A 2023 Update. Biol. Blood Marrow Transplant. 2024, 30, 349–385. [Google Scholar] [CrossRef]
- Elad, S.; Zeevi, I.; Finke, J.; Koldehoff, M.; Schwerdtfeger, R.; Wolff, D.; Mohrbacher, R.; Levitt, M.; Greinwald, R.; Shapira, M.Y. Improvement in Oral Chronic Graft-versus-Host Disease with the Administration of Effervescent Tablets of Topical Budesonide—An Open, Randomized, Multicenter Study. Biol. Blood Marrow Transplant. 2011, 18, 134–140. [Google Scholar] [CrossRef][Green Version]
- Noce, C.W.; Gomes, A.; Shcaira, V.; Corrêa, M.E.P.; Moreira, M.C.R.; Júnior, A.S.; Gonçalves, L.S.; Garnica, M.; Maiolino, A.; Torres, S.R. Randomized Double-Blind Clinical Trial Comparing Clobetasol and Dexamethasone for the Topical Treatment of Symptomatic Oral Chronic Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 1163–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mays, J.W.; Curtis, L.M.; Bassim, C.; Steinberg, S.M.; Venzon, D.; Rose, J.J.; Cho, M.; Kanakry, C.G.; Yazdanie, F.; Avila, D.; et al. A Randomized Phase 2 Placebo Controlled Trial of Clobetasol Rinse for Treatment of Oral Chronic Graft-Versus-Host Disease. Blood 2016, 128, 826. [Google Scholar] [CrossRef]
- Motta, A.; Zhan, Q.; Larson, A.; Lerman, M.; Woo, S.; Soiffer, R.; Murphy, G.; Treister, N. Immunohistopathological characterization and the impact of topical immunomodulatory therapy in oral chronic graft-versus-host disease: A pilot study. Oral Dis. 2017, 24, 580–590. [Google Scholar] [CrossRef]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Zeiser, R.; Polverelli, N.; Ram, R.; Hashmi, S.K.; Chakraverty, R.; Middeke, J.M.; Musso, M.; Giebel, S.; Uzay, A.; Langmuir, P.; et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2021, 385, 228–238. [Google Scholar] [CrossRef]
- Cutler, C.; Lee, S.J.; Arai, S.; Rotta, M.; Zoghi, B.; Lazaryan, A.; Ramakrishnan, A.; DeFilipp, Z.; Salhotra, A.; Chai-Ho, W.; et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar Study. Blood 2021, 138, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, A.B.; Witte, O.N. The role of Bruton’s tyrosine kinase in B-cell development and function: A genetic perspective. Immunol. Rev. 2000, 175, 120–127. [Google Scholar] [CrossRef]
- Garg, N.; Padron, E.J.; Rammohan, K.W.; Goodman, C.F. Bruton’s Tyrosine Kinase Inhibitors: The Next Frontier of B-Cell-Targeted Therapies for Cancer, Autoimmune Disorders, and Multiple Sclerosis. J. Clin. Med. 2022, 11, 6139. [Google Scholar] [CrossRef] [PubMed]
- King-Kallimanis, B.L.; Wroblewski, T.; Kwitkowski, V.; De Claro, R.A.; Gwise, T.; Bhatnagar, V.; Farrell, A.T.; Kluetz, P.G. FDA review summary of patient-reported outcome results for ibrutinib in the treatment of chronic graft versus host disease. Qual. Life Res. 2020, 29, 1903–1911. [Google Scholar] [CrossRef]
- Waller, E.K.; Miklos, D.; Cutler, C.; Arora, M.; Jagasia, M.H.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Chang, S.; et al. Ibrutinib for Chronic Graft-versus-Host Disease After Failure of Prior Therapy: 1-Year Update of a Phase 1b/2 Study. Biol. Blood Marrow Transplant. 2019, 25, 2002–2007. [Google Scholar] [CrossRef]
- Doki, N.; Toyosaki, M.; Shiratori, S.; Osumi, T.; Okada, M.; Kawakita, T.; Sawa, M.; Ishikawa, T.; Ueda, Y.; Yoshinari, N.; et al. An Open-Label, Single-Arm, Multicenter Study of Ibrutinib in Japanese Patients with Steroid-dependent/Refractory Chronic Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. 2021, 27, 867.e1–867.e9. [Google Scholar] [CrossRef] [PubMed]
- Abboud, R.; Choi, J.; Ruminski, P.; Schroeder, M.A.; Kim, S.; Abboud, C.N.; DiPersio, J.F. Insights into the role of the JAK/STAT signaling pathway in graft-versus-host disease. Ther. Adv. Hematol. 2020, 11, 2040620720914489. [Google Scholar] [CrossRef]
- Le, R.Q.; Wang, X.; Zhang, H.; Li, H.; Przepiorka, D.; Vallejo, J.; Leong, R.; Ma, L.; Goldberg, K.B.; Pazdur, R.; et al. FDA Approval Summary: Ruxolitinib for Treatment of Chronic Graft-Versus-Host Disease after Failure of One or Two Lines of Systemic Therapy. Oncol. 2022, 27, 493–500. [Google Scholar] [CrossRef]
