Physico-Chemical Characterization and Initial Evaluation of Carboxymethyl Chitosan–Hyaluronan Hydrocolloid Systems with Insulin Intended for Intranasal Administration
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Characterization of Hydrocolloidal Systems
2.1.1. Visual Aspect and pH
2.1.2. Contact Angle
2.1.3. Surface Tension
2.1.4. Work of Adhesion, Work of Cohesion, and Spreading Coefficient Based on Superficial Properties
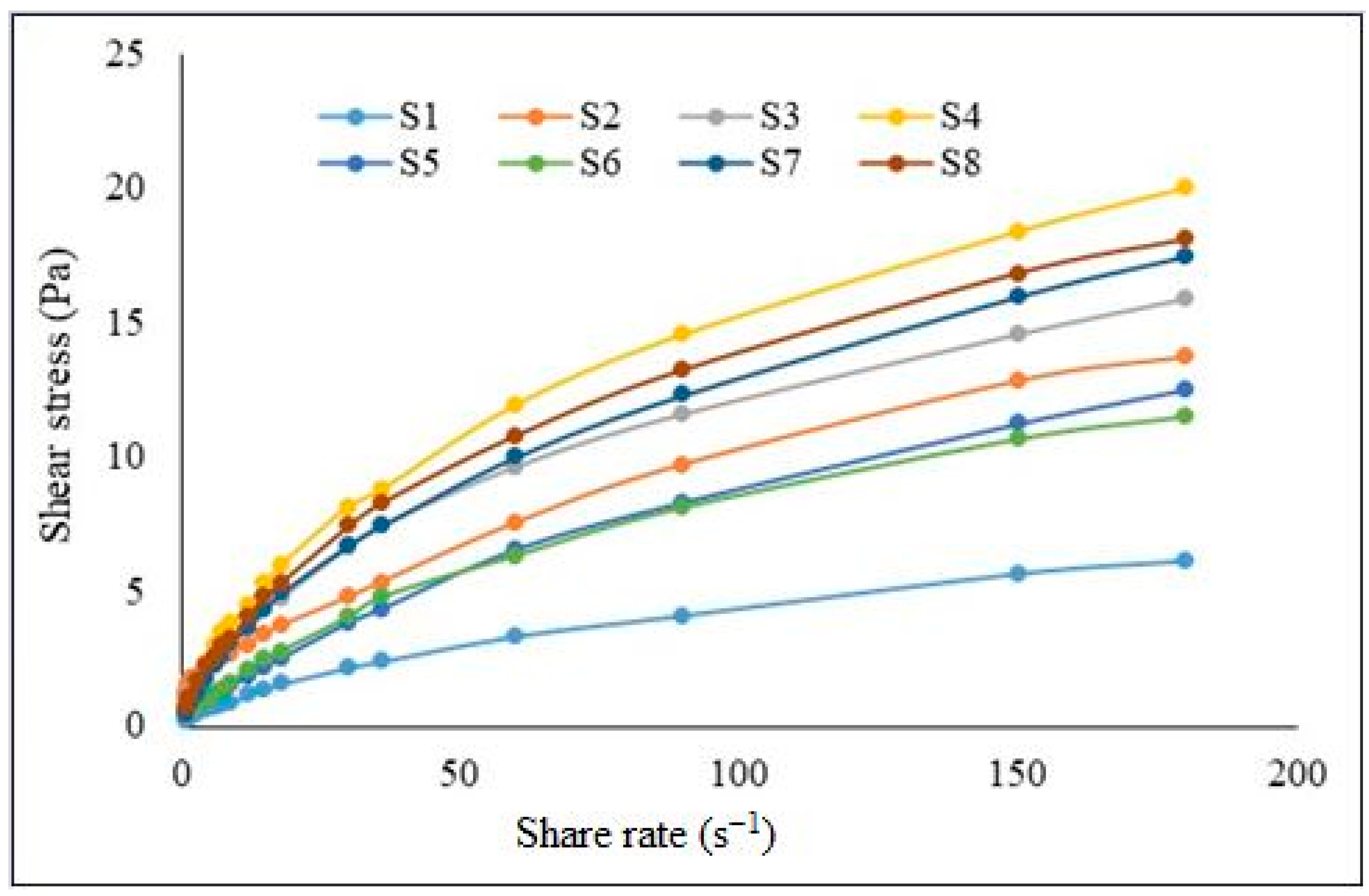
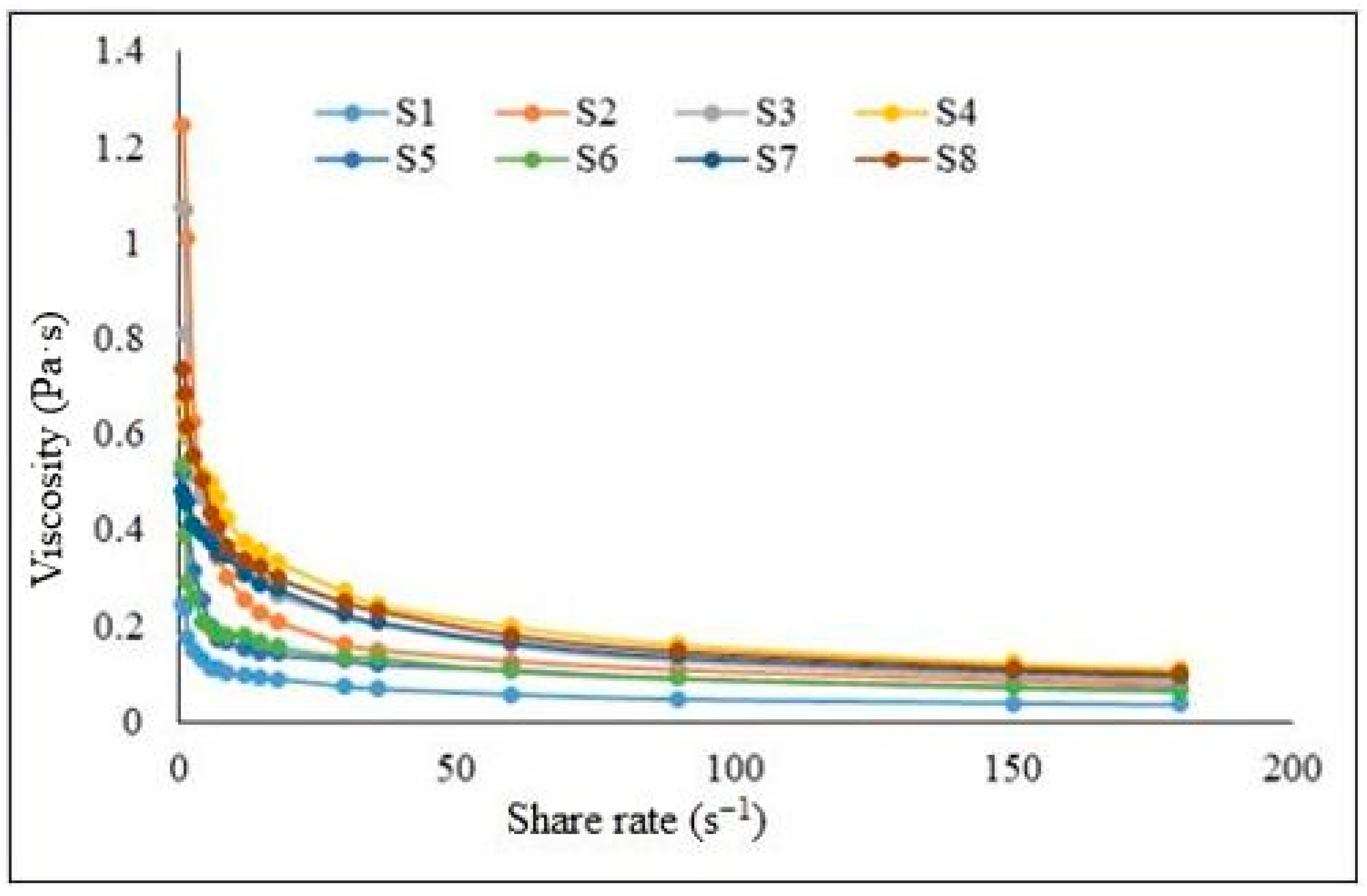
2.2. Rheology Analysis
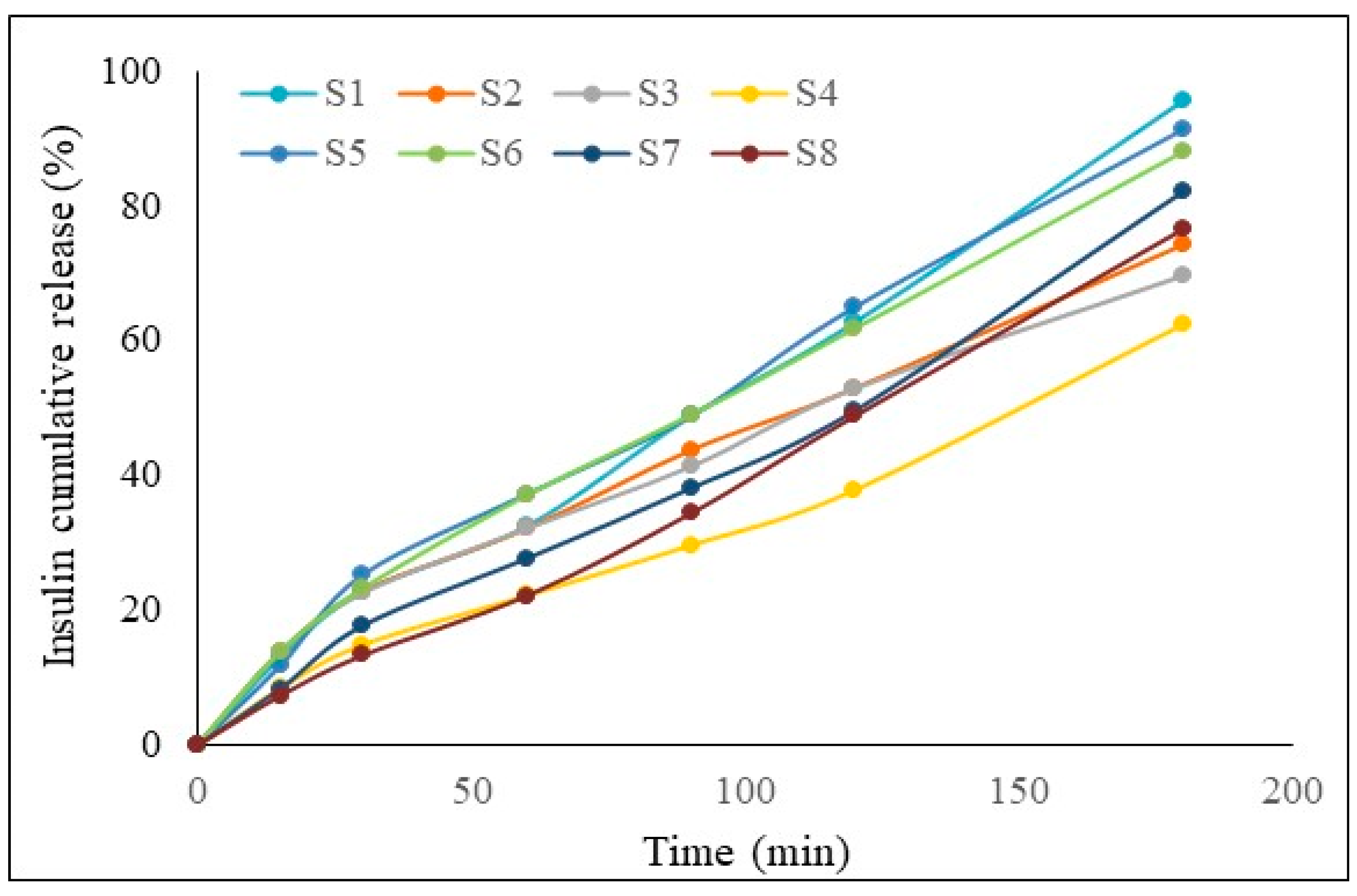
2.3. In Vitro Drug Release
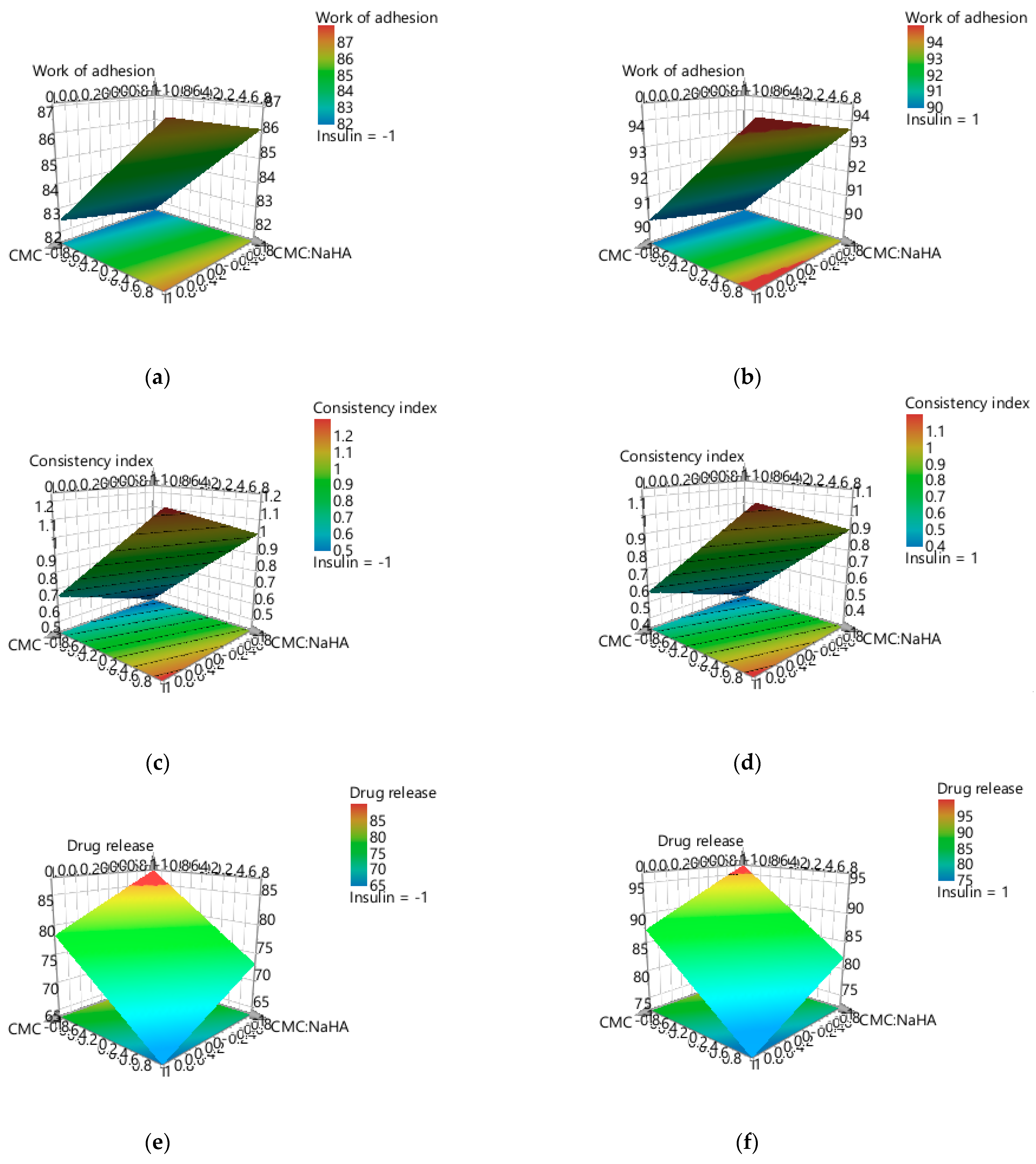
2.4. Experimental Design Screening
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Hydrocolloidal Systems and Experimental Design
4.3. Physico-Chemical Characterization of Hydrocolloidal Systems
4.3.1. Visual Aspect and pH Determination
4.3.2. Contact Angle Determination
4.3.3. Surface Tension Determination
4.3.4. Work of Adhesion, Work of Cohesion, and Spreading Coefficient Determinations Based on Superficial Properties
4.3.5. Rheology Analysis
4.4. In Vitro Drug Release
4.5. Experimental Design Screening
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.Y.J.; Baldelli, A.; Hoyos, C.M.; Tietz, O.; Ong, H.X.; Traini, D. Insulin Delivery to the Brain via the Nasal Route: Unraveling the Potential for Alzheimer’s Disease Therapy. Drug Deliv. Transl. Res. 2024, 14, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, I.O.; Bayunova, L.V.; Avrova, D.K.; Tretyakova, A.D.; Shpakov, A.O.; Avrova, N.F. The Autophagic and Apoptotic Death of Forebrain Neurons of Rats with Global Brain Ischemia Is Diminished by the Intranasal Administration of Insulin: Possible Mechanism of Its Action. Curr. Issues Mol. Biol. 2024, 46, 6580–6599. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Ni, X.; Yang, H.; Feng, L.; Chen, Z.; Bai, L. A comprehensive review of advanced nasal delivery: Specially insulin and calcitonin. Eur. J. Pharm. Sci. 2023, 192, 106630. [Google Scholar] [CrossRef]
