Genetic Mapping and Characterization of the Clubroot Resistance Gene BraPb8.3 in Brassica rapa
Abstract
1. Introduction
2. Results
2.1. Phenotype Evaluation and Genetic Analysis
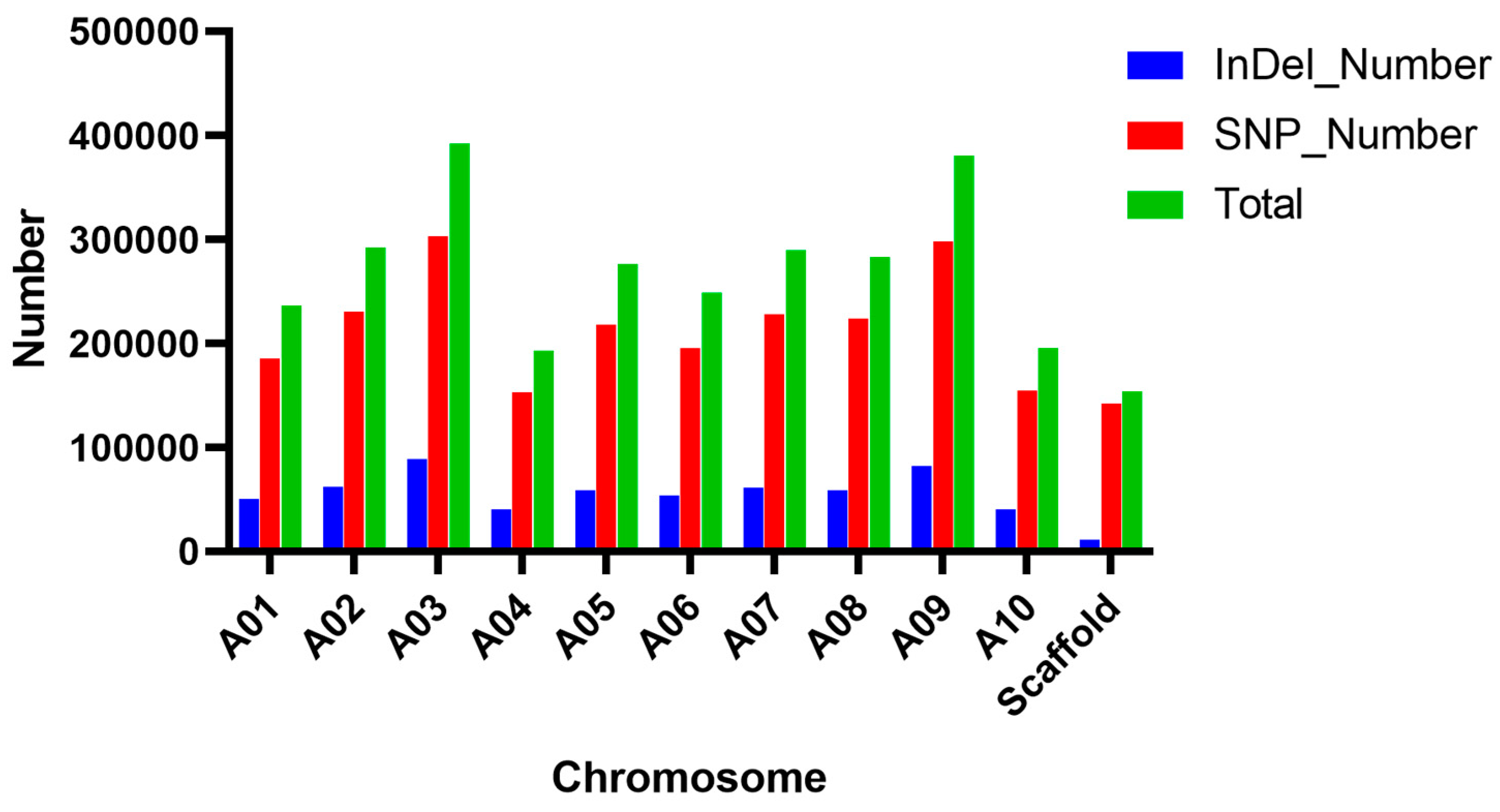
2.2. BSA-Seq Data Analysis
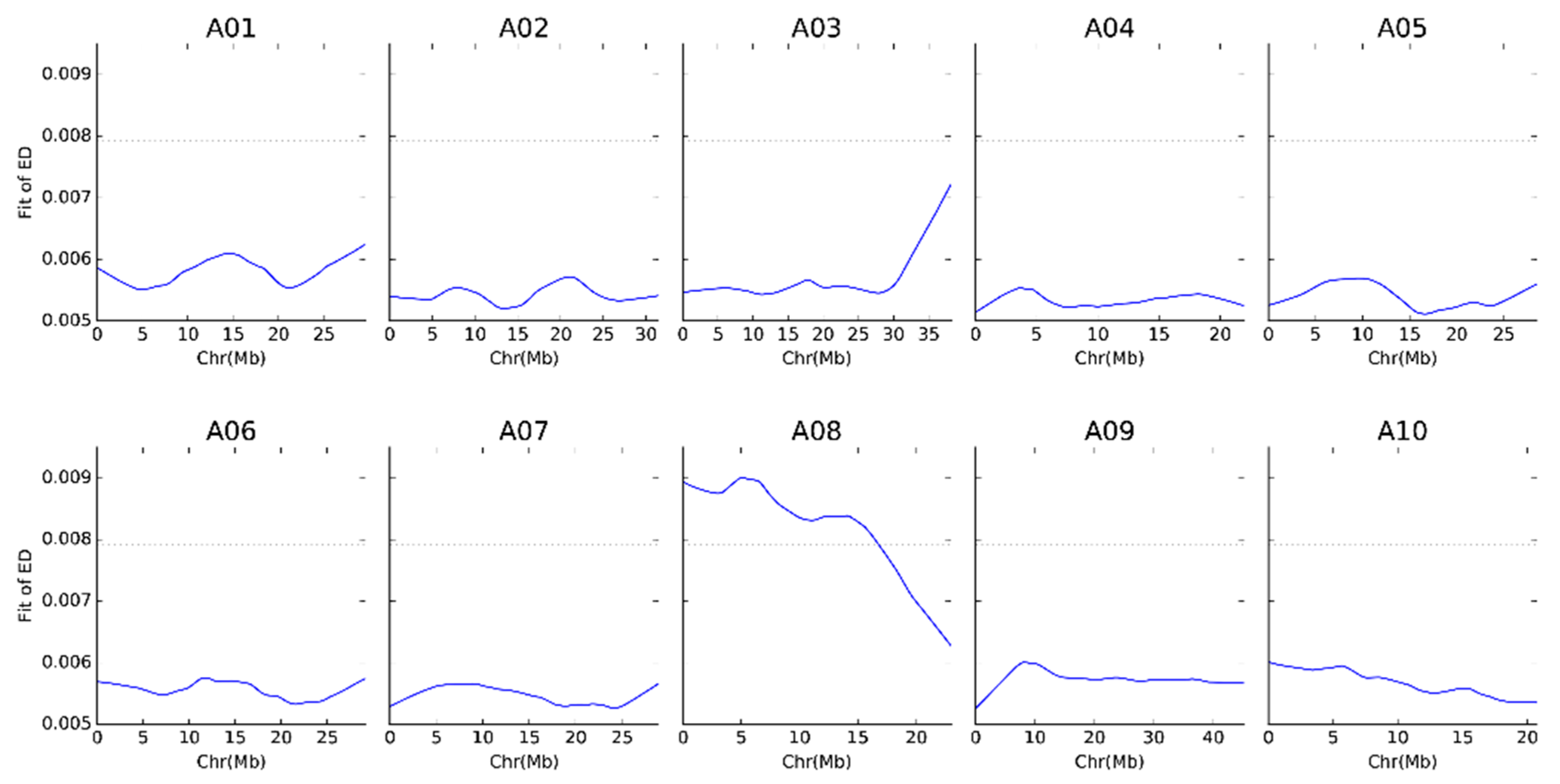
2.3. Euclidean Distance Analysis and Prediction of Candidate Areas
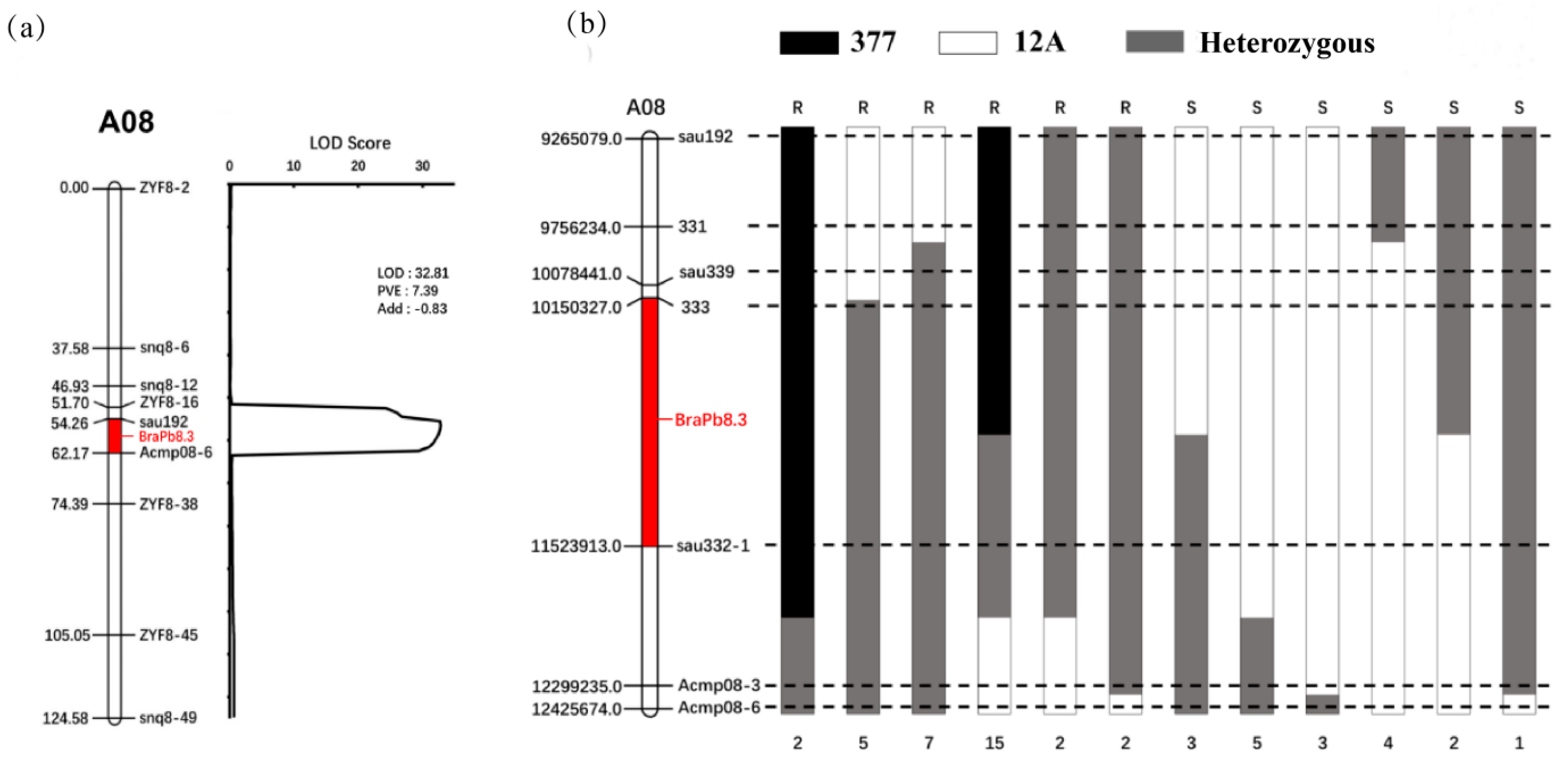
2.4. Preliminary Mapping of BraPb8.3
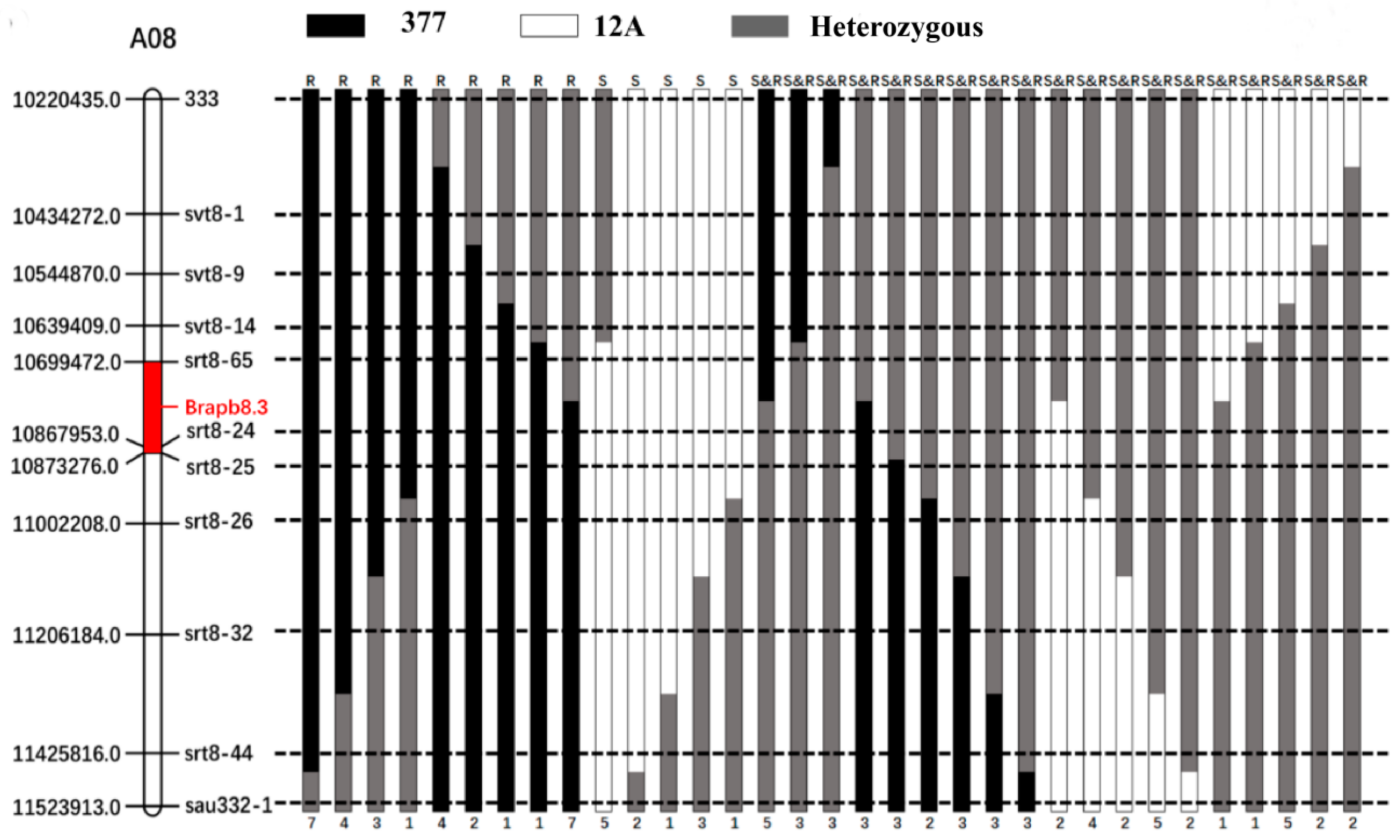
2.5. Fine Mapping of BraPb8.3
2.6. Candidate Genes Analysis
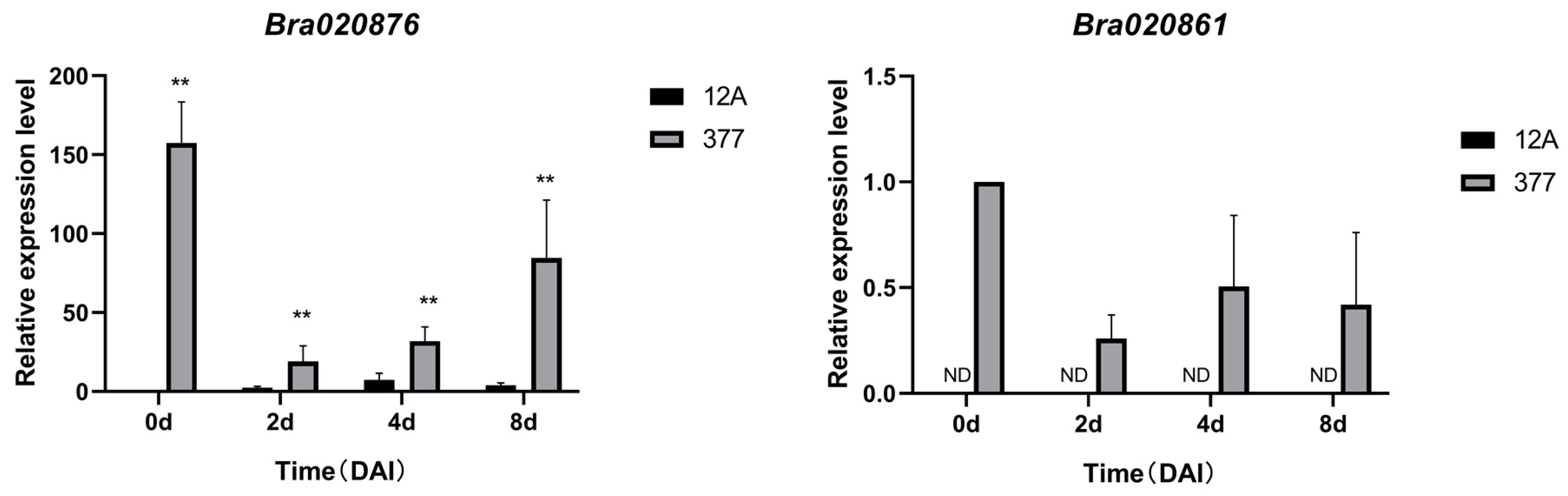
2.7. Expression Analysis of Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and P. brassicae Inoculation
4.2. BSA-Seq Analysis
4.3. Molecular Marker Development
4.4. Construction of Genetic Linkage Map and QTL Analysis
4.5. Fine Mapping of BraPb8.3
4.6. Prediction and Analysis of Candidate Genes
4.7. Total RNA Extraction and qRT-PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Guan, X.; Liu, S.; Yang, M.; Ren, J.; Guo, M.; Huang, Z.; Zhang, Y. Genome-Wide Identification and Analysis of TCP Transcription Factors Involved in the Formation of Leafy Head in Chinese Cabbage. Int. J. Mol. Sci. 2018, 19, 847. [Google Scholar] [CrossRef]
