Exploring Molecular Drivers of PARPi Resistance in BRCA1-Deficient Ovarian Cancer: The Role of LY6E and Immunomodulation
Abstract
1. Introduction
2. Results
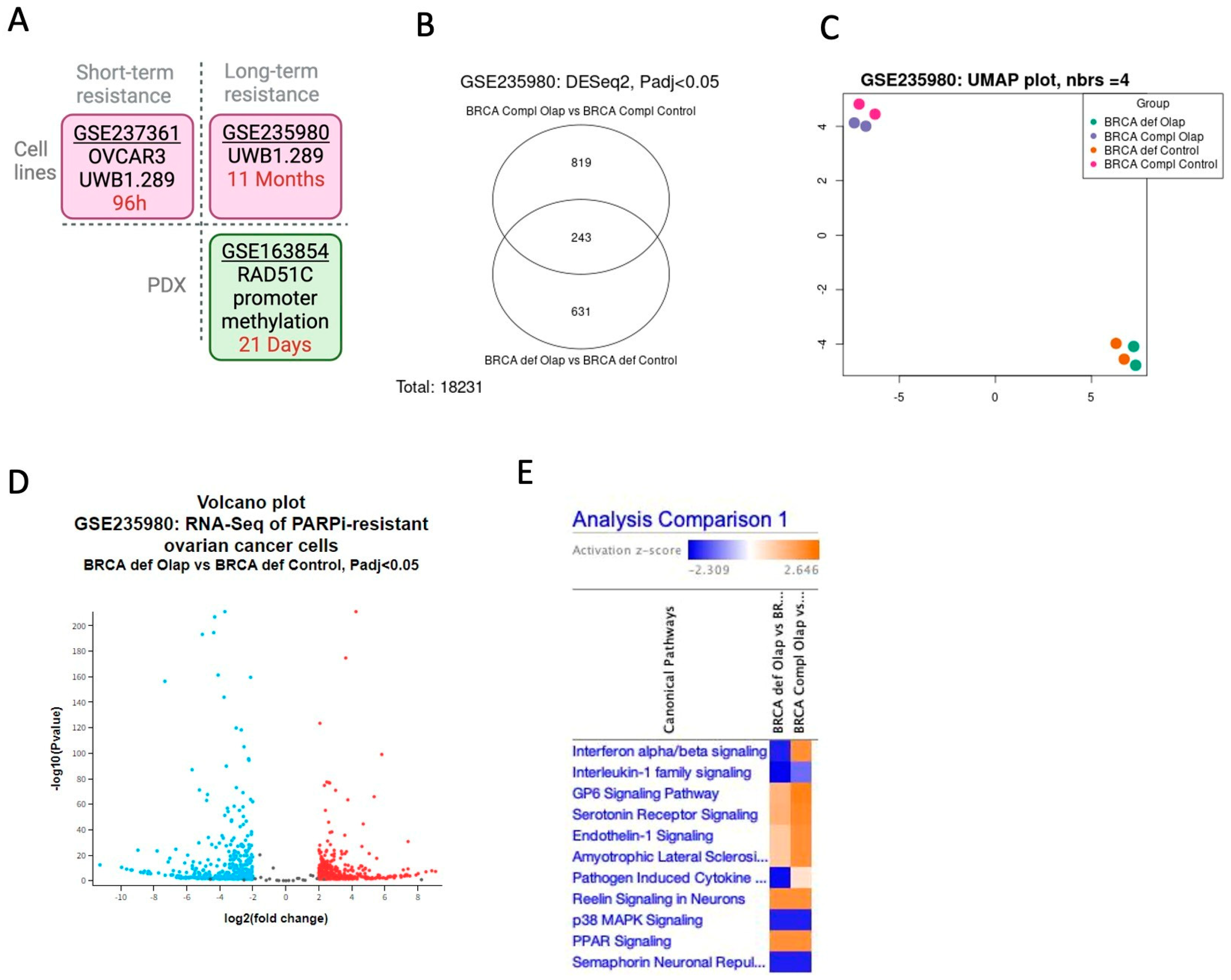
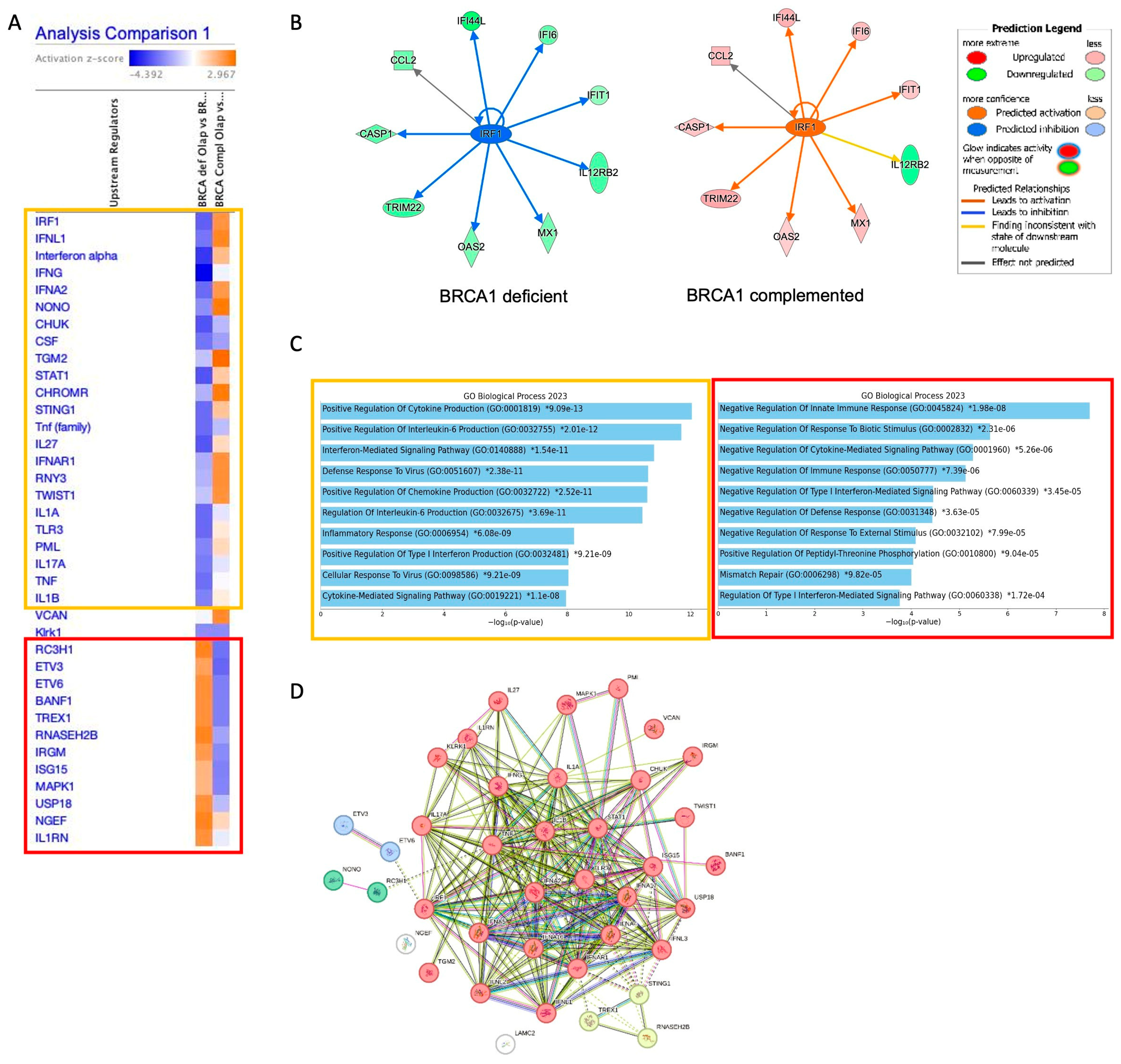
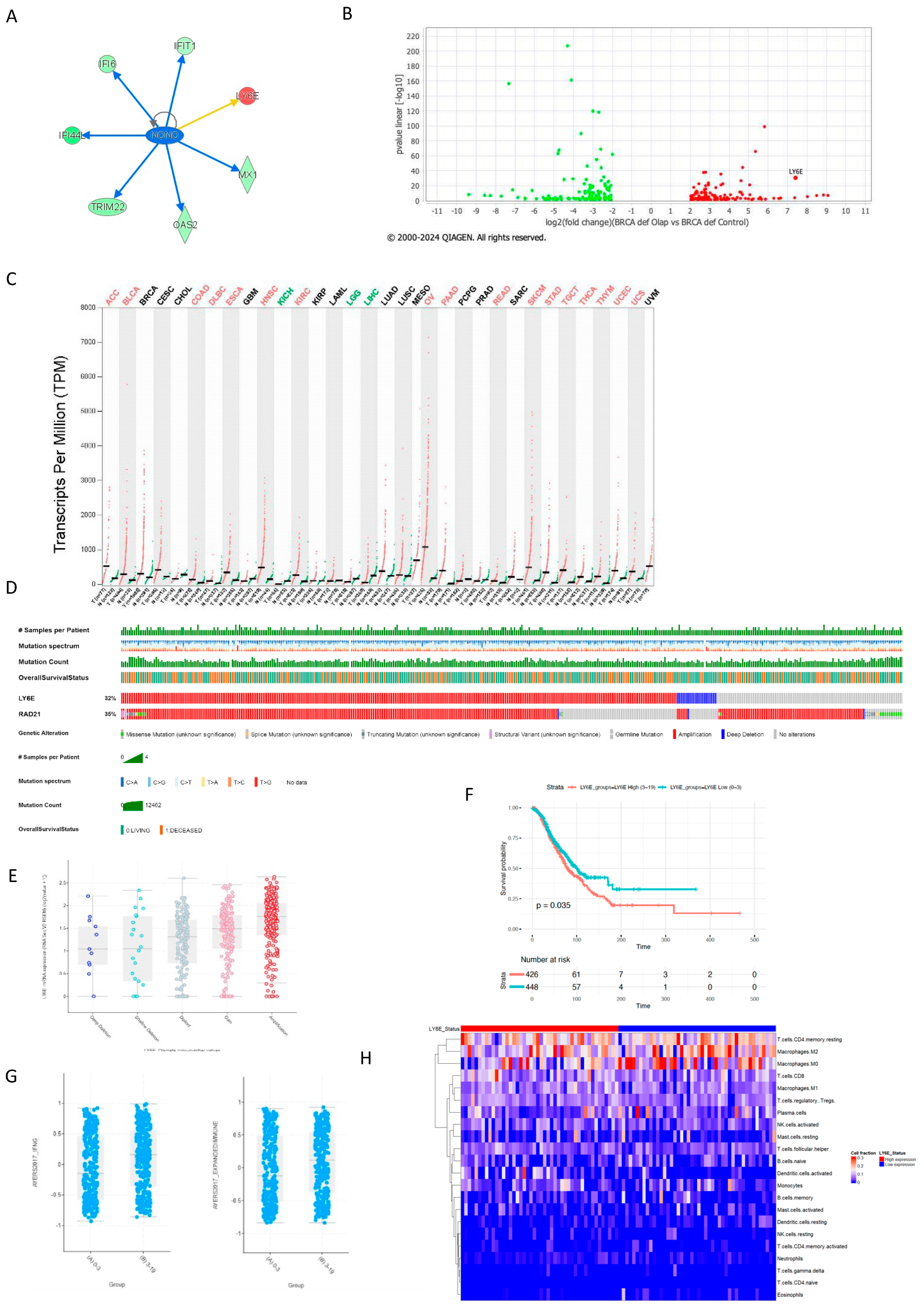
2.1. The Interferon Alpha/Beta Signaling Pathway Plays a Central Role in PARPi Response in BRCA1-Deficient Cells
2.2. BRCA1-Deficient Ovarian Cancer Cells Resistant to Olaparib Present a Positive Regulation of Cytokine as Interferon Production with Overexpression of LY6E
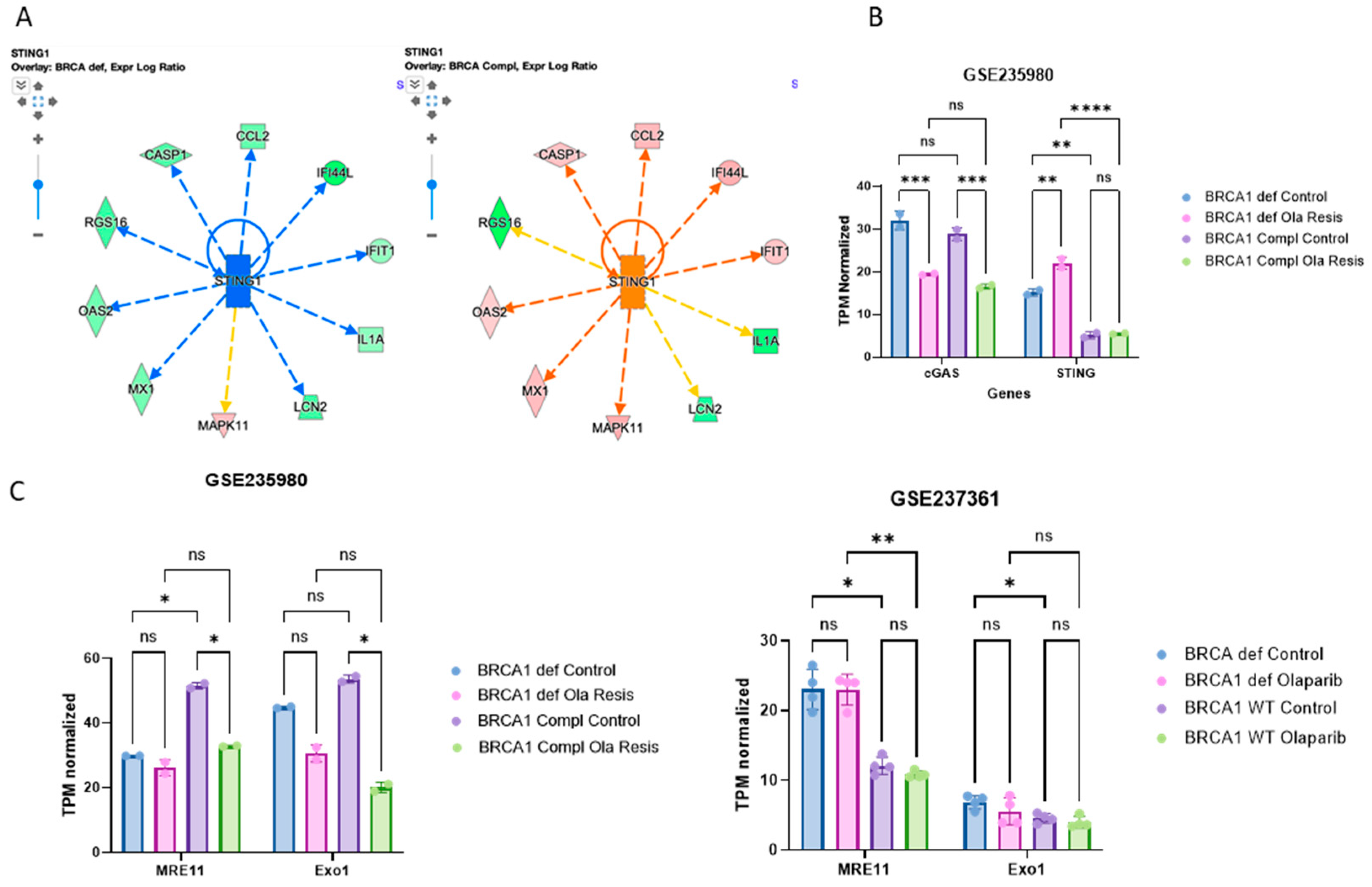
2.3. STING Response to Olaparib Requires BRCA1 but cGAS Downregulation Does Not
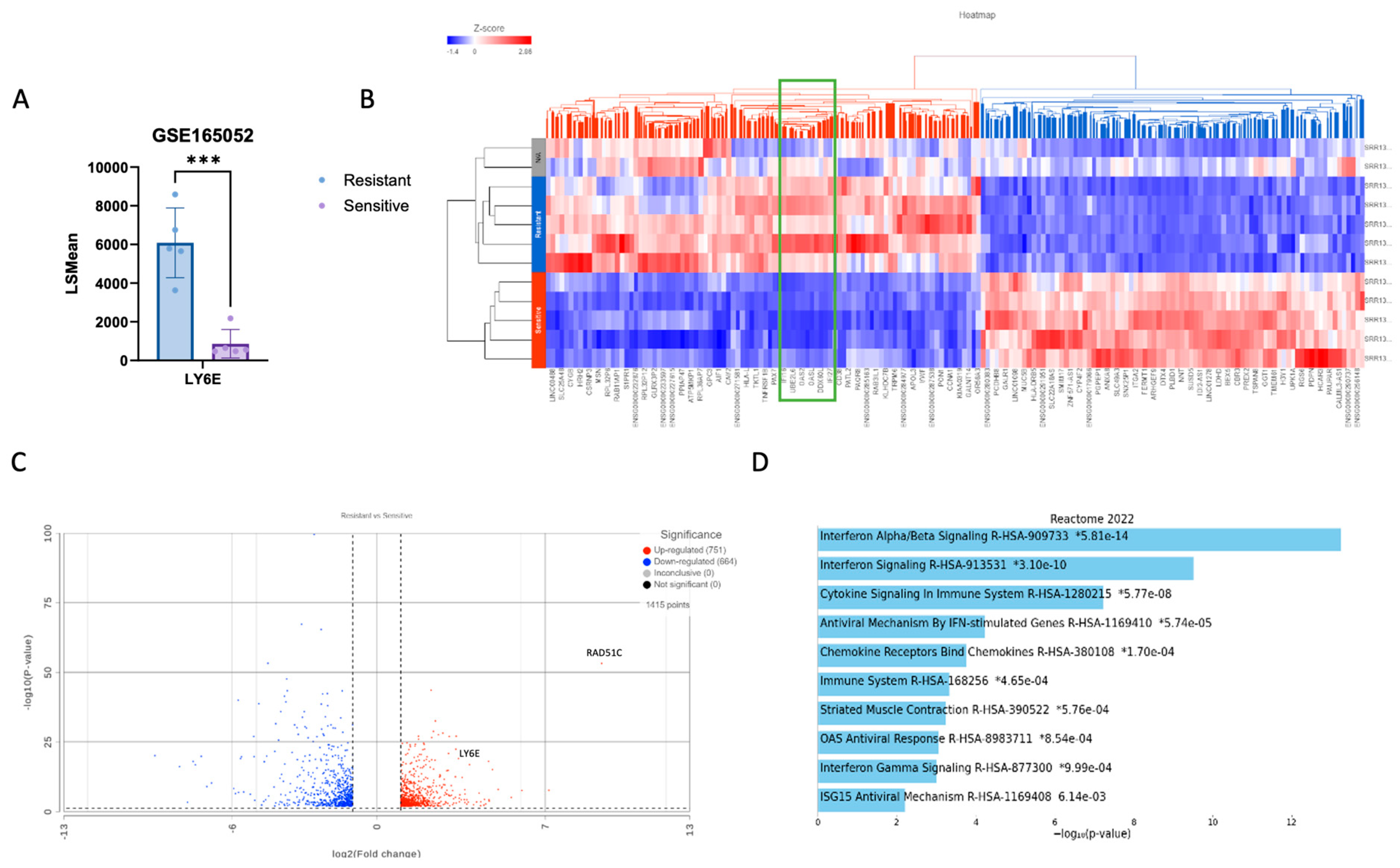
2.4. LY6E Amplification Is Also Present in PDX HRD PARPi-Resistant Tumors
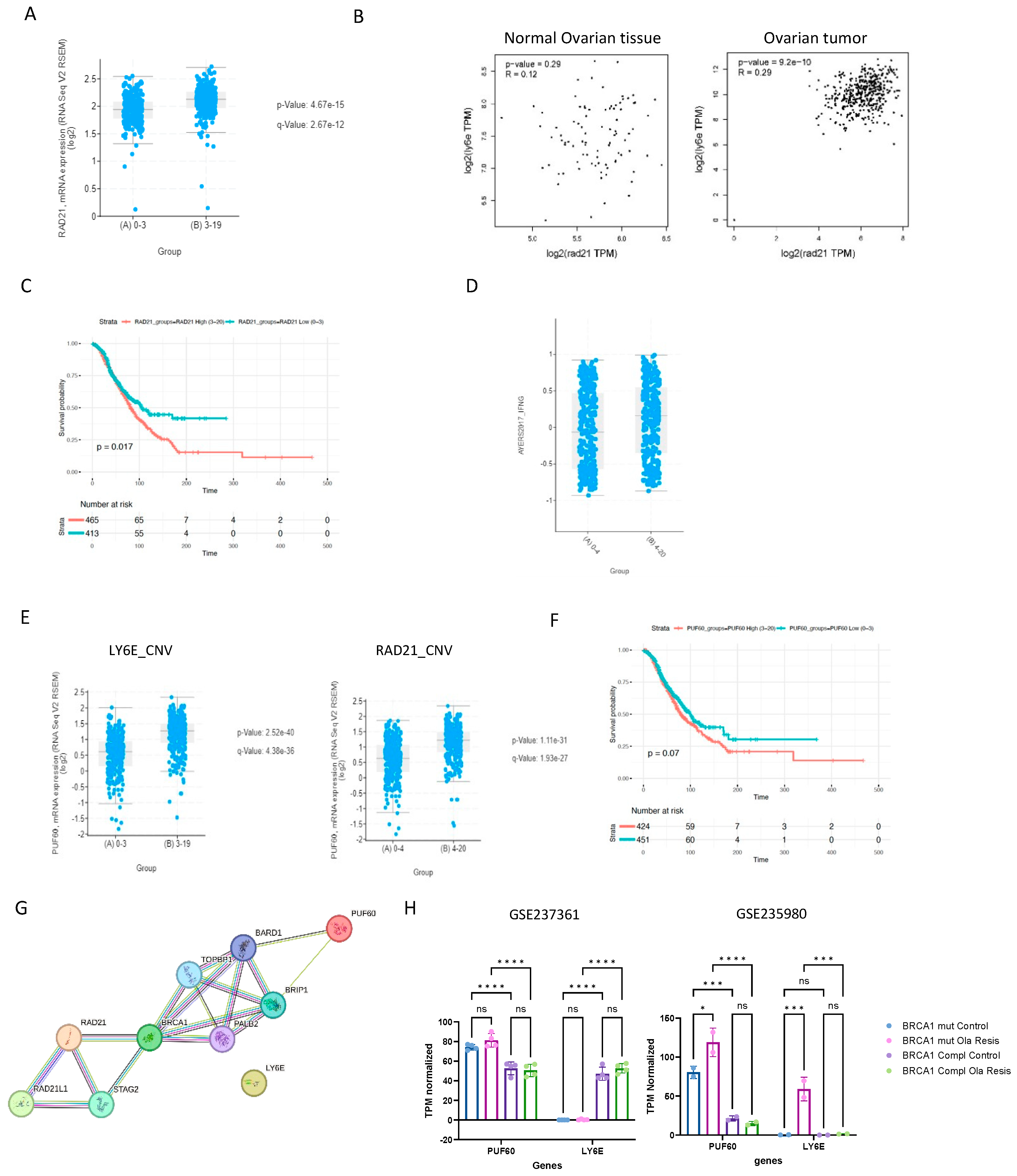
2.5. LY6E Is Amplified in Ovarian Cancer and Acts a Prognostic Biomarker
2.6. LY6E and Rad21 Share Correlation Expression in Ovarian Tumors
3. Discussion
4. Material and Methods
4.1. ORIEN/cBioPortal
4.2. Gene Expression Omnibus (GEO) Database (NCBI)
| GEO NCBI | PARPi | Treatment Period | Cells | Reference |
|---|---|---|---|---|
| GSE237361 | Olaparib | 96 h (short-time resistance) | UWB1.289 (BRCA-deficient) Ovca3 (BRCA WT) | [18] |
| GSE235980 | Olaparib, Niraparib, Talazoparib, Rucaparib, Veliparib | 11 months (long-time resistance) | UWB1.289 (BRCA-deficient) UWB1.289 + BRCA1 (BRCA-complemented) | [61] |
| GSE165054 | Niraparib | 21 days (long-time resistance) | PDX Rad51C promoter methylation (Resistant X Sensitive) | [62] |
4.3. Gene Expression Analysis
4.4. Ingenuity Pathway Analysis (IPA) QIAGEN
4.5. GEPIA2
4.6. Partek Flow v.11.0.24.0102
4.7. Protein–Protein Interaction Network Analysis
4.8. Ciberstortx
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statements
References
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Aghajanian, C.; Shapira-Frommer, R.; Rini, B.I.; Goss, G.; Hensley, M.L.; Schmutzler, R.K.; Gelmon, K.; Robson, M.E.; Olopade, O.I. Inhibition of Poly(ADP-Ribose) Polymerase in Patients with Breast and Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2359–2368. [Google Scholar] [CrossRef]
