Estimation of Chloride Channel Residual Function and Assessment of Targeted Drugs Efficiency in the Presence of a Complex Allele [L467F;F508del] in the CFTR Gene
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Clinical Picture of Patients with the Complex Allele [L467F;F508del] and Assessment of Targeted Therapy Efficiency
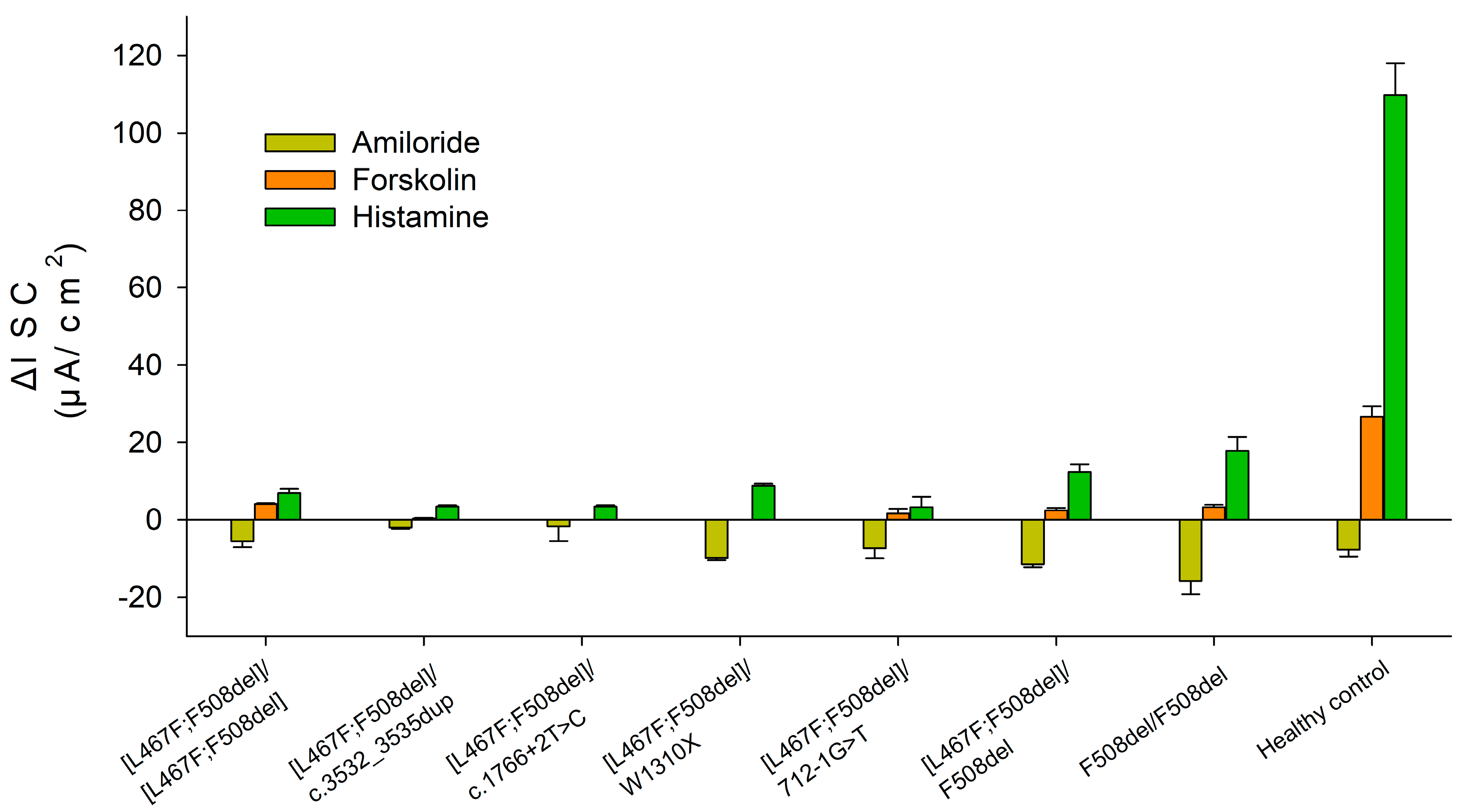
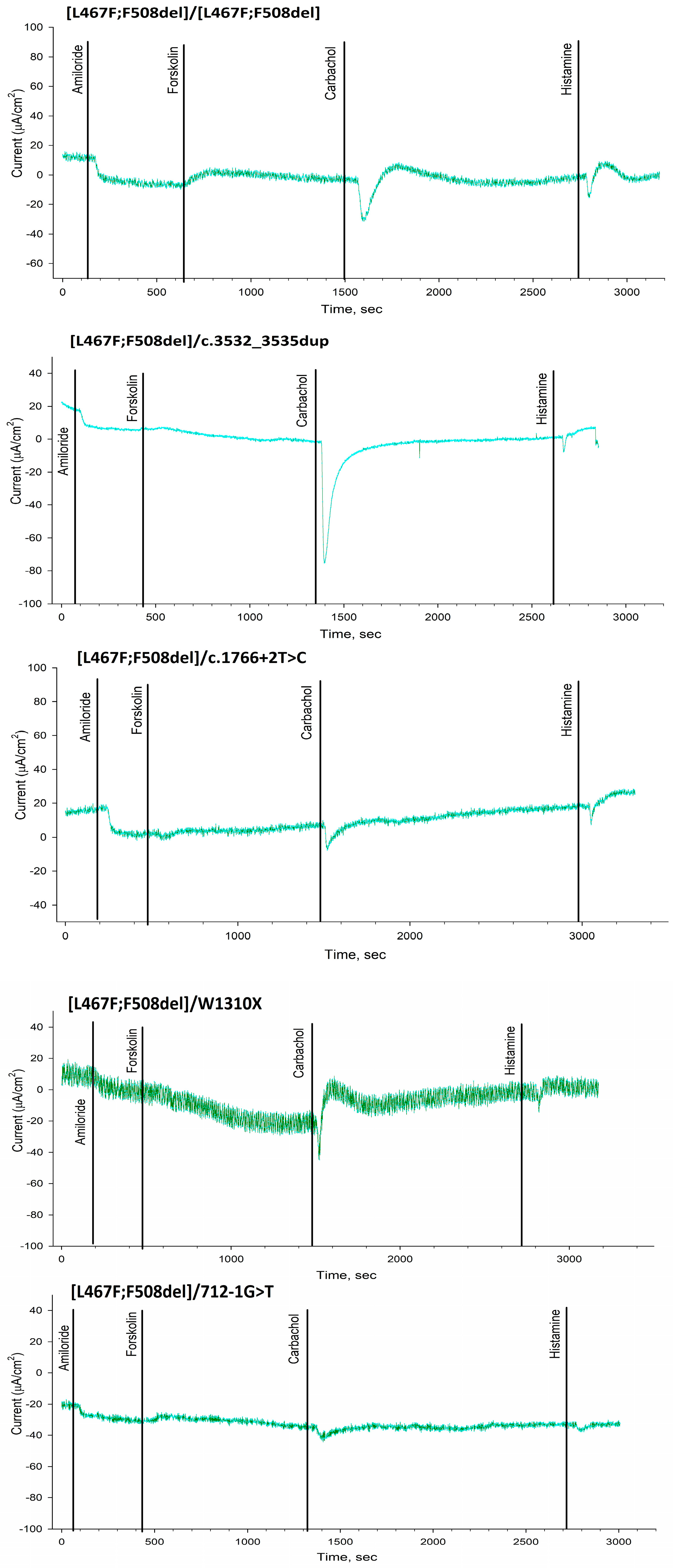
2.2. Evaluation of the Functional Activity of Ion Channels on the Surface of the Intestinal Epithelium by the Intestinal Current Measurements (ICM) Method
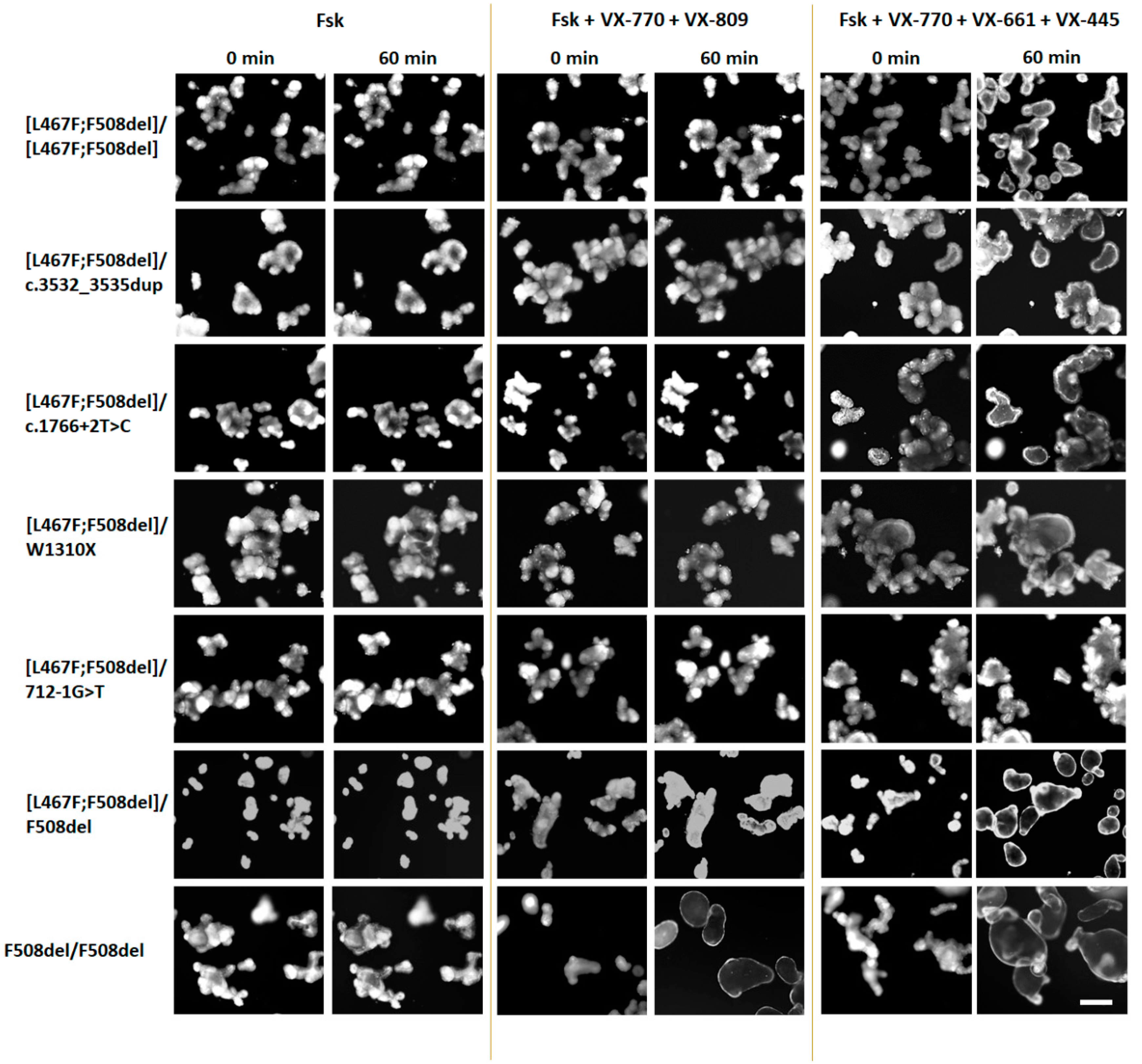
2.3. Evaluation of the Effectiveness of CFTR Modulators in Intestinal Organoids Derived from Patients with [L467F;F508del]/Class I and [L467F;F508del]/[L467F;F508del] Genotypes
3. Discussion
4. Materials and Methods
4.1. Clinical Picture
4.2. Intestinal Current Measurements (ICM)
4.3. Forskolin-Induced Swelling (FIS) Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a.u. | Arbitrary Units |
| ABLA | Allergic Bronchopulmonary Aspergillosis |
| ALT | Alanine Transaminase |
| AST | Aspartate Aminotransferase |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CF | Cystic Fibrosis |
| FCV | Forced Vital Capacity |
| FEV1 | Forced Expiratory Volume in one second |
| FIS | Forskolin-Induced Swelling assay |
| ICM | Intestinal Current Measurement |
| ISC | Short Circuit Current |
| MRSA | Methicillin-Resistant Staphylococcus Aureus |
| PH | Portal Hypertension |
| PI | Pancreatic Insufficiency |
| UDCA | Ursodeoxycholic Acid |
References
- Chevalier, B.; Hinzpeter, A. The influence of CFTR complex alleles on precision therapy of cystic fibrosis. J. Cyst. Fibros. 2020, 19, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Masson, A.; Schneider-Futschik, E.K.; Baatallah, N.; Nguyen-Khoa, T.; Girodon, E.; Hatton, A.; Flament, T.; Le Bourgeois, M.; Chedevergne, F.; Bailly, C.; et al. Predictive factors for lumacaftor/ivacaftor clinical response. J. Cyst. Fibros. 