- Lofts, A.; Abu-Hijleh, F.; Rigg, N.; Mishra, R.K.; Hoare, T. Using the Intranasal Route to Administer Drugs to Treat Neurological and Psychiatric Illnesses: Rationale, Successes, and Future Needs. CNS Drugs 2022, 36, 739–770. [Google Scholar] [CrossRef]
- Smith, K.; Fan, J.; Marriner, G.A.; Gerdes, J.; Kessler, R.; Zinn, K.R. Distribution of insulin in primate brain following nose-to-brain transport. Alzheimers Dement. 2024, 10, e12459. [Google Scholar] [CrossRef]
- Xu, D.; Song, X.J.; Chen, X.; Wang, J.W.; Cui, Y.L. Advances and future perspectives of intranasal drug delivery: A scientometric review. J. Control. Release 2024, 367, 366–384. [Google Scholar] [CrossRef]
- Vohra, M.; Amir, M.; Sharma, A.; Wadhwa, S. Formulation strategies for nose-to-brain drug delivery in Alzheimer’s disease. Health Sci. Rev. 2023, 6, 100075. [Google Scholar] [CrossRef]
- Shrewsbury, S.B. The Upper Nasal Space: Option for Systemic Drug Delivery, Mucosal Vaccines and “Nose-to-Brain”. Pharmaceutics 2023, 15, 1720. [Google Scholar] [CrossRef]
- Marcello, E.; Chiono, V. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. Int. J. Mol. Sci. 2023, 24, 3390. [Google Scholar] [CrossRef]
- Ziegler, A.-G.; Danne, T.; Daniel, C.; Bonifacio, E. 100 Years of insulin: Lifesaver, immune target, and potential remedy for prevention. Med 2021, 2, 1120–1137. [Google Scholar] [CrossRef]