- Jiang, X.; Li, W.; Li, X.; Bai, H.; Zhang, Z. Current Status and Future Prospects of PARP Inhibitor Clinical Trials in Ovarian Cancer. Cancer Manag. Res. 2019, 11, 4371–4390. [Google Scholar] [CrossRef]
- Mirza, M.R.; Pignata, S.; Ledermann, J.A. Latest Clinical Evidence and Further Development of PARP Inhibitors in Ovarian Cancer. Ann. Oncol. 2018, 29, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib Monotherapy for Late-Line Treatment of Ovarian Cancer (QUADRA): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Timms, K.M.; Liu, S.; Chen, H.; Litton, J.K.; Potter, J.; Lanchbury, J.S.; Stemke-Hale, K.; Hennessy, B.T.; Arun, B.K.; et al. Incidence and Outcome of BRCA Mutations in Unselected Patients with Triple Receptor-Negative Breast Cancer. Clin. Cancer Res. 2011, 17, 1082–1089. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Patients with Platinum-Sensitive Relapsed Serous Ovarian Cancer: A Preplanned Retrospective Analysis of Outcomes by BRCA Status in a Randomised Phase 2 Trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira, R.; Santin, A.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Sehouli, J.; et al. Final Results from the KEYNOTE-100 Trial of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer. J. Clin. Oncol. 2020, 38, 6005. [Google Scholar] [CrossRef]
- Jackson, L.M.; Moldovan, G.-L. Mechanisms of PARP1 Inhibitor Resistance and Their Implications for Cancer Treatment. NAR Cancer 2022, 4, zcac042. [Google Scholar] [CrossRef] [PubMed]
- Panzarino, N.J.; Krais, J.J.; Cong, K.; Peng, M.; Mosqueda, M.; Nayak, S.U.; Bond, S.M.; Calvo, J.A.; Doshi, M.B.; Bere, M.; et al. Replication Gaps Underlie BRCA Deficiency and Therapy Response. Cancer Res. 2021, 81, 1388–1397. [Google Scholar] [CrossRef]
- Buckley, N.E.; Hosey, A.M.; Gorski, J.J.; Purcell, J.W.; Mulligan, J.M.; Harkin, D.P.; Mullan, P.B. BRCA1 Regulates IFN-γ Signaling through a Mechanism Involving the Type I IFNs. Mol. Cancer Res. 2007, 5, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, T.; Lee, S.W.; Ouchi, M.; Aaronson, S.A.; Horvath, C.M. Collaboration of Signal Transducer and Activator of Transcription 1 (STAT1) and BRCA1 in Differential Regulation of IFN-Gamma Target Genes. Proc. Natl. Acad. Sci. USA 2000, 97, 5208–5213. [Google Scholar] [CrossRef]