- Dixon, G.R. The Occurrence and Economic Impact of Plasmodiophora brassicae and Clubroot Disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Struck, C.; Rüsch, S.; Strehlow, B. Control Strategies of Clubroot Disease Caused by Plasmodiophora brassicae. Microorganisms 2022, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Schwelm, A.; Zamani-Noor, N.; Salih, R.; Silvestre Vañó, M.; Wu, J.; González García, M.; Heick, T.M.; Luo, C.; Prakash, P.; et al. The clubroot pathogen Plasmodiophora brassicae: A profile update. Mol. Plant Pathol. 2023, 24, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qin, L.; Zhou, Z.; Hendriks, W.; Liu, S.; Wei, Y. Refining the Life Cycle of Plasmodiophora brassicae. Phytopathology 2020, 110, 1704–1712. [Google Scholar] [CrossRef]
- Zhang, C.; Du, C.; Li, Y.; Wang, H.; Zhang, C.; Chen, P. Advances in Biological Control and Resistance Genes of Brassicaceae Clubroot Disease-The Study Case of China. Int. J. Mol. Sci. 2023, 24, 785. [Google Scholar] [CrossRef]
- Pang, W.; Zhang, X.; Ma, Y.; Wang, Y.; Zhan, Z.; Piao, Z. Fine mapping and candidate gene analysis of CRA3.7 conferring clubroot resistance in Brassica rapa. Theor. Appl. Genet. 2022, 135, 4541–4548. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, A.; Liang, F.; Yao, X.; Wang, Y.; Liu, X.; Zhang, Y.; Dalelhan, J.; Zhang, B.; Qin, M.; et al. Screening of clubroot-resistant varieties and transfer of clubroot resistance genes to Brassica napus using distant hybridization. Breed. Sci. 2018, 68, 258–267. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, Y.; Yue, Y.; Sui, Y.; Hao, M.; Kang, X.; Wang, Q.; Chen, D.; Liu, B.; Yin, Z.; et al. Glutathione and neodiosmin feedback sustain plant immunity. J. Exp. Bot. 2023, 74, 976–990. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Bernoux, M.; Zetzsche, H.; Stuttmann, J. Connecting the dots between cell surface- and intracellular-triggered immune pathways in plants. Curr. Opin. Plant Biol. 2022, 69, 102276. [Google Scholar] [CrossRef] [PubMed]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Fujimura, M.; Nunome, T.; Fukuoka, H.; Matsumoto, S.; Hirai, M. Identification of two loci for resistance to clubroot (Plasmodiophora brassicae Woronin) in Brassica rapa L. Theor. Appl. Genet. 2003, 107, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jing, J.; Zhan, Z.; Zhang, T.; Zhang, C.; Piao, Z. Identification of novel QTLs for isolate-specific partial resistance to Plasmodiophora brassicae in Brassica rapa. PLoS ONE 2013, 8, e85307. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Liang, S.; Li, X.; Li, P.; Yu, S.; Lim, Y.P.; Piao, Z. Genetic detection of clubroot resistance loci in a new population of Brassica rapa. Hortic. Environ. Biotechnol. 2014, 55, 540–547. [Google Scholar] [CrossRef]