- White, M.F.; Kahn, C.R. Insulin action at a molecular level—100 years of progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.K.; Babu, M.R.; Vishwas, S.; Chaitanya, M.V.N.L.; Harish, V.; Gupta, G.; Chellappan, D.K.; Dua, K.; Singh, S.K. An overview of recent advances in insulin delivery and wearable technology for effective management of diabetes. J. Drug Deliv. Sci. Technol. 2022, 75, 103728. [Google Scholar] [CrossRef]
- Gaddam, M.; Singh, A.; Jain, N.; Avanthika, C.; Jhaveri, S.; De la Hoz, I.; Sanka, S.; Goli, S.R. A Comprehensive Review of Intranasal Insulin and Its Effect on the Cognitive Function of Diabetics. Cureus 2021, 13, e17219. [Google Scholar] [CrossRef] [PubMed]
- Gulley, L.D.; Shomaker, L.B.; Kelly, N.R.; Chen, K.Y.; Olsen, C.H.; Tanofsky-Kraff, M.; Yanovski, J.A. Examining cognitive-behavioral therapy change mechanisms for decreasing depression, weight, and insulin resistance in adolescent girls at risk for type 2 diabetes. J. Psychosom. Res. 2022, 157, 110781. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Zorina, I.I.; Derkach, K.V. Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium. Int. J. Mol. Sci. 2023, 24, 3278. [Google Scholar] [CrossRef]
- Dove, A.; Shang, Y.; Xu, W.; Grande, G.; Laukka, E.J.; Fratiglioni, L.; Marseglia, A. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement. 2021, 17, 1769–1778. [Google Scholar] [CrossRef]
- Ribaric, S. The Contribution of Type 2 Diabetes to Parkinson’s Disease Aetiology. Int. J. Mol. Sci. 2024, 25, 4358. [Google Scholar] [CrossRef]
- Muleiro Alvarez, M.; Cano-Herrera, G.; Martinez, M.F.O.; Gonzales-Portillo, J.V.; Monroy, G.R.; Perez, R.M.; Torres-Rios, J.A.; van Tienhoven, X.A.; Bernot, E.M.G.; Salazar, F.E.; et al. A Comprehensive Approach to Parkinson’s Disease: Addressing Its Molecular, Clinical, and Therapeutic Aspects. Int. J. Mol. Sci. 2024, 25, 7183. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov (accessed on 10 July 2024).