2019, 18, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Diana, A.; Polizzi, A.M.; Santostasi, T.; Ratclif, L.; Pantaleo, M.G.; Leonetti, G.; Iusco, D.R.; Gallo, C.; Conese, M.; Manca, A. The novel complex allele [A238V;F508del] of the CFTR gene: Clinical phenotype and possible implications for cystic fibrosis etiological therapies. J. Hum. Genet. 2016, 61, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, V.; Castaldo, G.; Salvatore, D.; Lucarelli, M.; Raia, V.; Angioni, A.; Carnovale, V.; Cirilli, N.; Casciaro, R.; Colombo, C.; et al. Genotype-phenotype correlation and functional studies in patients with cystic fibrosis bearing CFTR complex alleles. J. Med. Genet. 2017, 54, 224–235. [Google Scholar] [CrossRef]
- Kleinfelder, K.; Somenza, E.; Farinazzo, A.; Conti, J.; Lotti, V.; Latorre, R.V.; Rodella, L.; Massella, A.; Tomba, F.; Bertini, M.; et al. CFTR Modulators Rescue the Activity of CFTR in Colonoids Expressing the Complex Allele p.[R74W;V201M;D1270N]/dele22_24. Int. J. Mol. Sci. 2023, 24, 5199. [Google Scholar] [CrossRef]
- Romey, M.C.; Guittard, C.; Chazalette, J.P.; Frossard, P.; Dawson, K.P.; Patton, M.A.; Casals, T.; Bazarbachi, T.; Girodon, E.; Rault, G.; et al. Complex allele [-102T>A+S549R(T>G)] is associated with milder forms of cystic fibrosis than allele S549R(T>G) alone. Hum. Genet. 1999, 105, 145–150. [Google Scholar] [CrossRef] [PubMed]
- El-Seedy, A.; Girodon, E.; Norez, C.; Pajaud, J.; Pasquet, M.C.; de Becdelièvre, A.; Bienvenu, T.; des Georges, M.; Cabet, F.; Lalau, G.; et al. CFTR mutation combinations producing frequent complex alleles with different clinical and functional outcomes. Hum. Mutat. 2012, 33, 1557–1565. [Google Scholar] [CrossRef]
- Krasovsky, S.A.; Starinova, M.A.; Voronkova, A.Y.; Amelina, E.L.; Kashirskaya, N.Y.; Kondratyeva, E.I.; Nazarenko, L.P. (Eds.) Register of Patients with Cystic Fibrosis in the Russian Federation; 2021 Year; Publishing House Foundation «Ostrova»: St. Petersburg, Russia, 2023; p. 81. [Google Scholar]