- Coquet, J.M.; Chakravarti, S.; Kyparissoudis, K.; McNab, F.W.; Pitt, L.A.; McKenzie, B.S.; Berzins, S.P.; Smyth, M.J.; Godfrey, D.I. Diverse Cytokine Production by NKT Cell Subsets and Identification of an IL-17–Producing CD4−NK1.1− NKT Cell Population. Proc. Natl. Acad. Sci. USA 2008, 105, 11287–11292. [Google Scholar] [CrossRef]
- Kreienkamp, R.; Graziano, S.; Coll-Bonfill, N.; Bedia-Diaz, G.; Cybulla, E.; Vindigni, A.; Dorsett, D.; Kubben, N.; Batista, L.F.Z.; Gonzalo, S. A Cell-Intrinsic Interferon-like Response Links Replication Stress to Cellular Aging Caused by Progerin. Cell Rep. 2018, 22, 2006–2015. [Google Scholar] [CrossRef]
- Surrallés, J.; De La Fuente, M.; García, A.; González, M.A.; López, F.; Tarsounas, M.; Cantó, C.; Gómez, A.; Tominari, T.; López, J.A. The MRE11-RAD50-NBS1 Complex Is Required for the Resolution of Single-Strand DNA Gaps Generated by BRCA1-Deficient Cells. J. Biol. Chem. 2020, 295, 13054–13066. [Google Scholar]
- Liu, Z.; Du, W.; Liu, Y.; Chen, H.; Liang, J.; Jiang, J.; Hu, Y.; Wu, L.; Xia, Z.; Shi, J. MRE11 Degradation of Single-Strand DNA Facilitates cGAS Activation, Independent of Its Nuclease Activity. Cell Rep. 2021, 35, 109139. [Google Scholar] [CrossRef]
- Cho, M.-G.; Kumar, R.J.; Lin, C.-C.; Boyer, J.A.; Shahir, J.A.; Fagan-Solis, K.; Simpson, D.A.; Fan, C.; Foster, C.E.; Goddard, A.M.; et al. MRE11 Liberates cGAS from Nucleosome Sequestration during Tumorigenesis. Nature 2024, 625, 585–592. [Google Scholar] [CrossRef]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory Functions of Type I Interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Kuppusamy, P.; Narayanan, R.; Pradhan, S.; Ray, S.; Ghosh, S.; Das, S.; Ghosh, A.; Liu, X.; Zhuang, Y. BRCA1 Localizes at Promoters of Type I Interferon Signaling Molecules and Regulates Their Up-Regulation. Cell Rep. 2020, 32, 107950. [Google Scholar] [CrossRef]