- Schatz, S.; Gutierrez, G.R. Enhancing socio-communicative functions in an MCI patient with intra-nasal insulin: A case report. Front. Psychiatry 2024, 15, 1326702. [Google Scholar] [CrossRef]
- Lioutas, V.A.; Novak, V. Intranasal insulin neuroprotection in ischemic stroke. Neural Regen. Res. 2016, 11, 400–401. [Google Scholar] [CrossRef]
- Benedict, C.; Frey, W.H., II; Schioth, H.B.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp. Gerontol. 2011, 46, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Adem, M.A.; Decourt, B.; Sabbagh, M.N. Pharmacological Approaches Using Diabetic Drugs Repurposed for Alzheimer’s Disease. Biomedicines 2024, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kellar, D.; Register, T.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal insulin modulates cerebrospinal fluid markers of neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A randomized trial. Sci. Rep. 2022, 12, 1346. [Google Scholar] [CrossRef]
- Saha, P.; Kathuria, H.; Pandey, M.M. Intranasal nanotherapeutics for brain targeting and clinical studies in Parkinson’s disease. J. Control. Release 2023, 358, 293–318. [Google Scholar] [CrossRef]
- Fine, J.M.; Stroebel, B.M.; Faltesek, K.A.; Terai, K.; Haase, L.; Knutzen, K.E.; Kosyakovsky, J.; Bowe, T.J.; Fuller, A.K.; Frey, W.H.; et al. Intranasal delivery of low-dose insulin ameliorates motor dysfunction and dopaminergic cell death in a 6-OHDA rat model of Parkinson’s Disease. Neurosci. Lett. 2020, 714, 134567. [Google Scholar] [CrossRef]
- Novak, P.; Maldonado, D.A.P.; Novak, V. Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: A double-blinded placebo-controlled pilot study. PLoS ONE 2019, 14, e0214364. [Google Scholar] [CrossRef]
- Schneider, E.; Spetter, M.S.; Martin, E.; Sapey, E.; Yip, K.P.; Manolopoulos, K.N.; Tahrani, A.A.; Thomas, J.M.; Lee, M.; Hallschmid, M.; et al. The effect of intranasal insulin on appetite and mood in women with and without obesity: An experimental medicine study. Int. J. Obes. 2022, 46, 1319–1327. [Google Scholar] [CrossRef]
- Edwin Thanarajah, S.; Iglesias, S.; Kuzmanovic, B.; Rigoux, L.; Stephan, K.E.; Bruning, J.C.; Tittgemeyer, M. Modulation of midbrain neurocircuitry by intranasal insulin. Neuroimage 2019, 194, 120–127. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Li, M.; Li, X.; Li, R.; Wu, D. Efficacy and safety of intranasal insulin on postoperative cognitive dysfunction in elderly patients after laparoscopic radical resection of colorectal cancer: A double-blind pilot study. Front. Aging Neurosci. 2024, 16, 1375841. [Google Scholar] [CrossRef]
- Mantik, K.E.K.; Kim, S.; Gu, B.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Repositioning of Anti-Diabetic Drugs against Dementia: Insight from Molecular Perspectives to Clinical Trials. Int. J. Mol. Sci. 2023, 24, 11450. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Mantzoros, C.S.; Novak, P.; McGlinchey, R.; Dai, W.; Lioutas, V.; Buss, S.; Fortier, C.B.; Khan, F.; Becerra, L.A.; et al. MemAID: Memory advancement with intranasal insulin vs. placebo in type 2 diabetes and control participants: A randomized clinical trial. J. Neurol. 2022, 269, 4817–4835. [Google Scholar] [CrossRef] [PubMed]
- Schmid, V.; Kullmann, S.; Gfrorer, W.; Hund, V.; Hallschmid, M.; Lipp, H.P.; Haring, H.U.; Preissl, H.; Fritsche, A.; Heni, M. Safety of intranasal human insulin: A review. Diabetes Obes. Metab. 2018, 20, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Kupila, A.; Sipila, J.; Keskinen, P.; Simell, T.; Knip, M.; Pulkki, K.; Simell, O. Intranasally administered insulin intended for prevention of type 1 diabetes—A safety study in healthy adults. Diabetes Metab. Res. Rev. 2003, 19, 415–420. [Google Scholar] [CrossRef]