- Sakamoto, K.; Saito, A.; Hayashida, N.; Taguchi, G.; Matsumoto, E. Mapping of isolate-specific QTLs for clubroot resistance in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Theor. Appl. Genet. 2008, 117, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, X.; Peng, G.; Falk, K.C.; Strelkov, S.E.; Gossen, B.D. Genotyping-by-sequencing reveals three QTL for clubroot resistance to six pathotypes of Plasmodiophora brassicae in Brassica rapa. Sci. Rep. 2017, 7, 4516. [Google Scholar] [CrossRef]
- Matsumoto, E.; Yasui, C.; Ohi, M.; Tsukada, M. Linkage analysis of RFLP markers for clubroot resistance and pigmentation in Chinese cabbage (Brassica rapa ssp. pekinensis). Euphytica 1998, 104, 79–86. [Google Scholar] [CrossRef]
- Piao, Z.Y.; Deng, Y.Q.; Choi, S.R.; Park, Y.J.; Lim, Y.P. SCAR and CAPS mapping of CRb, a gene conferring resistance to Plasmodiophora brassicae in Chinese cabbage (Brassica rapa ssp. pekinensis). Theor. Appl. Genet. 2004, 108, 1458–1465. [Google Scholar] [CrossRef]
- Pang, W.; Fu, P.; Li, X.; Zhan, Z.; Yu, S.; Piao, Z. Identification and Mapping of the Clubroot Resistance Gene CRd in Chinese Cabbage (Brassica rapa ssp. pekinensis). Front. Plant Sci. 2018, 9, 653. [Google Scholar] [CrossRef]
- Saito, M.; Kubo, N.; Matsumoto, S.; Suwabe, K.; Tsukada, M.; Hirai, M. Fine mapping of the clubroot resistance gene, Crr3, in Brassica rapa. Theor. Appl. Genet. 2006, 114, 81–91. [Google Scholar] [CrossRef]
