Systemic Immune Factors and Risk of Allergic Contact Dermatitis: A Bidirectional Mendelian Randomization Study
Abstract
1. Introduction
2. Results
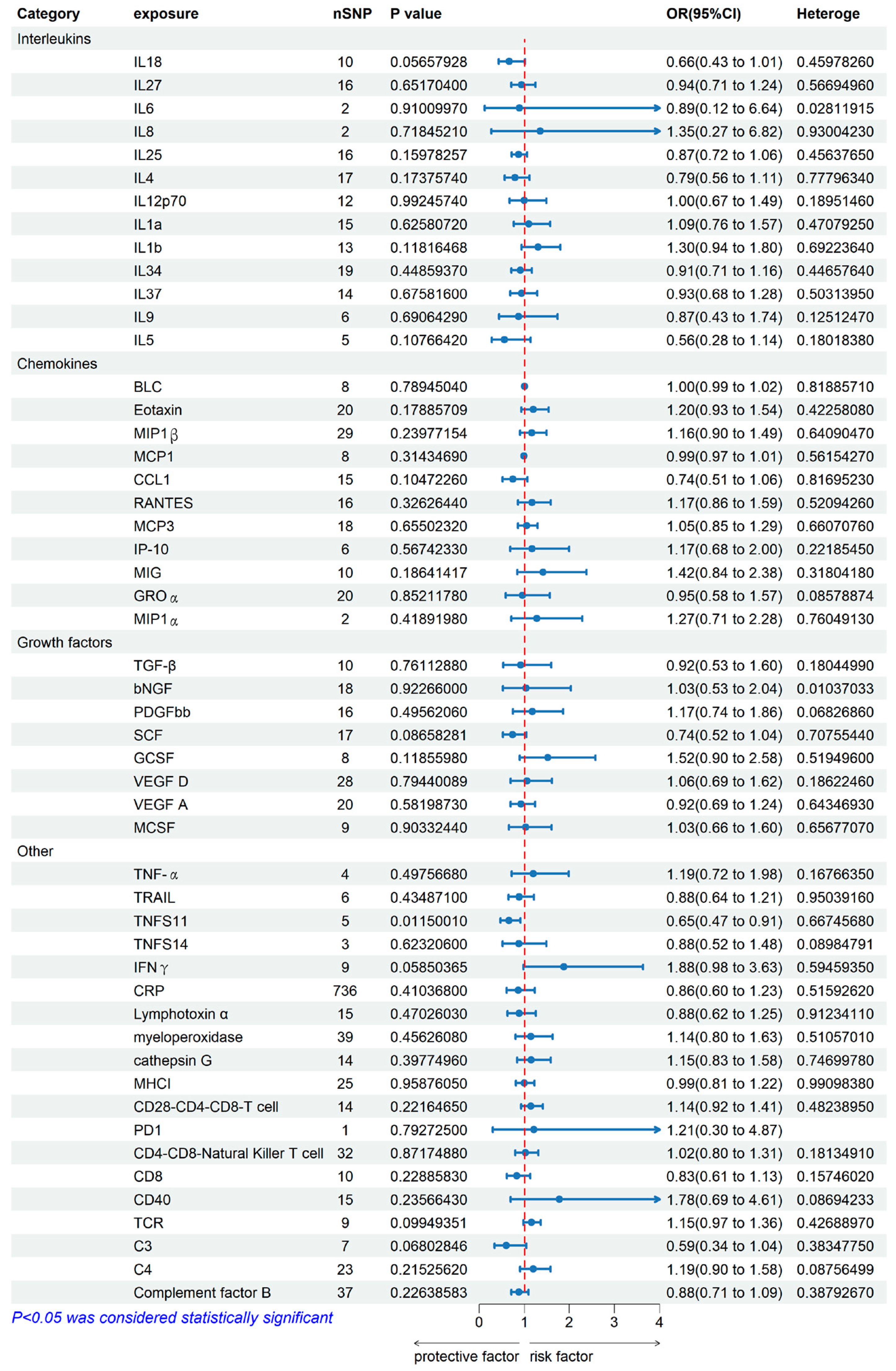
2.1. Genetically Predicted Systemic Immune Factors on Risk of Allergic Contact Dermatitis
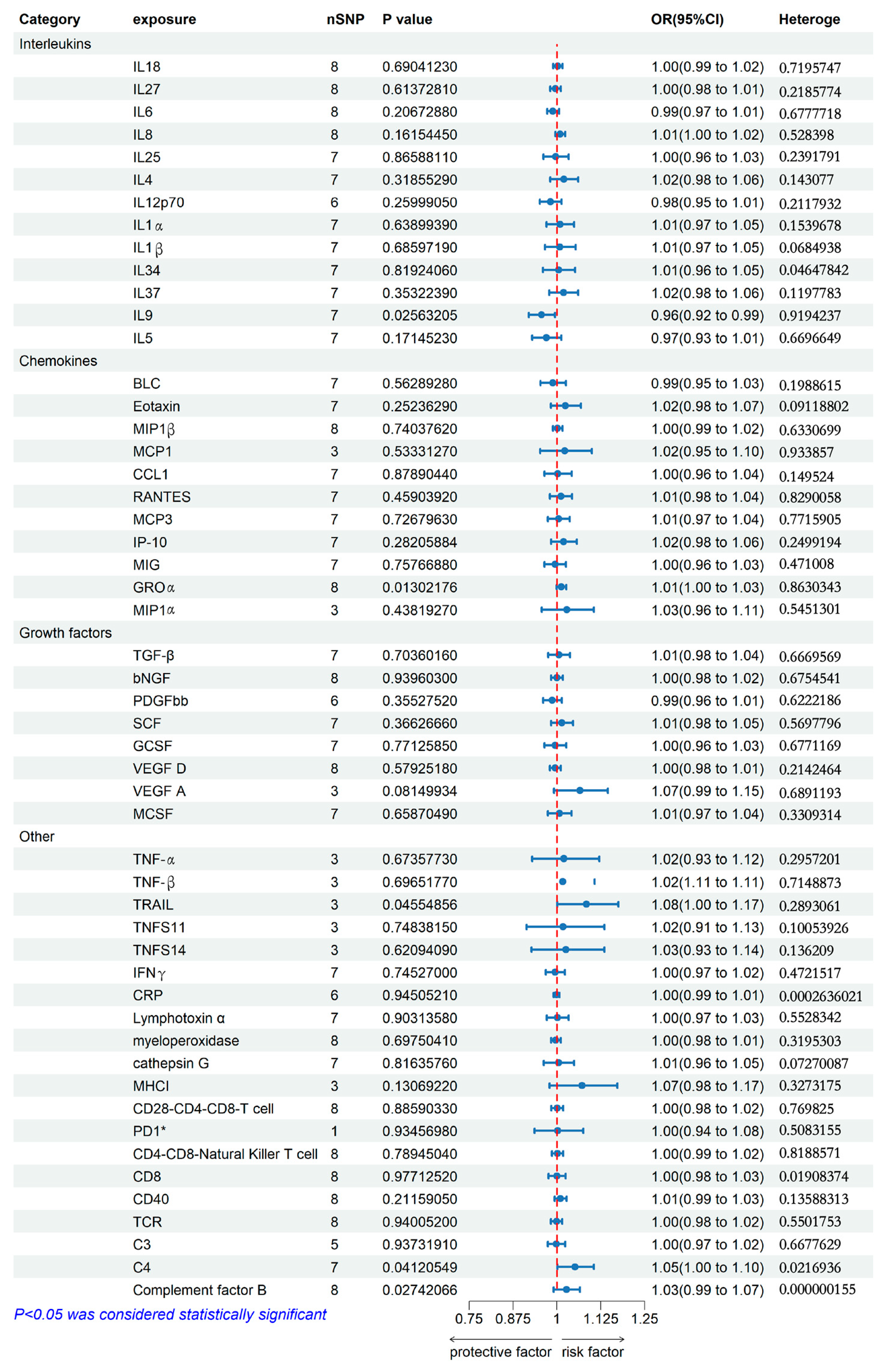
2.2. Genetically Predicted Allergic Contact Dermatitis on Systemic Immune Factor Levels
3. Discussion
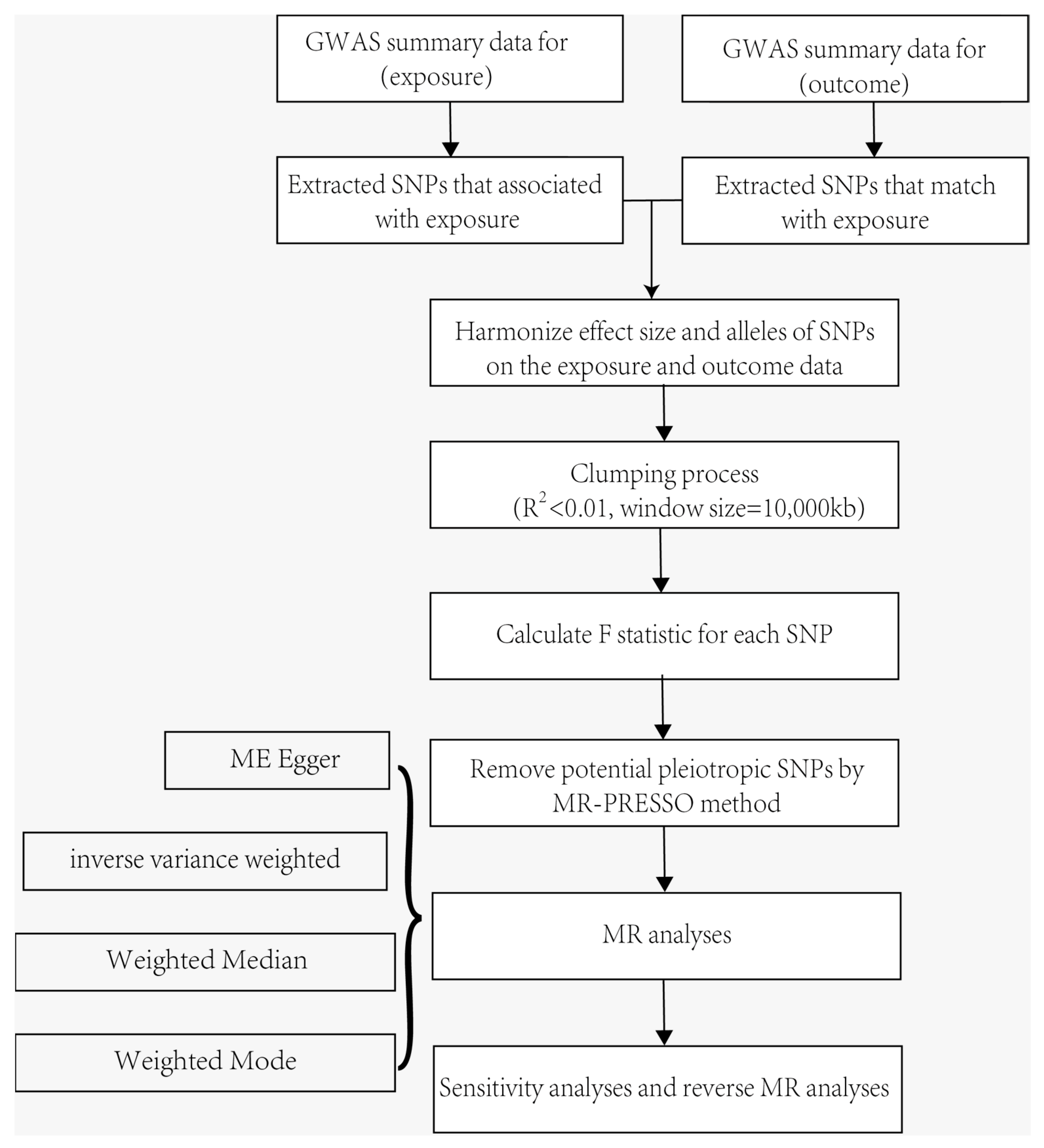
4. Methods
4.1. MR and Assumptions
4.2. Genetic Associations with Systemic Immune Factors and Allergic Contact Dermatitis
4.3. Selection of Genetic Instruments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; DeLeo, V.A. Allergic Contact Dermatitis. JAMA Dermatol. 2021, 157, 364. [Google Scholar] [CrossRef] [PubMed]
- Bonitsis, N.G.; Tatsioni, A.; Bassioukas, K.; Ioannidis, J.P. Allergens responsible for allergic contact dermatitis among children: A systematic review and meta-analysis. Contact Dermat. 2011, 64, 245–257. [Google Scholar] [CrossRef]
- Zhang, Z.; Malewicz, N.M.; Xu, X.; Pan, J.; Kumowski, N.; Zhu, T.; Shimada, S.G.; Nie, H.; LaMotte, R.H. Differences in itch and pain behaviors accompanying the irritant and allergic contact dermatitis produced by a contact allergen in mice. Pain Rep. 2019, 4, e781. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Limjunyawong, N.; Narang, C.; Jamaldeen, H.; Yu, S.; Patiram, S.; Nie, H.; Caterina, M.J.; Dong, X.; et al. Sensory neuron-expressed TRPC3 mediates acute and chronic itch. Pain 2023, 164, 98–110. [Google Scholar] [CrossRef]