- Kashyap, B.; Hanson, L.R.; Frey, W.H., II. Intranasal Insulin: A Treatment Strategy for Addiction. Neurotherapeutics 2020, 17, 105–115. [Google Scholar] [CrossRef]
- Hamidovic, A. Targeting Mediators of Smoking Persistence with Intranasal Insulin. Front. Pharmacol. 2017, 8, 706. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dong, J.; Ge, J.; Liu, X.; Liu, J. Neurobiology of Stress-Induced Nicotine Relapse. Int. J. Mol. Sci. 2024, 25, 1482. [Google Scholar] [CrossRef]
- Hamidovic, A.; Khafaja, M.; Brandon, V.; Anderson, J.; Ray, G.; Allan, A.M.; Burge, M.R. Reduction of smoking urges with intranasal insulin: A randomized, crossover, placebo-controlled clinical trial. Mol. Psychiatry 2017, 22, 1413–1421. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal insulin. J. Neuroendocrinol. 2021, 33, e12934. [Google Scholar] [CrossRef]
- Pang, H.T.; Chen, X.G.; Park, H.J.; Cha, D.S.; Kennedy, J.F. Preparation and rheological properties of deoxycholate-chitosan and carboxymethyl-chitosan in aqueous systems. Carbohydr. Polym. 2007, 69, 419–425. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef]
- Irimia, T. Contributions on Formulation and Preliminary Evaluation of Ocular Colloidal Systems of Chitosan and Poloxamer 407 with Bupivacaine Hydrochloride. Farmacia 2019, 67, 702–708. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Dinu-Pirvu, C.E. Periodontal Chitosan-Gels Designed for Improved Local Intra-Pocket Drug Delivery. Farmacia 2013, 61, 240–250. [Google Scholar]
- Alhowyan, A.A.; Kalam, M.A.; Iqbal, M.; Raish, M.; El-Toni, A.M.; Alkholief, M.; Almomen, A.A.; Alshamsan, A. Mesoporous Silica Nanoparticles Coated with Carboxymethyl Chitosan for 5-Fluorouracil Ocular Delivery: Characterization, In Vitro and In Vivo Studies. Molecules 2023, 28, 1260. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdara, N.; Tiwari, N.A. Carboxymethyl Chitosan and Its Applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, R.; Hassan, S.M.M.; Karim, M.R.; Rahman, M.M.; Rahman, S.; Hossain, M.N.; Islam, D.; Shaikh, M.A.A.; Georghiou, P.E. Carboxymethyl chitin and chitosan derivatives: Synthesis, characterization and antibacterial activity. Carbohydr. Polym. Technol. Appl. 2023, 5, 100283. [Google Scholar] [CrossRef]
- Horvat, S.; Feher, A.; Wolburg, H.; Sipos, P.; Veszelka, S.; Toth, A.; Kis, L.; Kurunczi, A.; Balogh, G.; Kurti, L.; et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur. J. Pharm. Biopharm. 2009, 72, 252–259. [Google Scholar] [CrossRef]
- Cuomo, F.; de Nigris, A.; Zeppa, L.; Lopez, F.; Ambrosone, L. Viscosimetric properties of sodium hyaluronate and hypromellose solutions for medical devices. J. Mol. Liq. 2024, 398, 124182. [Google Scholar] [CrossRef]
- Anicescu, M.C.; Dinu-Pirvu, C.E.; Talianu, M.T.; Ghica, M.V.; Anuta, V.; Prisada, R.M.; Nicoara, A.C.; Popa, L. Insights from a Box-Behnken Optimization Study of Microemulsions with Salicylic Acid for Acne Therapy. Pharmaceutics 2022, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Tudoroiu, E.-E.; Kaya, M.G.A.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Roșca, A.-M.; Popa, L.; Anuța, V.; Antoniac, A.; Chelaru, C.; et al. Design and evaluation of new wound dressings based on collagen-cellulose derivatives. Mater. Des. 2023, 236, 112469. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Popescu, R.; Irimia, T.; Dinu-Pîrvu, C.-E. Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride. Processes 2021, 9, 1694. [Google Scholar] [CrossRef]