- Baatallah, N.; Bitam, S.; Martin, N.; Servel, N.; Costes, B.; Mekki, C.; Chevalier, B.; Pranke, I.; Simonin, J.; Girodon, E.; et al. Cis Variants Identified in F508del Complex Alleles Modulate CFTR Channel Rescue by Small Molecules. Hum. Mutat. 2018, 39, 506–514. [Google Scholar] [CrossRef]
- Kondratyeva, E.; Efremova, A.; Melyanovskaya, Y.; Voronkova, A.; Polyakov, A.; Bulatenko, N.; Adyan, T.; Sherman, V.; Kovalskaia, V.; Petrova, N.; et al. Evaluation of the Complex p.[Leu467Phe;Phe508del] CFTR Allele in the Intestinal Organoids Model: Implications for Therapy. Int. J. Mol. Sci. 2022, 23, 10377. [Google Scholar] [CrossRef]
- Melyanovskaya, Y.; Kondratyeva, E.; Kutsev, S. Determination of reference values for the method of intestinal current measurement in the Russian Federation. Med. News North Cauc. 2020, 15, 162–166. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; De Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; Van De Graaf, E.A.; Nieuwenhuis, E.E.S.; Houwen, R.H.J.; et al. Characterizing Responses to CFTR-Modulating Drugs Using Rectal Organoids Derived from Subjects with Cystic Fibrosis. Sci. Transl. Med. 2016, 8, 344ra84. [Google Scholar] [CrossRef] [PubMed]
- Elahi, E.; Khodadad, A.; Kupershmidt, I.; Ghasemi, F.; Alinasab, B.; Naghizadeh, R.; Eason, R.G.; Amini, M.; Esmaili, M.; Esmaeili Dooki, M.R.; et al. A haplotype framework for cystic fibrosis mutations in Iran. J. Mol. Diagn. 2006, 8, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Vecchio-Pagán, B.; Blackman, S.M.; Lee, M.; Atalar, M.; Pellicore, M.J.; Pace, R.G.; Franca, A.L.; Raraigh, K.S.; Sharma, N.; Knowles, M.R.; et al. Deep resequencing of CFTR in 762 F508del homozygotes reveals clusters of non-coding variants associated with cystic fibrosis disease traits. Hum. Genome Var. 2016, 24, 16038. [Google Scholar] [CrossRef]
