Genome-Wide Characterization of the INDETERMINATE DOMAIN (IDD) Zinc Finger Gene Family in Solanum lycopersicum and the Functional Analysis of SlIDD15 in Shoot Gravitropism
Abstract
1. Introduction
2. Results
2.1. Identification and Physicochemical Analysis of IDD Members in Tomato
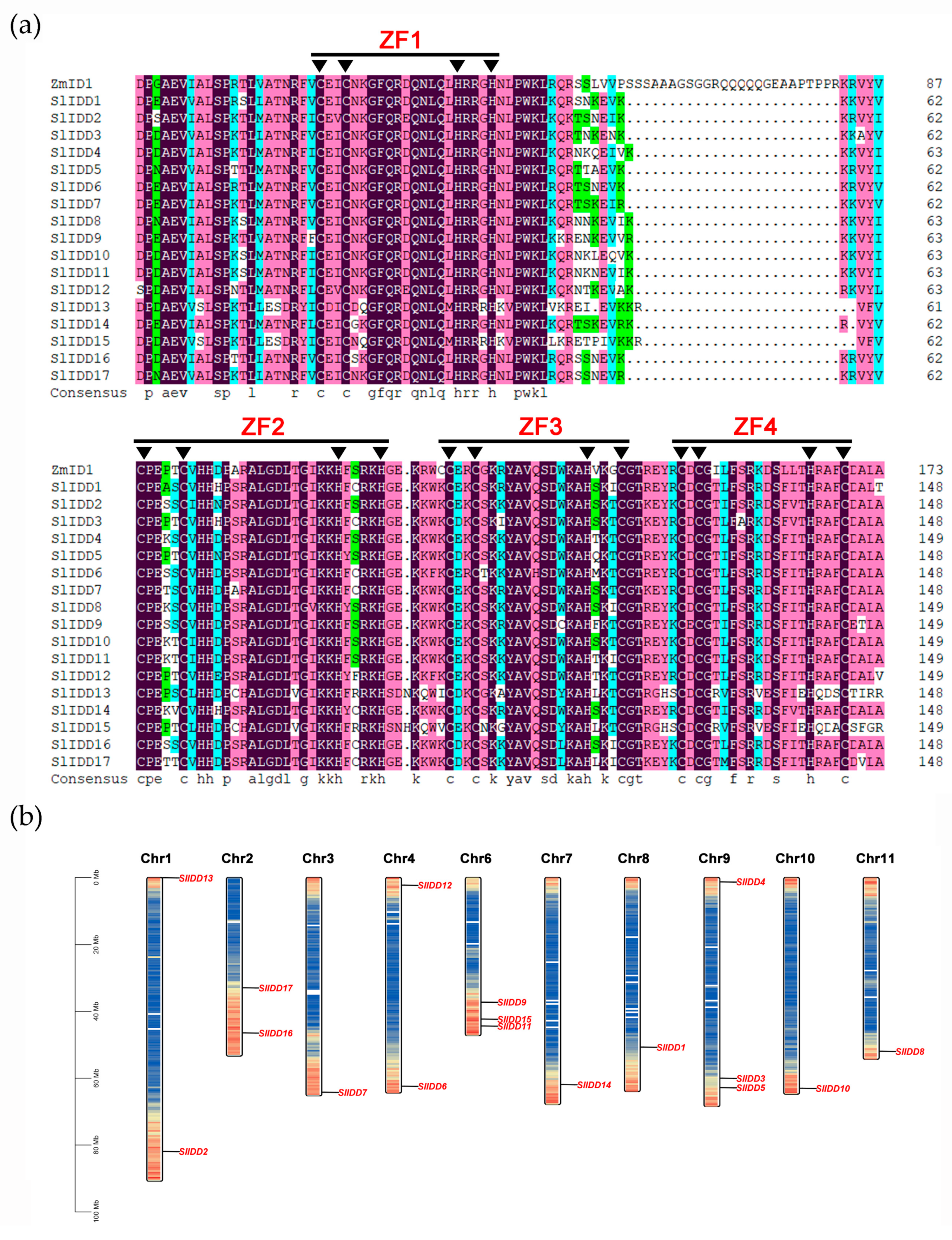
2.2. Chromosomal Location and Multiple Sequence Alignment of the SlIDD Gene Family
2.3. Conserved Motifs and Gene Structure of the SlIDD Gene Family
2.4. Phylogenetic Analysis of SlIDD Family
2.5. Gene Duplication and Evolutionary Analysis of SlIDD Gene Family
2.6. Analysis of Cis-Regulatory Elements in SlIDDs Gene Promoters
2.7. Expression Profile Analysis of SlIDD Genes in Different Tissues
2.8. Subcellular Localization of the SlIDD8 and SlIDD15 Proteins
2.9. CRISPR/Cas9-Mediated SlIDD15 Mutants Do Not Exhibit Defects in Shoot Gravitropism
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification and Physicochemical Properties Analysis of IDD Gene in Tomato
4.2. Multiple Sequence Alignment and Chromosomal Localization Analysis
4.3. Phylogenetic Relationship, Gene Structure, and Conserved Motif Analysis of SlIDDs
4.4. Phylogenetic Analysis of IDDs
4.5. Gene Duplication, Collinearity Analysis, and Ka/Ks Calculation
4.6. Analysis of the Cis-Acting Elements in the Promoters of SlIDDs
4.7. The Expression Profiles of SlIDD Genes in Different Tissues
4.8. RNA Extraction, Reverse Transcription, and Quantitative Real-Time RT-PCR (qRT-PCR) Analysis
4.9. Subcellular Vector Construction and Subcellular Localization
4.10. CRISPR/Cas9-Mediated Gene Editing in Tomato
4.11. Gravitropism Assay
4.12. Plant Materials and Growth Condition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.; Xian, Z.; Kang, X.; Tang, N.; Li, Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, J.; Tremblay, R.; Wong, A.Y.; Coneva, V.; Kozaki, A.; Mable, B.K. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genom. 2006, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.P.; Huang, P.; Lee, D.Y.; Brutnell, T.P. Making roots, shoots, and seeds: IDD gene family diversification in plants. Trends Plant Sci. 2018, 23, 66–78. [Google Scholar] [CrossRef]
- Zhang, T.; Tan, M.; Geng, L.; Li, J.; Xiang, Y.; Zhang, B.; Zhao, Y. New insight into comprehensive analysis of INDETERMINATE DOMAIN (IDD) gene family in rice. Plant Physiol. Biochem. 2020, 154, 547–556. [Google Scholar] [CrossRef]
- Kumar, M.; Le, D.T.; Hwang, S.; Seo, P.J.; Kim, H.U. Role of the INDETERMINATE DOMAIN genes in plants. Int. J. Mol. Sci. 2019, 20, 2286. [Google Scholar] [CrossRef]
- Coneva, V.; Zhu, T.; Colasanti, J. Expression differences between normal and indeterminate1 maize suggest downstream targets of ID1, a floral transition regulator in maize. J. Exp. Bot. 2007, 58, 3679–3693. [Google Scholar] [CrossRef]
- Colasanti, J.; Yuan, Z.; Sundaresan, V. The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 1998, 93, 593–603. [Google Scholar] [CrossRef]
- Wong, A.Y.; Colasanti, J. Maize floral regulator protein INDETERMINATE1 is localized to developing leaves and is not altered by light or the sink/source transition. J. Exp. Bot. 2007, 58, 403–414. [Google Scholar] [CrossRef]