- Marx, D.; Williams, G.; Birkhoff, M. Intranasal Drug Administration—An Attractive Delivery Route for Some Drugs. In Drug Discovery and Development—From Molecules to Medicine; IntechOpen Limited: London, UK, 2015. [Google Scholar] [CrossRef]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Popescu, R.; Ghica, M.V.; Dinu-Pirvu, C.E.; Anuta, V.; Lupuliasa, D.; Popa, L. New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. Int. J. Mol. Sci. 2020, 21, 5016. [Google Scholar] [CrossRef]
- Behnoudfar, D.; Dragila, M.I.; Meisenheimer, D.; Wildenschild, D. Contact angle hysteresis: A new paradigm? Adv. Water Resour. 2022, 161, 104138. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer: Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar] [CrossRef]
- Chen, H.; Muros-Cobos, J.L.; Holgado-Terriza, J.A.; Amirfazli, A. Surface tension measurement with a smartphone using a pendant drop. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 213–217. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Zhang, X.; Ji, M.; Ni, Y.; Han, R.; Li, Z.; Xiong, Y.; Tu, J.; He, D.; et al. Exploration of surface tension measurement methods for pharmaceutical excipients. Int. J. Pharm. 2024, 655, 123848. [Google Scholar] [CrossRef]
- Han, K.; Woghiren, O.E.; Priefer, R. Surface tension examination of various liquid oral, nasal, and ophthalmic dosage forms. Chem. Cent. J. 2016, 10, 31. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.A. Adhesion and cohesion. Int. J. Dent. 2012, 2012, 951324. [Google Scholar] [CrossRef]
- Marshall, S.J.; Bayne, S.C.; Baier, R.; Tomsia, A.P.; Marshall, G.W. A review of adhesion science. Dent. Mater. 2010, 26, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- Spindler, L.M.; Serpetsi, S.; Flamm, J.; Feuerhake, A.; Böhler, L.; Pravda, M.; Borchers, K.; Tovar, G.E.M.; Schindowski, K.; Gruber-Traub, C. Hyaluronate spreading validates mucin-agarose analogs as test systems to replace porcine nasal mucosa explants: An experimental and theoretical investigation. Colloids Surf. B Biointerfaces 2022, 217, 112689. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Rapiejko, P.; Sova, J.; Dobrowolska, K. Impact of physicochemical properties of nasal spray products on drug deposition and transport in the pediatric nasal cavity model. Int. J. Pharm. 2020, 574, 118911. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, G.M.; Bakhshaee, M.; Hudson, N.E.; Richards, D.H. Rheological behavior of nasal sprays in shear and extension. Drug Dev. Ind. Pharm. 2000, 26, 975–983. [Google Scholar] [CrossRef]
- Von Zuben, E.S.; Eloy, J.O.; Inacio, M.D.; Araujo, V.H.S.; Baviera, A.M.; Gremiao, M.P.D.; Chorilli, M. Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes. Pharmaceutics 2022, 14, 2492. [Google Scholar] [CrossRef]
- Ostrozka-Cieslik, A.; Maciazek-Jurczyk, M.; Pozycka, J.; Dolinska, B. Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Maftouhian, S.; Aliabadi, A.; Hassanzadeh-Khayyat, M.; Mohammadpour, F.; Khameneh, B.; Hadizadeh, F. Casein-based hydrogel carrying insulin: Preparation, in vitro evaluation and in vivo assessment. J. Pharm. Investig. 2018, 49, 635–641. [Google Scholar] [CrossRef]
- Alabdly, A.A.; Kassab, H.J. Rheological characterization, In vitro release, and Ex vivo permeation of Nefopam Thermosensitive and mucoadhesive intranasal in situ gel. J. Pharm. Negat. Results 2022, 13, 715–726. [Google Scholar]
- Ghica, M.V.; Albu, M.G.; Dinu-Pîrvu, C.; Moisescu, Ş. In vitro kinetic release and flow behaviour of some collagen-minocycline topical hydrogels. Rev. De Chim. 2012, 9, 929–935. [Google Scholar]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics 2021, 13, 1079. [Google Scholar] [CrossRef]
- Popescu, R.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Anuța, V.; Popa, L. Development and preliminary evaluation of intranasal hydrocolloidal systems based on chitosan and PVA with insulin, for central nervous system-associated diseases. Farmacia 2024, 72, 963–974. [Google Scholar]