- Gronauer, R.; Madersbacher, L.; Monfort-Lanzas, P.; Floriani, G.; Sprung, S.; Zeimet, A.G.; Marth, C.; Fiegl, H.; Hackl, H. Integrated Immunogenomic Analyses of High-Grade Serous Ovarian Cancer Reveal Vulnerability to Combination Immunotherapy. medRxiv 2024. [Google Scholar] [CrossRef]
- Zimmermann, M.; Murina, O.; Reijns, M.A.M.; Agathanggelou, A.; Challis, R.; Tarnauskaitė, Ž.; Muir, M.; Fluteau, A.; Aregger, M.; McEwan, A.; et al. CRISPR Screens Identify Genomic Ribonucleotides as a Source of PARP-Trapping Lesions. Nature 2018, 559, 285–289. [Google Scholar] [CrossRef]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Sasaki, N.; Homme, M.; Kitajima, S. Targeting the Loss of cGAS/STING Signaling in Cancer. Cancer Sci. 2023, 114, 3806–3815. [Google Scholar] [CrossRef]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, Evolution and Functions of the Human and Mouse Ly6/uPAR Family Genes. Hum. Genom. 2016, 10, 10. [Google Scholar] [CrossRef]
- Luo, L.; McGarvey, P.; Madhavan, S.; Kumar, R.; Gusev, Y.; Upadhyay, G. Distinct Lymphocyte Antigens 6 (Ly6) Family Members Ly6D, Ly6E, Ly6K and Ly6H Drive Tumorigenesis and Clinical Outcome. Oncotarget 2016, 7, 11165–11193. [Google Scholar] [CrossRef]
- AlHossiny, M.; Luo, L.; Frazier, W.R.; Steiner, N.; Gusev, Y.; Kallakury, B.; Glasgow, E.; Creswell, K.; Madhavan, S.; Kumar, R.; et al. Ly6E/K Signaling to TGFβ Promotes Breast Cancer Progression, Immune Escape, and Drug Resistance. Cancer Res. 2016, 76, 3376–3386. [Google Scholar] [CrossRef]
- Ablasser, A.; Chen, Z.J. cGAS in Action: Expanding Roles in Immunity and Inflammation. Science 2019, 363, eaat8657. [Google Scholar] [CrossRef]
- Unterholzner, L.; Dunphy, G. cGAS-Independent STING Activation in Response to DNA Damage. Mol. Cell. Oncol. 2019, 6, 1558682. [Google Scholar] [CrossRef] [PubMed]
- Buis, J.; Wu, Y.; Deng, Y.; Leddon, J.; Westfield, G.; Eckersdorff, M.; Sekiguchi, J.M.; Chang, S.; Ferguson, D.O. Mre11 Nuclease Activity Has Essential Roles in DNA Repair and Genomic Stability Distinct from ATM Activation. Cell 2008, 135, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lemaçon, D.; Jackson, J.; Quinet, A.; Brickner, J.R.; Li, S.; Yazinski, S.; You, Z.; Ira, G.; Zou, L.; Mosammaparast, N.; et al. MRE11 and EXO1 Nucleases Degrade Reversed Forks and Elicit MUS81-Dependent Fork Rescue in BRCA2-Deficient Cells. Nat. Commun. 