- Chu, M.; Song, T.; Falk, K.C.; Zhang, X.; Liu, X.; Chang, A.; Lahlali, R.; McGregor, L.; Gossen, B.D.; Peng, G.; et al. Fine mapping of Rcr1 and analyses of its effect on transcriptome patterns during infection by Plasmodiophora brassicae. BMC Genom. 2014, 15, 1166. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Peng, G.; Liu, X.; Deora, A.; Falk, K.C.; Gossen, B.D.; McDonald, M.R.; Yu, F. Fine Mapping of a Clubroot Resistance Gene in Chinese Cabbage Using SNP Markers Identified from Bulked Segregant RNA Sequencing. Front. Plant Sci. 2017, 8, 1448. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Y.; Wang, J.; Chen, Q.; Karim, M.M.; Gossen, B.D.; Peng, G. Identification of Two Major QTLs in Brassica napus Lines with Introgressed Clubroot Resistance from Turnip Cultivar ECD01. Front. Plant Sci. 2021, 12, 785989. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Monakhos, G.F.; Komakhin, R.A.; Monakhos, S.G. The New Clubroot Resistance Locus Is Located on Chromosome A05 in Chinese Cabbage (Brassica rapa L.). Russ. J. Genet. 2018, 54, 296–304. [Google Scholar] [CrossRef]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Kondo, M.; Fujimura, M.; Nunome, T.; Fukuoka, H.; Hirai, M.; Matsumoto, S. Simple sequence repeat-based comparative genomics between Brassica rapa and Arabidopsis thaliana: The genetic origin of clubroot resistance. Genetics 2006, 173, 309–319. [Google Scholar] [CrossRef]