- Sondo, E.; Cresta, F.; Pastorino, C.; Tomati, V.; Capurro, V.; Pesce, E.; Lena, M.; Iacomino, M.; Baffico, A.M.; Coviello, D.; et al. The L467F-F508del Complex Allele Hampers Pharmacological Rescue of Mutant CFTR by Elexacaftor/Tezacaftor/Ivacaftor in Cystic Fibrosis Patients: The Value of the Ex Vivo Nasal Epithelial Model to Address Non-Responders to CFTR-Modulating Drugs. Int. J. Mol. Sci. 2022, 23, 3175. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, V.; Centrone, C.; Ferrari, B.; Castellani, C.; Gunawardena, T.N.A.; Taccetti, G.; Laselva, O. Modulator Therapy in Cystic Fibrosis Patients with Cis Variants in F508del Complex Allele: A Short-Term Observational Case Series. J. Pers. Med. 2022, 12, 1421. [Google Scholar] [CrossRef]
- Krasnova, M.G.; Mokrousova, D.O.; Efremova, A.S.; Melyanovskaya, Y.L.; Sherman, V.D.; Bukharova, T.B.; Goldshtein, D.V. Functional activity of the CFTR channel in a patient with the [L467F;F508del]/W1310X genotype. Pulmonologiya 2024, 34, 264–270. (In Russian) [Google Scholar] [CrossRef]
- Krasnova, M.G.; Melianovskaya, Y.L.; Krasovskiy, S.A.; Bulatenko, N.V.; Efremova, A.S.; Bukharova, T.B.; Goldshtein, D.V. Description of the clinical picture and assessment of the functional activity of the CFTR channel in a patient with a complex allele [S466X;R1070Q]. Pulmonologiya 2023, 33, 233–242. (In Russian) [Google Scholar] [CrossRef]
- Derichs, N.; Sanz, J.; Von Kanel, T.; Stolpe, C.; Zapf, A.; Tümmler, B.; Gallati, S.; Ballmann, M. Intestinal Current Measurement for Diagnostic Classification of Patients with Questionable Cystic Fibrosis: Validation and Reference Data. Thorax 2010, 65, 594–599. [Google Scholar] [CrossRef]
- Guimbellot, J.; Sharma, J.; Rowe, S. Toward inclusive therapy with CFTR modulators: Progress and challenges. Pediatr. Pulmonol. 2017, 52, S4–S14. [Google Scholar] [CrossRef]
- Clancy, J.P.; Szczesniak, R.D.; Ashlock, M.A.; Ernst, S.E.; Fan, L.; Hornick, D.B.; Karp, P.H.; Khan, U.; Lymp, J.; Ostmann, A.J.; et al. Multicenter intestinal current measurements in rectal biopsies from CF and non-CF subjects to monitor CFTR function. PLoS ONE 2013, 8, e73905. [Google Scholar] [CrossRef] [PubMed]