- Kozaki, A.; Hake, S.; Colasanti, J. The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res. 2004, 32, 1710–1720. [Google Scholar] [CrossRef]
- Meng, X.; Muszynski, M.G.; Danilevskaya, O.N. The FT-like ZCN8 gene functions as a floral activator and Is Involved in photoperiod sensitivity in maize. Plant Cell 2011, 23, 942–960. [Google Scholar] [CrossRef]
- Matsubara, K.; Yamanouchi, U.; Wang, Z.X.; Minobe, Y.; Izawa, T.; Yano, M. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol. 2008, 148, 1425–1435. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.L.; Lee, S.; Je, B.I.; Piao, H.L.; Park, S.H.; Kim, C.M.; Ryu, C.H.; Park, S.H.; Xuan, Y.H.; et al. Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J. 2008, 56, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; You, C.; Li, C.; Long, T.; Chen, G.; Byrne, M.E.; Zhang, Q. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 12915–12920. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, L.; Zhang, S.; Shen, J.; Li, S.; Hu, S.; Peng, Q.; Xiao, J.; Wu, C. Suppressor of rid1 (SID1) shares common targets with RID1 on florigen genes to initiate floral transition in rice. PLoS Genet. 2017, 13, e1006642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, L.; Zhao, L.; Wu, C. Genome-wide binding analysis of transcription factor Rice Indeterminate 1 reveals a complex network controlling rice floral transition. J. Integr. Plant Biol. 2022, 64, 1690–1705. [Google Scholar] [CrossRef]
- Seo, P.J.; Ryu, J.; Kang, S.K.; Park, C.M. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011, 65, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Kaimi, R.; Colasanti, J.; Kozaki, A. Activity of transcription factor JACKDAW is essential for SHR/SCR-dependent activation of SCARECROW and MAGPIE and is modulated by reciprocal interactions with MAGPIE, SCARECROW and SHORT ROOT. Plant Mol. Biol. 2011, 77, 489–499. [Google Scholar] [CrossRef]
- Long, Y.; Smet, W.; Cruz-Ramírez, A.; Castelijns, B.; de Jonge, W.; Mähönen, A.P.; Bouchet, B.P.; Perez, G.S.; Akhmanova, A.; Scheres, B.; et al. Arabidopsis BIRD zinc finger proteins jointly stabilize tissue boundaries by confining the cell fate regulator SHORT-ROOT and contributing to fate Specification. Plant Cell 2015, 27, 1185–1199. [Google Scholar] [CrossRef]
- Welch, D.; Hassan, H.; Blilou, I.; Immink, R.; Heidstra, R.; Scheres, B. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007, 21, 2196–2204. [Google Scholar] [CrossRef]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Levesque, M.P.; Vernoux, T.; Busch, W.; Cui, H.; Wang, J.Y.; Blilou, I.; Hassan, H.; Nakajima, K.; Matsumoto, N.; Lohmann, J.U.; et al. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 2006, 4, e143. [Google Scholar] [CrossRef]
- Moreno-Risueno, M.A.; Sozzani, R.; Yardımcı, G.G.; Petricka, J.J.; Vernoux, T.; Blilou, I.; Alonso, J.; Winter, C.M.; Ohler, U.; Scheres, B.; et al. Transcriptional control of tissue formation throughout root development. Science 2015, 350, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef] [PubMed]