- Zhu, H.; Zhai, W.; Li, X.; Zhu, Y. Two QTLs controlling Clubroot resistance identified from Bulked Segregant Sequencing in Pakchoi (Brassica campestris ssp. chinensis Makino). Sci. Rep. 2019, 9, 9228. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Suwabe, K.; Tomita, R.N.; Kato, T.; Nunome, T.; Fukuoka, H.; Matsumoto, S. Identification and characterization of Crr1a, a gene for resistance to clubroot disease (Plasmodiophora brassicae Woronin) in Brassica rapa L. PLoS ONE 2013, 8, e54745. [Google Scholar] [CrossRef]
- Laila, R.; Park, J.I.; Robin, A.H.K.; Natarajan, S.; Vijayakumar, H.; Shirasawa, K.; Isobe, S.; Kim, H.T.; Nou, I.S. Mapping of a novel clubroot resistance QTL using ddRAD-seq in Chinese cabbage (Brassica rapa L.). BMC Plant Biol. 2019, 19, 13. [Google Scholar] [CrossRef]
- Karim, M.M.; Dakouri, A.; Zhang, Y.; Chen, Q.; Peng, G.; Strelkov, S.E.; Gossen, B.D.; Yu, F. Two Clubroot-Resistance Genes, Rcr3 and Rcr9(wa), Mapped in Brassica rapa Using Bulk Segregant RNA Sequencing. Int. J. Mol. Sci. 2020, 21, 5033. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Mehboob Ur, R.; Foolad, M.R. Marker-assisted selection in plant breeding for salinity tolerance. Methods Mol. Biol. 2012, 913, 305–333. [Google Scholar] [CrossRef]