- Vonk, A.M.; van Mourik, P.; Ramalho, A.S.; Silva, I.A.L.; Statia, M.; Kruisselbrink, E.; Suen, S.W.F.; Dekkers, J.F.; Vleggaar, F.P.; Houwen, R.H.J.; et al. Protocol for Application, Standardization and Validation of the Forskolin-Induced Swelling Assay in Cystic Fibrosis Human Colon Organoids. STAR Protoc. 2020, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, A.S.; Förstová, E.; Vonk, A.M.; Ferrante, M.; Verfailli, C.; Dupont, L.; Boon, M.; Proesmans, M.; Beekman, J.M.; Sarouk, I.; et al. Correction of CFTR Function in Intestinal Organoids to Guide Treatment of Cystic Fibrosis. Eur. Respir. J. 2021, 57, 1902426. [Google Scholar] [CrossRef] [PubMed]


| Genotype | [L467F;F508del]/ [L467F;F508del] | [L467F;F508del]/ c.3532_3535dup | [L467F;F508del]/ c.1766+2T>C | [L467F;F508del]/ W1310X | [L467F;F508del]/ 712-1G>T |
|---|---|---|---|---|---|
| № | 1 | 2 | 3 | 4 | 5 |
| Age at the time of the study, years | 7 | 16.5 | 16.5 | 10 | 7 |
| Gender m/f | m | f | f | f | m |
| Age of diagnosis of CF | 3 months | 2.5 years | 4 months | 2 months | 1 month |
| Sweat test, mmol/L | 118 * | 89 * | not performed | 98 ** | 120 * |
| Fecal elastase-1, μg/g of feces | <15 | <15 | <15 | <15 | <15 |
| BMI, kg/m2 | 13.3 | 20.3 | 16.7 | 15.9 | 14.6 |
| BMI, %o | 2.93 | 44 | 4.5 | 36.31 | 24.46 |
| FEV1, % | 91 | 78 | not performed | 110 | not performed |
| FVC, % | 87 | 71 | not performed | 116 | not performed |
| Chronic Pseudomonas aeruginosa | - | + | + | - | - |
| Chronic Staphylococcus aureus | + | - | + | + | + |
| Liver damage | Without cirrhosis | Cirrhosis without PH | - | Cirrhosis without PH | - |
| Polyposis of the paranasal sinuses | Sinusitis without polyps | + | + | + | + Operated on a year after the start of targeted therapy |
| Indicator | Period of the Targeted Therapy | Patient | ||
|---|---|---|---|---|
| L467F;F508del]/ [L467F;F508del] Patient 1 | [L467F;F508del]/ W1310X Patient 4 | [L467F;F508del]/ 712-1G>T Patient 5 | ||
| BMI (kg/m2) | 1 day (start) | 13.5 | - | 14.6 |
| 6 months | 13.3 | 15.9 | 14.6 | |
| 12 months | - | - | 14.6 | |
| Sweat test (mmol/L) | 1 day (start) | 104 * | 89 ** | 120 * |