- Feurtado, J.A.; Huang, D.; Wicki-Stordeur, L.; Hemstock, L.E.; Potentier, M.S.; Tsang, E.W.; Cutler, A.J. The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell 2011, 23, 1772–1794. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Neelakandan, A.K.; Gontarek, B.C.; Vollbrecht, E.; Becraft, P.W. The naked endosperm genes encode duplicate INDETERMINATE domain transcription factors required for maize endosperm cell patterning and differentiation. Plant Physiol. 2015, 167, 443–456. [Google Scholar] [CrossRef]
- Gontarek, B.C.; Neelakandan, A.K.; Wu, H.; Becraft, P.W. NKD transcription factors are central regulators of maize endosperm development. Plant Cell 2016, 28, 2916–2936. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirano, K.; Sato, T.; Mitsuda, N.; Nomoto, M.; Maeo, K.; Koketsu, E.; Mitani, R.; Kawamura, M.; Ishiguro, S.; et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc. Natl. Acad. Sci. USA 2014, 111, 7861–7866. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.L., Jr.; Hollender, C.A. Branching out: New insights into the genetic regulation of shoot architecture in trees. Curr. Opin. Plant Biol. 2019, 47, 73–80. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z. Plant responses to gravity. Semin. Cell Dev. Biol. 2019, 92, 122–125. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.; Lee, H.; Kim, S.; Kang, J.; Kadam, U.S.; Ju Park, S.; Sik Chung, W.; Chan Hong, J. Cellular and physiological functions of SGR family in gravitropic response in higher plants. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, M.; Kato, T.; Fukaki, H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999, 4, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.T.; Sakaguchi, K.; Kiyose, S.; Taira, K.; Kato, T.; Nakamura, M.; Tasaka, M. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006, 47, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, D.; Li, M.; Wang, K.; Cheng, Z. Loose Plant Architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 2013, 161, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Gao, Q.; Chen, F.; Bai, M.; Zhuang, Z.; Peng, Y. Mutant lpa1 analysis of ZmLPA1 gene regulates maize leaf-angle development through the auxin pathway. Int. J. Mol. Sci. 2022, 23, 4886. [Google Scholar] [CrossRef]
- Van Eck, J.; Keen, P.; Tjahjadi, M. Agrobacterium tumefaciens-Mediated Transformation of Tomato. Methods Mol. Biol. 2019, 1864, 225–234. [Google Scholar] [CrossRef]
- The Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Q.; Zeng, J.; He, X.; Ma, W.; Ge, L.; Liu, W. Comprehensive Analysis of the INDETERMINATE DOMAIN (IDD) Gene Family and Their Response to Abiotic Stress in Zea mays. Int. J. Mol. Sci. 2023, 24, 6185. [Google Scholar] [CrossRef]