- Novak-Bilic, G.; Vucic, M.; Japundzic, I.; Mestrovic-Stefekov, J.; Stanic-Duktaj, S.; Lugovic-Mihic, L. Irritant and Allergic Contact Dermatitis—Skin Lesion Characteristics. Acta Clin. Croat. 2018, 57, 713–720. [Google Scholar] [CrossRef]
- van Rijt, L.S.; Utsch, L.; Lutter, R.; van Ree, R. Oxidative Stress: Promoter of Allergic Sensitization to Protease Allergens? Int. J. Mol. Sci. 2017, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Zhang, J.; Liu, A.; Wang, P.; Xu, J. A Bidirectional Mendelian Randomization Study of Sarcopenia-Related Traits and Knee Osteoarthritis. Clin. Interv. Aging 2023, 18, 1577–1586. [Google Scholar] [CrossRef]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Q.; Wang, Z.; Lu, J.; Wang, R. Systemic inflammatory regulators and proliferative diabetic retinopathy: A bidirectional Mendelian randomization study. Front. Immunol. 2023, 14, 1088778. [Google Scholar] [CrossRef]
- Yeung, C.; Schooling, C.M. Systemic inflammatory regulators and risk of Alzheimer’s disease: A bidirectional Mendelian-randomization study. Int. J. Epidemiol. 2021, 50, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, Y.; Rasheed, H.; Walker, V.; Sugawara, Y.; Li, J.; Leng, Y.; Elsworth, B.; Wootton, R.E.; Fang, S.; et al. Trans-ethnic Mendelian-randomization study reveals causal relationships between cardiometabolic factors and chronic kidney disease. Int. J. Epidemiol. 2022, 50, 1995–2010. [Google Scholar] [CrossRef] [PubMed]
- Balmert, S.C.; Donahue, C.; Vu, J.R.; Erdos, G.; Falo, L.J.; Little, S.R. In vivo induction of regulatory T cells promotes allergen tolerance and suppresses allergic contact dermatitis. J. Control. Release 2017, 261, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xue, H.B.; Guan, X.H.; Shu, C.M.; Wang, F.; Zhang, J.H.; An, R.Z. The Imbalance of Th17 cells and CD4+ CD25high Foxp3+ Treg cells in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Rajagopal, S.; Atwater, A.R. Chemokine Signaling in Allergic Contact Dermatitis: Toward Targeted Therapies. Dermatitis 2018, 29, 179–186. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef]
- Sakai, T. Fracture risks and their mechanisms in atopic dermatitis, focusing on receptor activator of nuclear factor kappa-B ligand. Clin. Exp. Dermatol. 2023, 48, 1209–1213. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Loser, K.; Mehling, A.; Loeser, S.; Apelt, J.; Kuhn, A.; Grabbe, S.; Schwarz, T.; Penninger, J.M.; Beissert, S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 2006, 12, 1372–1379. [Google Scholar] [CrossRef]
- Soontrapa, K.; Honda, T.; Sakata, D.; Yao, C.; Hirata, T.; Hori, S.; Matsuoka, T.; Kita, Y.; Shimizu, T.; Kabashima, K.; et al. Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc. Natl. Acad. Sci. USA 2011, 108, 6668–6673. [Google Scholar] [CrossRef]
- Angkasekwinai, P.; Dong, C. IL-9-producing T cells: Potential players in allergy and cancer. Nat. Rev. Immunol. 2021, 21, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, P. Th9 Cells in Allergic Disease. Curr. Allergy Asthma Rep. 2019, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Renert-Yuval, Y.; Del, D.E.; Pavel, A.B.; Fang, M.; Lefferdink, R.; Wu, J.; Diaz, A.; Estrada, Y.D.; Canter, T.; Zhang, N.; et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J. Allergy Clin. Immunol. 2021, 148, 148–163. [Google Scholar] [CrossRef]