- Hudda, Z.; Flannery, A.; Teusink-Cross, A.; Davies, S.M.; Khandelwal, P. Topical ruxolitinib is promising as sole or adjunctive therapy in treating maculopapular rash of acute and chronic skin GVHD. Bone Marrow Transplant. 2024, 59, 425–427. [Google Scholar] [CrossRef]
- Cook, E.; Dong, M.; Chiang, S.C.; Luedeke, D.; Lake, K.E.; Hoerth, C.; Deavy, M.; Setchell, K.D.; Zhao, J.; Punt, N.; et al. Ruxolitinib Pharmacokinetics and Pharmacodynamics in Children with Acute and Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2024, 30, 528.e1–528.e12. [Google Scholar] [CrossRef]
- Julian, L.; Olson, M.F. Rho-associated coiled-coil containing kinases (ROCK). Small GTPases 2014, 5, e29846. [Google Scholar] [CrossRef] [PubMed]
- Zanin-Zhorov, A.; Blazar, B.R. ROCK2, a critical regulator of immune modulation and fibrosis has emerged as a therapeutic target in chronic graft-versus-host disease. Clin. Immunol. 2021, 230, 108823. [Google Scholar] [CrossRef] [PubMed]
- Przepiorka, D.; Le, R.Q.; Ionan, A.; Li, R.-J.; Wang, Y.-H.; Gudi, R.; Mitra, S.; Vallejo, J.; Okusanya, O.O.; Ma, L.; et al. FDA Approval Summary: Belumosudil for Adult and Pediatric Patients 12 Years and Older with Chronic GvHD after Two or More Prior Lines of Systemic Therapy. Clin. Cancer Res. 2022, 28, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Kattner, A.-S.; Holler, E.; Holler, B.; Klobuch, S.; Weber, D.; Martinovic, D.; Edinger, M.; Herr, W.; Wolff, D. IL6-receptor antibody tocilizumab as salvage therapy in severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: A retrospective analysis. Ann. Hematol. 2020, 99, 847–853. [Google Scholar] [CrossRef]
- Drobyski, W.R.; Pasquini, M.; Kovatovic, K.; Palmer, J.; Rizzo, J.D.; Saad, A.; Saber, W.; Hari, P. Tocilizumab for the Treatment of Steroid Refractory Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2011, 17, 1862–1868. [Google Scholar] [CrossRef]
- Wobma, H.M.; Kapadia, M.; Kim, H.T.; Alvarez-Calderon, F.; Baumeister, S.H.C.; Duncan, C.; Forrest, S.; Gorfinkel, L.; Huang, J.; Lehmann, L.E.; et al. Real-world experience with low-dose IL-2 for children and young adults with refractory chronic graft-versus-host disease. Blood Adv. 2023, 7, 4647–4657. [Google Scholar] [CrossRef]
- Belizaire, R.; Kim, H.T.; Poryanda, S.J.; Mirkovic, N.V.; Hipolito, E.; Savage, W.J.; Reynolds, C.G.; Fields, M.J.; Whangbo, J.; Kubo, T.; et al. Efficacy and immunologic effects of extracorporeal photopheresis plus interleukin-2 in chronic graft-versus-host disease. Blood Adv. 2019, 3, 969–979. [Google Scholar] [CrossRef]
- Malik, M.I.; Litzow, M.; Hogan, W.; Patnaik, M.; Murad, M.H.; Prokop, L.J.; Winters, J.L.; Hashmi, S. Extracorporeal photopheresis for chronic graft-versus-host disease: A systematic review and meta-analysis. BLOOD Res. 2014, 49, 100–106. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, J.T.; Jessri, M.; Costa-da-Silva, A.C.; Sharma, R.; Mays, J.W.; Treister, N.S. Oral Chronic Graft-Versus-Host Disease: Pathogenesis, Diagnosis, Current Treatment, and Emerging Therapies. Int. J. Mol. Sci. 2024, 25, 10411. https://doi.org/10.3390/ijms251910411
Nguyen JT, Jessri M, Costa-da-Silva AC, Sharma R, Mays JW, Treister NS. Oral Chronic Graft-Versus-Host Disease: Pathogenesis, Diagnosis, Current Treatment, and Emerging Therapies. International Journal of Molecular Sciences. 2024; 25(19):10411. https://doi.org/10.3390/ijms251910411
Chicago/Turabian StyleNguyen, Joe T., Maryam Jessri, Ana C. Costa-da-Silva, Rubina Sharma, Jacqueline W. Mays, and Nathaniel S. Treister. 2024. "Oral Chronic Graft-Versus-Host Disease: Pathogenesis, Diagnosis, Current Treatment, and Emerging Therapies" International Journal of Molecular Sciences 25, no. 19: 10411. https://doi.org/10.3390/ijms251910411
APA StyleNguyen, J. T., Jessri, M., Costa-da-Silva, A. C., Sharma, R., Mays, J. W., & Treister, N. S. (2024). Oral Chronic Graft-Versus-Host Disease: Pathogenesis, Diagnosis, Current Treatment, and Emerging Therapies. International Journal of Molecular Sciences, 25(19), 10411. https://doi.org/10.3390/ijms251910411