| 6 months | 121 | 95 ** | - | |
| 12 months | - | - | 114 | |
| FVC, %pred | 1 day (start) | 84 | 120 | does not perform the maneuver |
| 6 months | 87.5 | 116 | does not perform the maneuver | |
| 12 months | - | - | does not perform the maneuver | |
| FEV1,% pred | 1 day (start) | 85 | 113 | does not perform the maneuver |
| 6 months | 91 | 110 | does not perform the maneuver | |
| 12 months | - | - | does not perform the maneuver | |
| Bronchopulmonary exacerbations | the period of therapy | 2–3 exacerbations on targeted therapy, as before the start of therapy | 3 exacerbations on targeted therapy, as before the start of therapy | 2 exacerbations on targeted therapy, as before the start of therapy |
| Changes of nasal polyposis and rhinosinusitis | the period of therapy | Without changes | Without changes | operated on due to nasal polyposis |
| ALT, U/L | 1 day (start) | 18 | 24 | 17 |
| 6 months | 25 | 20 | 25 | |
| 12 months | - | - | 18 | |
| AST, U/L | 1 day (start) | 26 | 30 | 29 |
| 6 months | 31 | 17 | 28 | |
| 12 months | 26 | 28 | 30 | |
| Total bilirubin, µmol/L | 1 day (start) | 6.9 | 7.8 | 12.3 |
| 6 months | 12.7 | 10.0 | 11.2 | |
| 12 months | - | - | 9.8 | |
| Systolic blood pressure, mmHg. | 1 day (start) | 90 | 95 | - |
| 6 months | 95 | 90 | - | |
| 12 months | - | - | - | |
| Diastolic blood pressure, mmHg | 1 day (start) | 60 | 65 | - |
| 6 months | 60 | 60 | - | |
| 12 months | - | - | - | |
| Genotype | Amiloride | Forskolin | Histamine | |
|---|---|---|---|---|
| [L467F;F508del]/ [L467F;F508del] Patient 1 | Biopsy № 1 | −4 | 4 | 8.5 |
| Biopsy № 2 | −4.5 | 4 | 5.5 | |
| Biopsy № 3 | −8 | 4.5 | 7 | |
| M ± m | −5.5 ± 1.54 | 4.17 ± 0.2 | 7.0 ± 1.06 | |
| [L467F;F508del]/ c.3532_3535dup Patient 2 | Biopsy № 1 | −1.5 | 0.5 | 3 |
| Biopsy № 2 | −2.5 | 0 | 4 | |
| Biopsy № 3 | −2 | 0.5 | 3.5 | |
| M ± m | −2 ± 0.35 | 0.33 ± 0.2 | 3.5 ± 0.35 | |
| [L467F;F508del]/ c.1766+2T>C Patient 3 | Biopsy № 1 | −3.5 | 0 | 3 |
| Biopsy № 2 | 0 | 0 | 3.5 | |
| Biopsy № 3 | −1.5 | 0 | 4 | |