- Sun, B.; Fan, Y.; Duan, H.; Liu, X.; Chen, Y.; Shang, G.; Liu, Y.; Yang, H.; Qu, C.; Li, J.; et al. Genome-wide characterization of Brassica napus INDETERMINATE DOMAIN genes reveals a negative role for BnA08.IDD7 in plant development. Ind. Crops Prod. 2022, 175, 114263. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, D.; Xing, L.; Qi, S.; Du, L.; Wu, H.; Shao, H.; Li, Y.; Ma, J.; Han, M. Phylogenetic analysis of IDD gene family and characterization of its expression in response to flower induction in Malus. Mol. Genet. Genom. 2017, 292, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, M.; Chen, J.; Shao, M.; Zou, L.; Ying, Y.; Liu, S. Genome-wide identification of the highly conserved INDETERMINATE DOMAIN (IDD) zinc finger gene family in moso bamboo (Phyllostachys edulis). Int. J. Mol. Sci. 2022, 23, 13952. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, Y.; Ma, S.; Lu, L.; Yang, Z.; Wang, Z.; Li, F. Genome-wide identification of Gossypium INDETERMINATE DOMAIN genes and their expression profiles in ovule development and abiotic stress responses. J. Cotton Res. 2019, 2, 3. [Google Scholar] [CrossRef]
- Su, X.; Meng, T.; Zhao, Y.; Li, G.; Cheng, X.; Abdullah, M.; Sun, X.; Cai, Y.; Lin, Y. Comparative genomic analysis of the IDD genes in five Rosaceae species and expression analysis in Chinese white pear (Pyrus bretschneideri). PeerJ 2019, 7, e6628. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, L.; Zhang, Y.; Xu, L.; Li, N.; Zhang, X.; Pan, Y. Genome-wide identification of C2H2 zinc-finger genes and their expression patterns under heat stress in tomato (Solanum lycopersicum L.). PeerJ 2019, 7, e7929. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Kang, Y.M.; Yang, I.B.; Eo, H.B.; Baek, K.L.; Jang, S.; Eybishitz, A.; Kim, H.C.; Je, B.I.; Park, S.J.; et al. Functional characterization of plant specific Indeterminate Domain (IDD) transcription factors in tomato (Solanum lycopersicum L.). Sci. Rep. 2024, 14, 8015. [Google Scholar] [CrossRef]
- Prochetto, S.; Reinheimer, R. Step by step evolution of Indeterminate Domain (IDD) transcriptional regulators: From algae to angiosperms. Ann. Bot. 2020, 126, 85–101. [Google Scholar] [CrossRef]
- Fukazawa, J.; Teramura, H.; Murakoshi, S.; Nasuno, K.; Nishida, N.; Ito, T.; Yoshida, M.; Kamiya, Y.; Yamaguchi, S.; Takahashi, Y. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 2014, 26, 2920–2938. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, G.; Liu, X.; Ding, X.; Zhang, D.; Wang, X.; Zhou, Y.; Yan, H.; Li, T.; Wu, K.; et al. Histone demethylase SlJMJ6 promotes fruit ripening by removing H3K27 methylation of ripening-related genes in tomato. New Phytol. 2020, 227, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Nakano, T.; Shima, Y.; Ito, Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 2013, 25, 371–386. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Ju, Z.; Cao, D.; Zhu, H.; Fu, D.; Grierson, D.; Qin, G.; Luo, Y.; Zhu, B. The RIN-MC fusion of MADS-Box transcription factors has transcriptional activity and modulates expression of many ripening genes. Plant Physiol. 2018, 176, 891–909. [Google Scholar] [CrossRef]