2017, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Quinet, A.; Tirman, S.; Jackson, J.; Šviković, S.; Lemaçon, D.; Carvajal-Maldonado, D.; González-Acosta, D.; Vessoni, A.T.; Cybulla, E.; Wood, M.; et al. PRIMPOL-Mediated Adaptive Response Suppresses Replication Fork Reversal in BRCA-Deficient Cells. Mol. Cell 2020, 77, 461–474.e9. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, Q.; Martincuks, A.; Kearns, M.J.; Jiang, T.; Lin, Z.; Cheng, X.; Qian, C.; Xie, S.; Kim, H.-J.; et al. STING Agonism Overcomes STAT3-Mediated Immunosuppression and Adaptive Resistance to PARP Inhibition in Ovarian Cancer. J. Immunother. Cancer 2023, 11, e005627. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Wang, Y.; Li, Y.; Wang, W.; Liu, Y.; Li, Y.; Ma, J.; Zhang, L.; Liu, X. IFI16 and DNA Repair Proteins ATM and PARP1 Activate STING in a Non-Canonical Pathway. Nat. Commun. 2021, 12, 5678. [Google Scholar]
- Huang, Y.; Zhao, Y.; Jiang, J.; Zhang, X.; Zhang, H.; Liu, S.; Zhang, S.; Zhang, J.; Li, C.; Lin, X. STING Agonism Reprograms Myeloid Cells in the Tumor Microenvironment of PARPi-Resistant Ovarian Tumors and Overcomes Resistance. Cancer Immunol. Res. 2023, 11, 2114–2127. [Google Scholar]
- Yu, D.; Huang, C.-J.; Tucker, H.O. Established and Evolving Roles of the Multifunctional Non-POU Domain-Containing Octamer-Binding Protein (NonO) and Splicing Factor Proline- and Glutamine-Rich (SFPQ). J. Dev. Biol. 2024, 12, 3. [Google Scholar] [CrossRef]
- Petti, E.; Buemi, V.; Zappone, A.; Schillaci, O.; Broccia, P.V.; Dinami, R.; Matteoni, S.; Benetti, R.; Schoeftner, S. SFPQ and NONO Suppress RNA:DNA-Hybrid-Related Telomere Instability. Nat. Commun. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–Related mRNA Profile Predicts Clinical Response to PD-1 Blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Speiser, D.E.; Chijioke, O.; Schaeuble, K.; Münz, C. CD4+ T Cells in Cancer. Nat. Cancer 2023, 4, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Kumagai, K.; Kobayashi, T.; Saito, T. CD4+ T Cells: Key Players in the Regulation of Immune Responses. Front. Immunol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Neoantigen Burden as a Predictor of Clinical Benefit to Immune Checkpoint Blockade. Science 2014, 346, 875–881. [Google Scholar] [CrossRef]
- Kim, C.; Wang, X.-D.; Yu, Y. PARP1 Inhibitors Trigger Innate Immunity via PARP1 Trapping-Induced DNA Damage Response. eLife 2020, 9, e60637. [Google Scholar] [CrossRef]
- Benguigui, M.; Cooper, T.J.; Kalkar, P.; Schif-Zuck, S.; Halaban, R.; Bacchiocchi, A.; Kamer, I.; Deo, A.; Manobla, B.; Menachem, R.; et al. Interferon-Stimulated Neutrophils as a Predictor of Immunotherapy Response. Cancer Cell 2024, 42, 253–265.e12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, K.; Xiao, Y.; Feng, B.; Mikule, K.; Ma, X.; Feng, N.; Vellano, C.P.; Federico, L.; Marszalek, J.R.; et al. Niraparib Activates Interferon Signaling and Potentiates Anti-PD-1 Antibody Efficacy in Tumor Models. Sci. Rep. 2019, 9, 1853. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Liu, J.; Song, W.; Wang, Q.; Li, Y.; Zhang, X.; Xu, L. CNV and Elevated Expression of PUF60 Are Associated with Cancer Progression and Poor Prognosis. Oncotarget 2016, 7, 25710–25721. [Google Scholar] [CrossRef]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The Landscape of Somatic Copy-Number Alteration across Human Cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- Matejcic, M.; Saunders, E.J.; Dadaev, T.; Brook, M.N.; Wang, K.; Sheng, X.; Al Olama, A.A.; Schumacher, F.R.; Ingles, S.A.; Govindasami, K.; et al. Germline variation at 8q24 and prostate cancer risk in men of European ancestry. Nat. Commun. 2018, 9, 4616. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, M.; Wang, Z.; Yu, L.; Zhang, L.; Yan, Y.; Chen, L.; Zhang, C.; Chen, L. LY6E Overexpression Promotes Cancer Cell Growth and Metastasis in Breast Cancer, Gastric Cancer, and Lung Cancer. Oncogene 2018, 37, 4569–4578. [Google Scholar] [CrossRef]