| M ± m | −1.67 ± 3.78 | 0 | 3.5 ± 0.35 | |
| [L467F;F508del]/ W1310X Patient 4 | Biopsy № 1 | −10 | 0 | 9 |
| Biopsy № 2 | −10.5 | 0 | 8 | |
| Biopsy № 3 | −9 | 0 | 9.5 | |
| M ± m | −9.83 ± 0.54 | 0 | 8.83 ± 0.54 | |
| [L467F;F508del]/ 712-1G>T Patient 5 | Biopsy № 1 | −11.5 | 3.5 | 5 |
| Biopsy № 2 | −5.5 | 0 | 2.5 | |
| Biopsy № 3 | −5 | 1.5 | 2.5 | |
| M ± m | −7.33 ± 2.56 | 1.67 ± 1.24 | 3.33 ± 2.7 | |
| [L467F;F508del]/ class I | −5.21 ± 0.98 | 1.23 ± 0.48 | 5.23 ± 0.67 | |
| [L467F;F508del]/F508del * | −11.39 ± 0.79 | 2.5 ± 0.61 | 12.39 ± 1.98 | |
| F508del/F508del ** | −15.7 ± 3.51 | 3.33 ± 0.63 | 17.83 ± 3.57 | |
| Control group (wtCFTR/wtCFTR) ** | −7.67 ± 1.76 | 26.72 ± 2.66 | 109.76 ± 8.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremova, A.; Melyanovskaya, Y.; Krasnova, M.; Voronkova, A.; Mokrousova, D.; Zhekaite, E.; Bulatenko, N.; Makhnach, O.; Bukharova, T.; Kutsev, S.; et al. Estimation of Chloride Channel Residual Function and Assessment of Targeted Drugs Efficiency in the Presence of a Complex Allele [L467F;F508del] in the CFTR Gene. Int. J. Mol. Sci. 2024, 25, 10424. https://doi.org/10.3390/ijms251910424
Efremova A, Melyanovskaya Y, Krasnova M, Voronkova A, Mokrousova D, Zhekaite E, Bulatenko N, Makhnach O, Bukharova T, Kutsev S, et al. Estimation of Chloride Channel Residual Function and Assessment of Targeted Drugs Efficiency in the Presence of a Complex Allele [L467F;F508del] in the CFTR Gene. International Journal of Molecular Sciences. 2024; 25(19):10424. https://doi.org/10.3390/ijms251910424
Chicago/Turabian StyleEfremova, Anna, Yuliya Melyanovskaya, Maria Krasnova, Anna Voronkova, Diana Mokrousova, Elena Zhekaite, Nataliya Bulatenko, Oleg Makhnach, Tatiana Bukharova, Sergei Kutsev, and et al. 2024. "Estimation of Chloride Channel Residual Function and Assessment of Targeted Drugs Efficiency in the Presence of a Complex Allele [L467F;F508del] in the CFTR Gene" International Journal of Molecular Sciences 25, no. 19: 10424. https://doi.org/10.3390/ijms251910424