- Schlapbach, C.; Gehad, A.; Yang, C.; Watanabe, R.; Guenova, E.; Teague, J.E.; Campbell, L.; Yawalkar, N.; Kupper, T.S.; Clark, R.A. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci. Transl. Med. 2014, 6, 219ra8. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gery, I. The unique features of Th9 cells and their products. Crit. Rev. Immunol. 2012, 32, 1–10. [Google Scholar] [CrossRef]
- Mohamad, Z.N.; Shiota, J.; Calder, A.N.; Keeley, T.M.; Allen, B.L.; Nakao, K.; Samuelson, L.C.; Razumilava, N. C-X-C motif chemokine ligand 1 induced by Hedgehog signaling promotes mouse extrahepatic bile duct repair after acute injury. Hepatology 2022, 76, 936–950. [Google Scholar] [CrossRef]
- Terui, T.; Ozawa, M.; Tagami, H. Role of neutrophils in induction of acute inflammation in T-cell-mediated immune dermatosis, psoriasis: A neutrophil-associated inflammation-boosting loop. Exp. Dermatol. 2000, 9, 1–10. [Google Scholar] [CrossRef]
- Raychaudhuri, S.P.; Jiang, W.Y.; Farber, E.M.; Schall, T.J.; Ruff, M.R.; Pert, C.B. Upregulation of RANTES in psoriatic keratinocytes: A possible pathogenic mechanism for psoriasis. Acta Derm.-Venereol. 1999, 79, 9–11. [Google Scholar] [CrossRef]
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Taraborrelli, L.; Peltzer, N.; Montinaro, A.; Kupka, S.; Rieser, E.; Hartwig, T.; Sarr, A.; Darding, M.; Draber, P.; Haas, T.L.; et al. LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat. Commun. 2018, 9, 3910. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef] [PubMed]