- Ye, J.; Hu, T.; Yang, C.; Li, H.; Yang, M.; Ijaz, R.; Ye, Z.; Zhang, Y. Transcriptome profiling of tomato fruit development reveals transcription factors associated with ascorbic acid, carotenoid and flavonoid biosynthesis. PLoS ONE 2015, 10, e0130885. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Vrebalov, J.; Zheng, H.; Xu, Y.; Yin, X.; Liu, W.; Liu, Z.; Sorensen, I.; Su, G.; Ma, Q.; et al. A tomato LATERAL ORGAN BOUNDARIES transcription factor, SlLOB1, predominantly regulates cell wall and softening components of ripening. Proc. Natl. Acad. Sci. USA 2021, 118, e2102486118. [Google Scholar] [CrossRef]
- Sacco, A.; Raiola, A.; Calafiore, R.; Barone, A.; Rigano, M.M. New insights in the control of antioxidants accumulation in tomato by transcriptomic analyses of genotypes exhibiting contrasting levels of fruit metabolites. BMC Genom. 2019, 20, 43. [Google Scholar] [CrossRef]
- Song, Z.; Li, W.; Lai, X.; Chen, H.; Wang, L.; Chen, W.; Li, X.; Zhu, X. MaC2H2-IDD regulates fruit softening and involved in softening disorder induced by cold stress in banana. Plant J. 2024, 118, 1937–1954. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Tremblay, R.; Colasanti, J. Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels. Plant Mol. Biol. 2008, 67, 57–69. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)—From genotype to phenotype to breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-S.; Chen, Y.-C.; Lu, C.-H.; Hwang, J.-K. Prediction of protein subcellular localization. Proteins-Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Joung, J.G.; Tang, X.; Zheng, Y.; Huang, M.; Lee, J.M.; McQuinn, R.; Tieman, D.M.; Alba, R.; Klee, H.J.; et al. Tomato Functional Genomics Database: A comprehensive resource and analysis package for tomato functional genomics. Nucleic Acids Res. 2011, 39, D1156-1163. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.G. CRISPR-GE: A convenient software toolkit for CRISPR-based genome editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]








| Gene Name | Gene ID | Identity with ZmID1 (%) | Number of Amino Acid | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Number of Exons | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|---|
| SlIDD1 | Solyc08g063040.4.1 | 44.95 | 467 | 51,814.78 | 9.17 | 45.49 | 53.55 | −0.774 | 3 | Nuclear |
| SlIDD2 | Solyc01g099340.3.1 | 44.66 | 424 | 47,449.69 | 8.92 | 49.55 | 58.68 | −0.898 | 3 | Nuclear |
| SlIDD3 | Solyc09g065670.3.1 | 44.41 | 506 | 55,734.15 | 8.7 | 39.66 | 60.02 | −0.679 | 3 | Nuclear |
| SlIDD4 | Solyc09g007550.3.1 | 42.45 | 459 | 51,314.57 | 9.38 | 46.92 | 58.47 | −0.775 | 3 | Nuclear |
| SlIDD5 | Solyc09g074780.3.1 | 40.33 | 520 | 57,535.07 | 9.06 | 54.7 | 60.25 | −0.771 | 4 | Nuclear |
| SlIDD6 | Solyc04g080130.3.1 | 40.2 | 501 | 54,292.99 | 8.24 | 50.04 | 67.01 | −0.477 | 4 | Nuclear |
| SlIDD7 | Solyc03g121660.3.1 | 39.17 | 544 | 59,497.5 | 8.93 | 47.93 | 56.01 | −0.695 | 3 | Nuclear |
| SlIDD8 | Solyc11g069240.2.1 | 38.66 | 449 | 50,087.32 | 9.31 | 45.76 | 66.06 | −0.72 | 4 | Nuclear |
| SlIDD9 | Solyc06g062670.3.1 | 38.62 | 503 | 56,295.12 | 8.75 | 51.69 | 61.91 | −0.67 | 4 | Nuclear |
| SlIDD10 | Solyc10g084180.2.1 | 38.26 | 493 | 54,589.11 | 9.2 | 46.13 | 58.03 | −0.686 | 3 | Nuclear |
| SlIDD11 | Solyc06g075250.3.1 | 37.96 | 528 | 58,184.82 | 9.09 | 41.29 | 58.07 | −0.793 | 3 | Nuclear |