- Pei, D.; Song, S.; Kang, J.; Zhang, C.; Wang, J.; Dong, T.; Ge, M.; Pervaiz, T.; Zhang, P.; Fang, J. Characterization of Simple Sequence Repeat (SSR) Markers Mined in Whole Grape Genomes. Genes 2023, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Gemmill, C.E.C.; Grierson, E.R.P. Inter-Simple Sequence Repeats (ISSR), Microsatellite-Primed Genomic Profiling Using Universal Primers. Methods Mol. Biol. 2021, 2222, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, M.K.; Kale, S.M.; Sriswathi, M.; Naresh, T.; Shasidhar, Y.; Garg, V.; Pandey, M.K.; Varshney, R.K. Genome-Wide Discovery and Deployment of Insertions and Deletions Markers Provided Greater Insights on Species, Genomes, and Sections Relationships in the Genus Arachis. Front. Plant Sci. 2017, 8, 2064. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Robbins, M.D.; Staub, J.E. Population development by phenotypic selection with subsequent marker-assisted selection for line extraction in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2006, 112, 843–855. [Google Scholar] [CrossRef]
- Sureshkumar, S.; Tamilkumar, P.; Senthil, N.; Nagarajan, P.; Thangavelu, A.U.; Raveendran, M.; Vellaikumar, S.; Ganesan, K.N.; Balagopal, R.; Vijayalakshmi, G.; et al. Marker assisted selection of low phytic acid trait in maize (Zea mays L.). Hereditas 2014, 151, 20–27. [Google Scholar] [CrossRef]
- Khan, M.A.; Durel, C.E.; Duffy, B.; Drouet, D.; Kellerhals, M.; Gessler, C.; Patocchi, A. Development of molecular markers linked to the ‘Fiesta’ linkage group 7 major QTL for fire blight resistance and their application for marker-assisted selection. Genome 2007, 50, 568–577. [Google Scholar] [CrossRef]