- Fultang, L.; Booth, S.; Yogev, O.; Danilo, M.; Singh, S.; Jin, W.; Xie, C.; Edwards, N.J.; Tarrats, N.; Jimenez, L.; et al. LY6E Impacts T Cell Functions and Modulates Immune Checkpoint Signaling in Cancer. Nat. Commun. 2020, 11, 4533. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, H.; Wang, S.; Wang, S.; Yang, H.; Zhang, H.; Nie, C.; Yang, C.; Zhu, M.; Wang, D.; et al. LY6E and IFI27 Are Upregulated in Response to Immune Activation via the cGAS–STING Pathway and Promote Interferon Production. Front. Immunol. 2018, 9, 3165. [Google Scholar] [CrossRef]
- Jiang, D.; Guo, S.; Wang, X.; Chen, M.; Zhang, Y.; Cao, C.; Zhang, Q.; Chen, Y. IFI27 Is Localized to the Mitochondrial Membrane and Involved in Regulating Mitochondrial Integrity and Apoptosis. Cell Death Dis. 2020, 11, 957. [Google Scholar]
- Kuo, C.Y.; Chen, J.H.; Liu, T.C.; Huang, Y.T.; Chou, Y.C.; Wu, Y.C.; Chen, T.W.; Chang, H.C.; Chang, S.Y. Altered Mitochondrial Dynamics and Energy Metabolism in PARPi-Resistant Ovarian Cancer Cells as Adaptive Mechanisms for Survival. Cancer Lett. 2021, 518, 85–94. [Google Scholar] [CrossRef]
- Li, T.; Zhao, J.; Wang, C.; Wu, Z.; Liu, X.; Zhang, L.; Zhang, H.; Yang, X. Overexpression of IFI27 Contributes to Drug Resistance and Apoptosis Resistance in Cancer Cells, Supporting Survival Despite Chemotherapy. Cancer Sci. 2020, 111, 3206–3217. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, H.; Wu, J.; Wang, J.; Liu, X.; Liu, C.; Zhang, X.; Zhao, Y.; Zheng, Y.; Liu, C. IFI27 Interacts with RAD21 to Regulate Nuclear DNA Damage Response and DNA Repair Mechanisms. Cell Rep. 2021, 37, 110074. [Google Scholar]
- Hauf, S.; Waizenegger, I.C.; Peters, J.M. Cohesin Cleavage by Separase Required for Anaphase and Cytokinesis in Human Cells. Science 2001, 293, 1320–1323. [Google Scholar] [CrossRef]
- Yan, M.; Xu, H.; Waddell, N.; Shield-Artin, K.; Haviv, I.; kConFab authors; McKay, M.J.; Fox, S.B. Enhanced RAD21 Cohesin Expression Confers Poor Prognosis in BRCA2 and BRCAX, but Not BRCA1 Familial Breast Cancers. Breast Cancer Res. BCR 2012, 14, R69. [Google Scholar] [CrossRef]