- Singh, S.K.; Rajoria, K.; Kumar, A.; Sharma, S. A double-blind controlled clinical trial to evaluate the effects of nasal therapy with Vrihatajivakadya oil on different viscosities in patients with migraine. J. Ayurveda Integr. Med. 2023, 14, 100662. [Google Scholar] [CrossRef] [PubMed]
- De Barros, C.; Portugal, I.; Batain, F.; Portella, D.; Severino, P.; Cardoso, J.; Arcuri, P.; Chaud, M.; Alves, T. Formulation, design and strategies for efficient nanotechnology-based nasal delivery systems. RPS Pharm. Pharmacol. Rep. 2022, 1, rqac003. [Google Scholar] [CrossRef]
- Wong, C.Y.J.; Baldelli, A.; Tietz, O.; van der Hoven, J.; Suman, J.; Ong, H.X.; Traini, D. An overview of in vitro and in vivo techniques for characterization of intranasal protein and peptide formulations for brain targeting. Int. J. Pharm. 2024, 654, 123922. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Xu, X.; Wen, N.; Song, R.; Meng, Q.; Guan, Y.; Cheng, S.; Cao, D.; Dong, Y.; Qie, J.; et al. A Drug Carrier for Sustained Zero-Order Release of Peptide Therapeutics. Sci. Rep. 2017, 7, 5524. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Badawi, A.M.; Mansour, H.F. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: Design, in-vitro characterization and clinical evaluation. Int. J. Pharm. 2021, 601, 120600. [Google Scholar] [CrossRef]
- Nazar, H.; Caliceti, P.; Carpenter, B.; El-Mallah, A.I.; Fatouros, D.G.; Roldo, M.; van der Merwe, S.M.; Tsibouklis, J. A once-a-day dosage form for the delivery of insulin through the nasal route: In vitro assessment and in vivo evaluation. Biomater. Sci. 2013, 1, 306–314. [Google Scholar] [CrossRef]
- Muntu, C.M.; Avanti, C.; Hayun; Surini, S. Promising brain biodistribution of insulin via intranasal dry powder for nose-to-brain delivery. Heliyon 2024, 10, e33657. [Google Scholar] [CrossRef]
- De Souza Von Zuben, E.; Eloy, J.O.; Araujo, V.H.S.; Gremião, M.P.D.; Chorilli, M. Insulin-loaded liposomes functionalized with cell-penetrating peptides: Influence on drug release and permeation through porcine nasal mucosa. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126624. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, S.S. Preparation and Characterization of Chitosan Nanoparticles of Insulin for Nasal Delivery. J. Drug Deliv. Ther. 2018, 8, 400–406. [Google Scholar]
- Li, Y.; Wu, X.; Zhu, Q.; Chen, Z.; Lu, Y.; Qi, J.; Wu, W. Improving the hypoglycemic effect of insulin via the nasal administration of deep eutectic solvents. Int. J. Pharm. 2019, 569, 118584. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, J.; Swedrowska, M.; Scherliess, R.; Parry, M.; Ramjeeawon, M.; Taylor, D.; Gauthier, G.; Brown, L.; Amiel, S.; Zelaya, F.; et al. Characterisation of nasal devices for delivery of insulin to the brain and evaluation in humans using functional magnetic resonance imaging. J. Control. Release 2019, 302, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ioan, D.C.; Rau, I.; Kaya, M.G.A.; Radu, N.; Bostan, M.; Zgarian, R.G.; Tihan, G.T.; Dinu-Pirvu, C.E.; Lupuliasa, A.; Ghica, M.V. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymers 2020, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- MODDE®. Available online: https://www.sartorius.com/en/products/process-analytical-technology/data-analytics-software/doe-software/modde (accessed on 1 May 2020).
- Minitab. Available online: https://www.minitab.com/en-us/ (accessed on 16 June 2024).
- Javed, M.N.; Alam, M.S.; Waziri, A.; Pottoo, F.H.; Yadav, A.K.; Hasnain, M.S.; Almalki, F.A. QbD Applications for the Development of Nanopharmaceutical Products. In Pharmaceutical Quality by Design; Academic Press: Cambridge, MA, USA, 2019; pp. 229–253. [Google Scholar] [CrossRef]