- Kapp, A.; Wokalek, H.; Schopf, E. Involvement of complement in psoriasis and atopic dermatitis--measurement of C3a and C5a, C3, C4 and C1 inactivator. Arch. Dermatol. Res. 1985, 277, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Del, M.A.; Sellitto, A.; Solaro, E.; Esposito, S.; Cuomo, G. Tocilizumab reduces complement C3 and C4 serum levels in rheumatoid arthritis patients. Clin. Rheumatol. 2018, 37, 1695–1700. [Google Scholar] [CrossRef]
- Schaid, T.J.; Hansen, K.C.; Sauaia, A.; Moore, E.E.; DeBot, M.; Cralley, A.L.; Erickson, C.; Silliman, C.C.; Banerjee, A.; Ghasabyan, A.; et al. Postinjury complement C4 activation is associated with adverse outcomes and is potentially influenced by plasma resuscitation. J. Trauma Acute Care Surg. 2022, 93, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Krein, P.M.; Muruve, D.A.; Winston, B.W. Complement factor B gene regulation: Synergistic effects of TNF-alpha and IFN-gamma in macrophages. J. Immunol. 2002, 169, 2627–2635. [Google Scholar] [CrossRef]
- Chen, K.; Deng, Y.; Shang, S.; Tang, L.; Li, Q.; Bai, X.; Chen, X. Complement factor B inhibitor LNP023 improves lupus nephritis in MRL/lpr mice. Biomed. Pharmacother. 2022, 153, 113433. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Z.; Noordam, R.; van Heemst, D.; Li-Gao, R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-sample Mendelian Randomization Study. J. Crohns Colitis 2022, 16, 633–642. [Google Scholar] [CrossRef]
- Chen, M.; Xie, C.R.; Shi, Y.Z.; Tang, T.C.; Zheng, H. Gut microbiota and major depressive disorder: A bidirectional Mendelian randomization. J. Affect. Disord. 2022, 316, 187–193. [Google Scholar] [CrossRef]