| SlIDD12 | Solyc04g008500.4.1 | 37.58 | 517 | 57,519.53 | 9.13 | 44.62 | 56.25 | −0.855 | 4 | Nuclear |
| SlIDD13 | Solyc01g005060.3.1 | 36.92 | 339 | 38,602.66 | 8.77 | 79.82 | 69.38 | −0.824 | 3 | Nuclear |
| SlIDD14 | Solyc07g053570.4.1 | 35.76 | 502 | 55,280.78 | 9.08 | 54.53 | 66.87 | −0.651 | 3 | Nuclear |
| SlIDD15 | Solyc06g072360.3.1 | 35.16 | 401 | 45,860.71 | 9.12 | 67.09 | 66.88 | −0.827 | 3 | Nuclear |
| SlIDD16 | Solyc02g085580.4.1 | 30.73 | 483 | 50,520.04 | 8.87 | 59.45 | 63.54 | −0.351 | 4 | Nuclear |
| SlIDD17 | Solyc02g062940.3.1 | 28.02 | 656 | 68,007.62 | 8.31 | 56.09 | 60.87 | −0.34 | 4 | Nuclear |
| Duplicate Gene Pair | Ka | Ks | Ka/Ks | Purify Selection | Duplication Type | Time = Ks/2λ (MYAa) |
|---|---|---|---|---|---|---|
| SlIDD7/SlIDD1 | 0.327 | 1.710 | 0.191 | Yes | Segmental | 57.015 |
| SlIDD7/SlIDD9 | 0.238 | 1.005 | 0.237 | Yes | Segmental | 33.515 |
| SlIDD2/SlIDD5 | 0.337 | 2.803 | 0.120 | Yes | Segmental | 93.425 |
| SlIDD11/SlIDD4 | 0.325 | 2.007 | 0.162 | Yes | Segmental | 66.906 |
| SlIDD11/SlIDD8 | 0.201 | 1.233 | 0.163 | Yes | Segmental | 41.090 |
| SlIDD11/SlIDD10 | 0.373 | 1.691 | 0.220 | Yes | Segmental | 56.361 |
| SlIDD4/SlIDD10 | 0.229 | 0.924 | 0.248 | Yes | Segmental | 30.816 |
| SlIDD4/SlIDD8 | 0.311 | 1.675 | 0.186 | Yes | Segmental | 55.819 |
| SlIDD17/SlIDD16 | 0.162 | 0.777 | 0.208 | Yes | Segmental | 25.896 |
| SlIDD16/SlIDD6 | 0.361 | 1.917 | 0.188 | Yes | Segmental | 63.900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, M.; Fang, Y.; Yang, J.; Xie, X.; Zhang, H.; Zhou, D.; Zhou, Y.; He, Y.; Chen, J.; et al. Genome-Wide Characterization of the INDETERMINATE DOMAIN (IDD) Zinc Finger Gene Family in Solanum lycopersicum and the Functional Analysis of SlIDD15 in Shoot Gravitropism. Int. J. Mol. Sci. 2024, 25, 10422. https://doi.org/10.3390/ijms251910422
Wu H, Liu M, Fang Y, Yang J, Xie X, Zhang H, Zhou D, Zhou Y, He Y, Chen J, et al. Genome-Wide Characterization of the INDETERMINATE DOMAIN (IDD) Zinc Finger Gene Family in Solanum lycopersicum and the Functional Analysis of SlIDD15 in Shoot Gravitropism. International Journal of Molecular Sciences. 2024; 25(19):10422. https://doi.org/10.3390/ijms251910422
Chicago/Turabian StyleWu, Huan, Mingli Liu, Yuqi Fang, Jing Yang, Xiaoting Xie, Hailong Zhang, Dian Zhou, Yueqiong Zhou, Yexin He, Jianghua Chen, and et al. 2024. "Genome-Wide Characterization of the INDETERMINATE DOMAIN (IDD) Zinc Finger Gene Family in Solanum lycopersicum and the Functional Analysis of SlIDD15 in Shoot Gravitropism" International Journal of Molecular Sciences 25, no. 19: 10422. https://doi.org/10.3390/ijms251910422
APA StyleWu, H., Liu, M., Fang, Y., Yang, J., Xie, X., Zhang, H., Zhou, D., Zhou, Y., He, Y., Chen, J., & Bai, Q. (2024). Genome-Wide Characterization of the INDETERMINATE DOMAIN (IDD) Zinc Finger Gene Family in Solanum lycopersicum and the Functional Analysis of SlIDD15 in Shoot Gravitropism. International Journal of Molecular Sciences, 25(19), 10422. https://doi.org/10.3390/ijms251910422