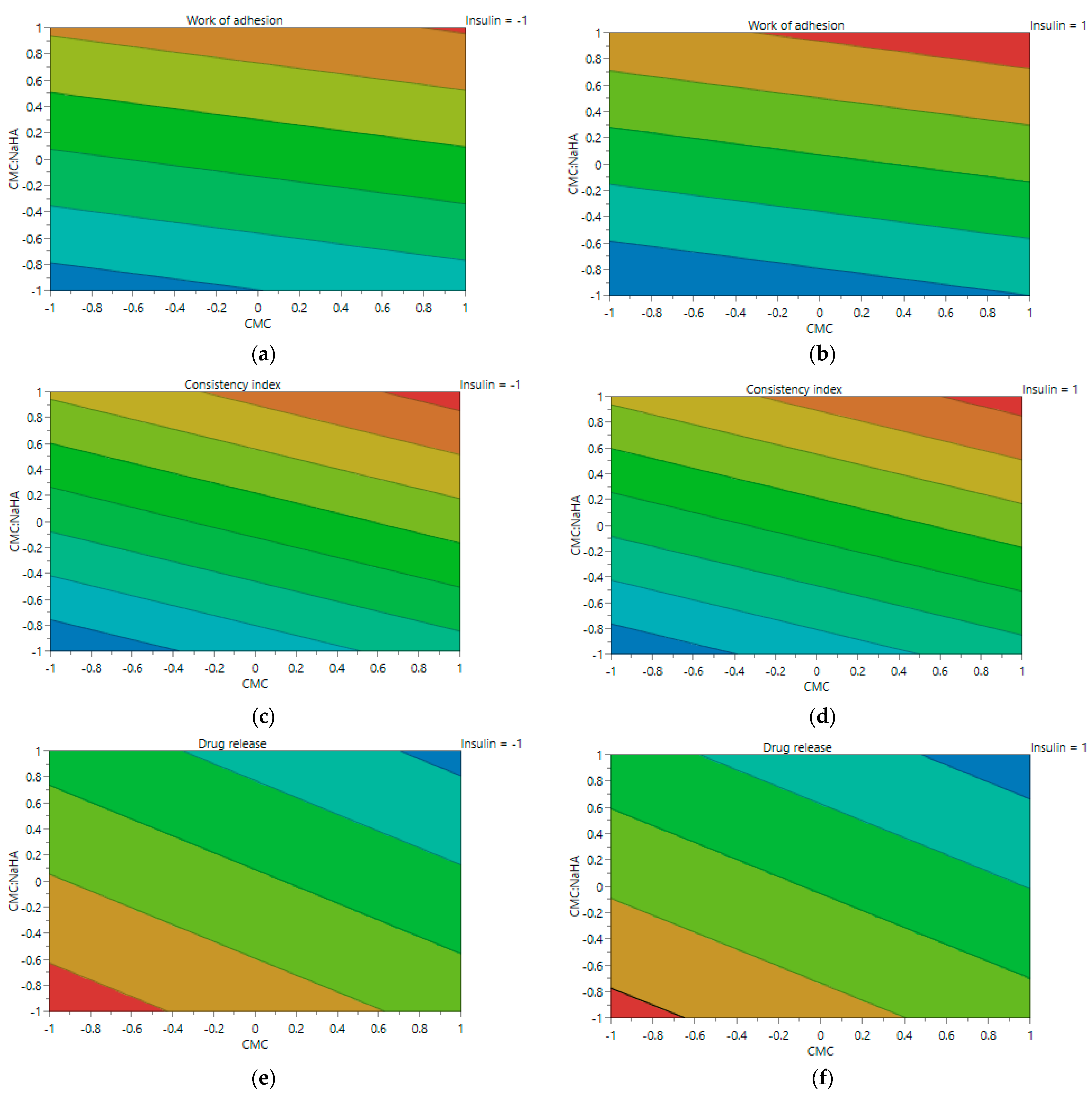

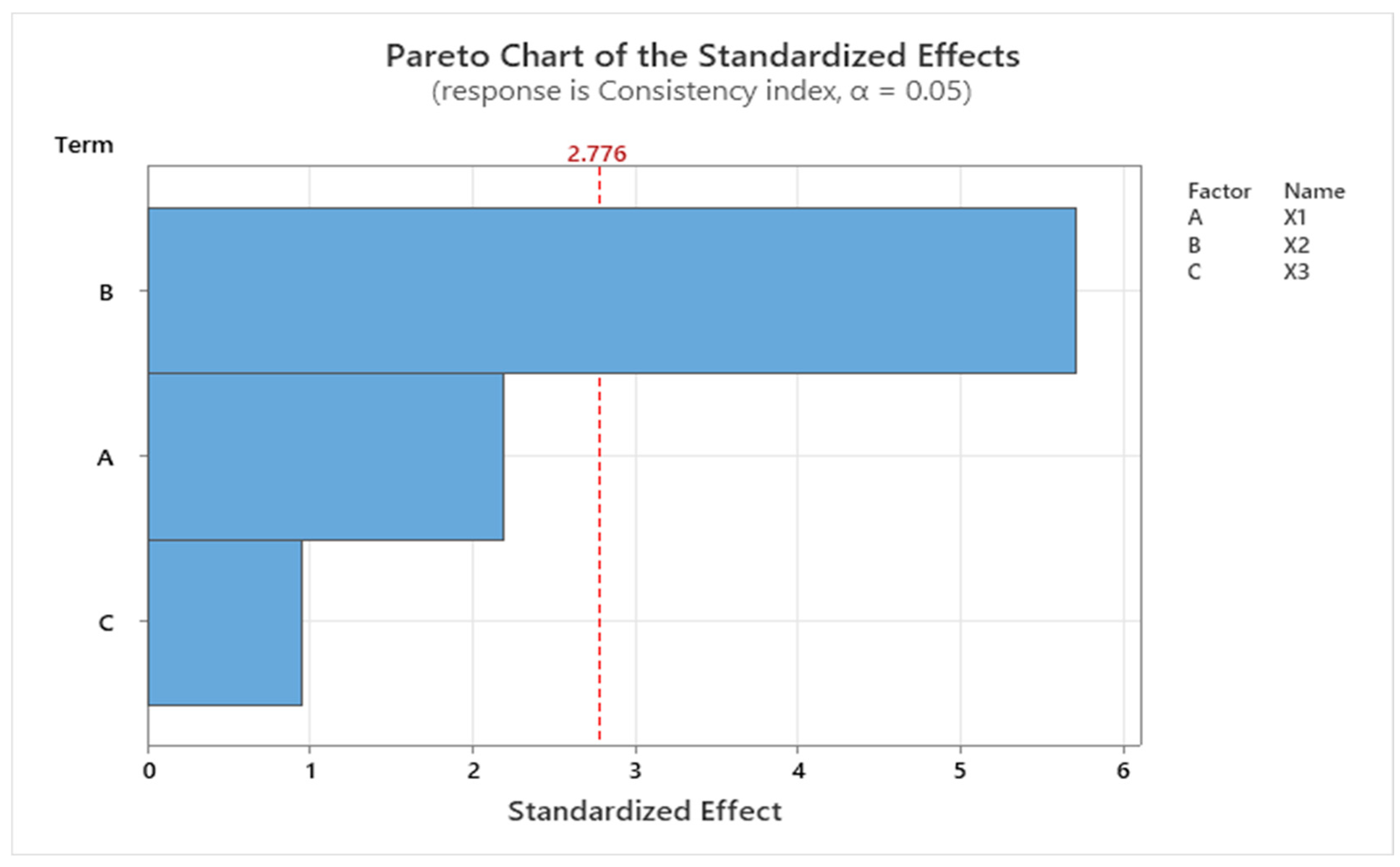

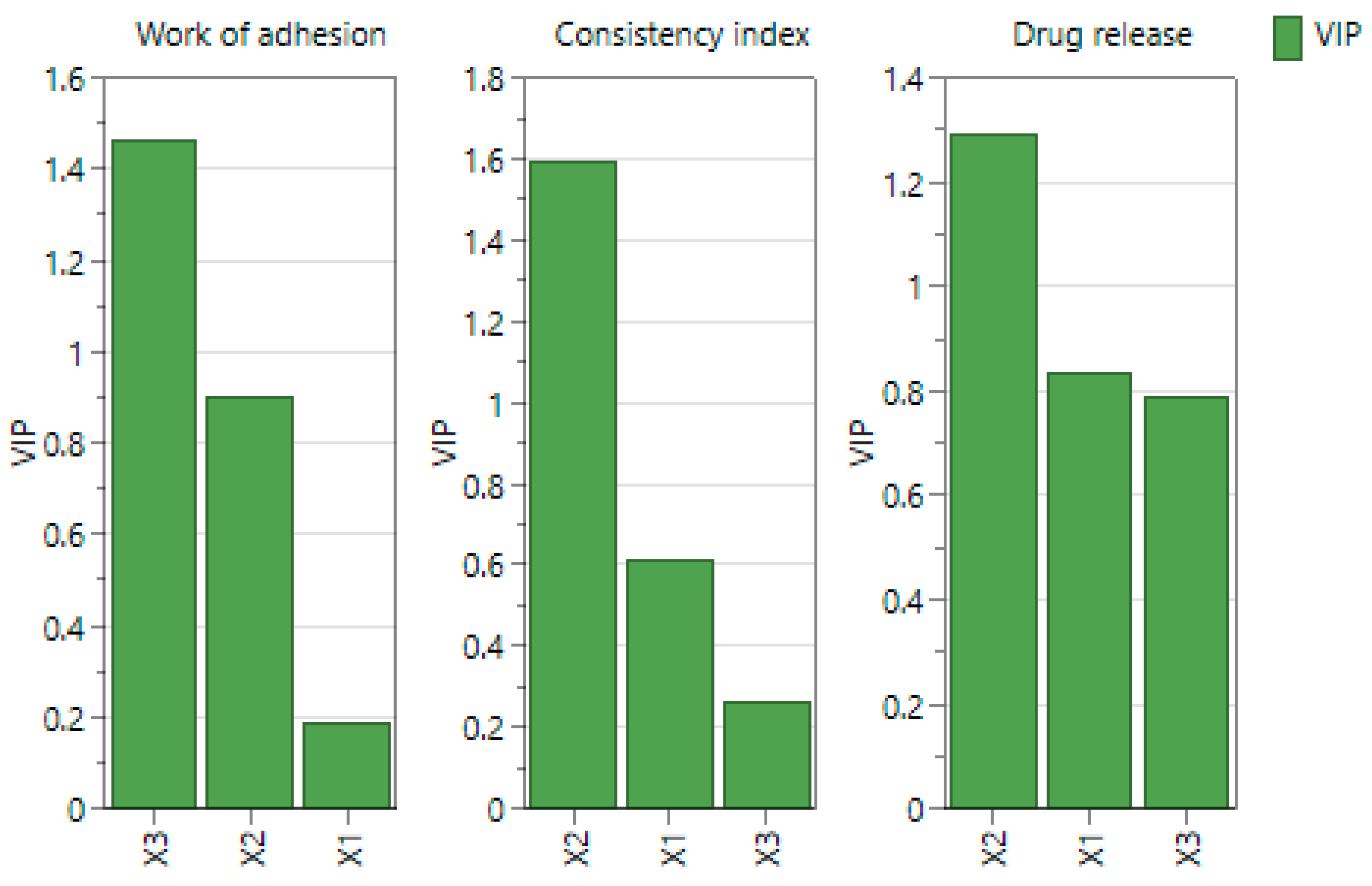
| System | X1 CMC (% w/v) | X2 CMC/NaHA Ratio | X3 Insulin (IU/mL) | pH | Contact Angle (°) | Surface Tension (mN/m) |
|---|---|---|---|---|---|---|
| S1 | 1 | 1/1 | 20 | 6.65 | 66.35 ± 0.64 | 57.19 ± 0.24 |
| S2 | 2 | 1/1 | 20 | 6.57 | 65.48 ± 2.06 | 60.51 ± 0.05 |
| S3 | 1 | 1/2 | 20 | 6.68 | 68.79 ± 1.56 | 58.85 ± 0.77 |
| S4 | 2 | 1/2 | 20 | 6.64 | 64.68 ± 0.73 | 62.07 ± 0.71 |
| S5 | 1 | 1/1 | 30 | 6.68 | 61.73 ± 0.75 | 60.91 ± 0.49 |
| S6 | 2 | 1/1 | 30 | 6.62 | 64.10 ± 0.65 | 59.01 ± 0.32 |
| S7 | 1 | 1/2 | 30 | 6.72 | 50.47 ± 0.26 | 59.65 ± 0.72 |
| S8 | 2 | 1/2 | 30 | 6.65 | 58.43 ± 0.52 | 62.46 ± 0.30 |
| System | Wa | Wc | S |
|---|---|---|---|
| S1 | 82.84 ± 0.27 | 114.38 ± 0.48 | −31.54 ± 0.7 |
| S2 | 85.62 ± 1.95 | 121.02 ± 0.1 | −35.40 ± 2.02 |
| S3 | 80.14 ± 2.75 | 117.7 ± 2.72 | −37.56 ± 1.45 |
| S4 | 88.62 ± 1.16 | 124.14 ± 1.43 | −35.52 ± 0.88 |
| S5 | 89.76 ± 0.26 | 121.83 ± 0.98 | −32.06 ± 0.96 |
| S6 | 84.79 ± 0.8 | 118.03 ± 1.31 | −33.24 ± 0.57 |
| S7 | 97.62 ± 1.1 | 119.3 ± 1.44 | −21.68 ± 0.23 |
| S8 | 95.17 ± 0.45 | 124.93 ± 0.23 | −29.76 ± 0.21 |
| Sample | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| K | 0.261 | 0.841 | 1.054 | 1.187 | 0.393 | 0.475 | 0.897 | 1.095 |
| n | 0.611 | 0.539 | 0.526 | 0.549 | 0.670 | 0.620 | 0.576 | 0.546 |
| R2 | 0.9980 | 0.9926 | 0.9960 | 0.9952 | 0.9969 | 0.9953 | 0.9958 | 0.9954 |
| System | Determination Coefficient (R2) | Kinetic Parameters | Drug Released (%) | ||||
|---|---|---|---|---|---|---|---|
| Higuchi Model | Zero-Order Model | Power Law Model | Zero-Order Model | Power Law Model | |||
| Kinetic Constant (1/min) | Kinetic Constant (1/minn) | Release Exponent (n) | |||||
| 1 | 0.9248 | 0.9959 | 0.9934 | 0.538 | 0.891 | 0.896 | 95.91 |
| 2 | 0.9710 | 0.9819 | 0.9962 | 0.445 | 1.885 | 0.703 | 74.35 |
| 3 | 0.9790 | 0.9771 | 0.9972 | 0.427 | 2.146 | 0.668 | 69.86 |
| 4 | 0.9128 | 0.9934 | 0.9852 | 0.342 | 0.522 | 0.913 | 62.53 |
| 5 | 0.9515 | 0.9913 | 0.9958 | 0.534 | 1.419 | 0.799 | 91.53 |
| 6 | 0.9585 | 0.9899 | 0.9980 | 0.517 | 1.554 | 0.774 | 88.06 |
| 7 | 0.9026 | 0.9962 | 0.9905 | 0.444 | 0.482 | 0.983 | 82.18 |
| 8 | 0.8857 | 0.9978 | 0.9968 | 0.412 | 0.282 | 1.076 | 76.65 |
| Response | Optimal Setpoint |
|---|---|
| Work of adhesion (mN/m) | 92.55 |
| Consistency index (Pa·sn) | 0.89 |
| Drug release (%) | 81.64 |
| Factors | Independent Variables | Levels of Variation | |
|---|---|---|---|
| Lower (−1) | Upper (+1) | ||
| X1 | Carboxymethyl chitosan (% w/v) | 1 | 2 |
| X2 | CMC/NaHA ratio | 1/1 | 1/2 |
| X3 | Insulin (IU/mL) | 20 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuța, V.; Popa, L. Physico-Chemical Characterization and Initial Evaluation of Carboxymethyl Chitosan–Hyaluronan Hydrocolloid Systems with Insulin Intended for Intranasal Administration. Int. J. Mol. Sci. 2024, 25, 10452. https://doi.org/10.3390/ijms251910452
Popescu R, Dinu-Pîrvu C-E, Ghica MV, Anuța V, Popa L. Physico-Chemical Characterization and Initial Evaluation of Carboxymethyl Chitosan–Hyaluronan Hydrocolloid Systems with Insulin Intended for Intranasal Administration. International Journal of Molecular Sciences. 2024; 25(19):10452. https://doi.org/10.3390/ijms251910452
Chicago/Turabian StylePopescu, Roxana, Cristina-Elena Dinu-Pîrvu, Mihaela Violeta Ghica, Valentina Anuța, and Lăcrămioara Popa. 2024. "Physico-Chemical Characterization and Initial Evaluation of Carboxymethyl Chitosan–Hyaluronan Hydrocolloid Systems with Insulin Intended for Intranasal Administration" International Journal of Molecular Sciences 25, no. 19: 10452. https://doi.org/10.3390/ijms251910452