- Xian, W.; Wu, D.; Liu, B.; Hong, S.; Huo, Z.; Xiao, H.; Li, Y. Graves Disease and Inflammatory Bowel Disease: A Bidirectional Mendelian Randomization. J. Clin. Endocrinol. Metab. 2023, 108, 1075–1083. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, L.; Huang, P.; Luo, D.; Bi, C.; Chen, X. Genetic causal association between rheumatoid arthritis and periodontitis: A bidirectional two-sample Mendelian randomization analysis. Clin. Oral Investig. 2024, 28, 107. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Dai, W.; Cai, K.; Xiao, Y.; Luo, A.; Lai, Z.; Wang, J.; Xu, L.; Nie, H. Systemic Immune Factors and Risk of Allergic Contact Dermatitis: A Bidirectional Mendelian Randomization Study. Int. J. Mol. Sci. 2024, 25, 10436. https://doi.org/10.3390/ijms251910436
Long Y, Dai W, Cai K, Xiao Y, Luo A, Lai Z, Wang J, Xu L, Nie H. Systemic Immune Factors and Risk of Allergic Contact Dermatitis: A Bidirectional Mendelian Randomization Study. International Journal of Molecular Sciences. 2024; 25(19):10436. https://doi.org/10.3390/ijms251910436
Chicago/Turabian StyleLong, Yingxin, Wenzhang Dai, Kexin Cai, Yuan Xiao, Anqi Luo, Ziwei Lai, Junlin Wang, Lipeng Xu, and Hong Nie. 2024. "Systemic Immune Factors and Risk of Allergic Contact Dermatitis: A Bidirectional Mendelian Randomization Study" International Journal of Molecular Sciences 25, no. 19: 10436. https://doi.org/10.3390/ijms251910436
APA StyleLong, Y., Dai, W., Cai, K., Xiao, Y., Luo, A., Lai, Z., Wang, J., Xu, L., & Nie, H. (2024). Systemic Immune Factors and Risk of Allergic Contact Dermatitis: A Bidirectional Mendelian Randomization Study. International Journal of Molecular Sciences, 25(19), 10436. https://doi.org/10.3390/ijms251910436