- Zhang, C.; Ni, X.; Tao, C.; Zhou, Z.; Wang, F.; Gu, F.; Cui, X.; Jiang, S.; Li, Q.; Lu, H.; et al. Targeting PUF60 Prevents Tumor Progression by Retarding mRNA Decay of Oxidative Phosphorylation in Ovarian Cancer. Cell. Oncol. 2024, 47, 157–174. [Google Scholar] [CrossRef]
- Sun, D.; Lei, W.; Hou, X.; Li, H.; Ni, W. PUF60 Accelerates the Progression of Breast Cancer through Downregulation of PTEN Expression. Cancer Manag. Res. 2019, 11, 821–830. [Google Scholar] [CrossRef]
- Klotz, D.M.; Schwarz, F.M.; Dubrovska, A.; Schuster, K.; Theis, M.; Krüger, A.; Kutz, O.; Link, T.; Wimberger, P.; Drukewitz, S.; et al. Establishment and Molecular Characterization of an In Vitro Model for PARPi-Resistant Ovarian Cancer. Cancers 2023, 15, 3774. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.M.; McGehee, C.D.; Nesic, K.; Correia, C.; Weiskittel, T.M.; Kelly, R.L.; Venkatachalam, A.; Hou, X.; Pathoulas, N.M.; Meng, X.W.; et al. Characterization of a RAD51C -Silenced High-Grade Serous Ovarian Cancer Model during Development of PARP Inhibitor Resistance. NAR Cancer 2021, 3, zcab028. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. Clifton NJ 2018, 1711, 243–259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petta, T.B.; Carlson, J. Exploring Molecular Drivers of PARPi Resistance in BRCA1-Deficient Ovarian Cancer: The Role of LY6E and Immunomodulation. Int. J. Mol. Sci. 2024, 25, 10427. https://doi.org/10.3390/ijms251910427
Petta TB, Carlson J. Exploring Molecular Drivers of PARPi Resistance in BRCA1-Deficient Ovarian Cancer: The Role of LY6E and Immunomodulation. International Journal of Molecular Sciences. 2024; 25(19):10427. https://doi.org/10.3390/ijms251910427
Chicago/Turabian StylePetta, Tirzah Braz, and Joseph Carlson. 2024. "Exploring Molecular Drivers of PARPi Resistance in BRCA1-Deficient Ovarian Cancer: The Role of LY6E and Immunomodulation" International Journal of Molecular Sciences 25, no. 19: 10427. https://doi.org/10.3390/ijms251910427
APA StylePetta, T. B., & Carlson, J. (2024). Exploring Molecular Drivers of PARPi Resistance in BRCA1-Deficient Ovarian Cancer: The Role of LY6E and Immunomodulation. International Journal of Molecular Sciences, 25(19), 10427. https://doi.org/10.3390/ijms251910427