- Davierwala, A.P.; Reddy, A.P.; Lagu, M.D.; Ranjekar, P.K.; Gupta, V.S. Marker assisted selection of bacterial blight resistance genes in rice. Biochem. Genet. 2001, 39, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Shah, N.; Jia, R.; Li, X.; Zhang, C.; Piao, Z. Transferring of clubroot-resistant locus CRd from Chinese cabbage (Brassica rapa) to canola (Brassica napus) through interspecific hybridization. Breed. Sci. 2022, 72, 189–197. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Ma, Y.; Cao, G.; Ma, S.; Zhang, T.; Zhan, Z.; Piao, Z. Marker-Assisted Pyramiding of CRa and CRd Genes to Improve the Clubroot Resistance of Brassica rapa. Genes 2022, 13, 2414. [Google Scholar] [CrossRef]
- Kawasaki, M.; Ohara, T.; Ishida, M.; Takahata, Y.; Hatakeyama, K. Development of novel clubroot resistant rapeseed lines (Brassica napus L.) effective against Japanese field isolates by marker assisted selection. Breed. Sci. 2021, 71, 528–537. [Google Scholar] [CrossRef]
- Mehraj, H.; Akter, A.; Miyaji, N.; Miyazaki, J.; Shea, D.J.; Fujimoto, R.; Doullah, M.A. Genetics of Clubroot and Fusarium Wilt Disease Resistance in Brassica Vegetables: The Application of Marker Assisted Breeding for Disease Resistance. Plants 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Greer, S.F.; Surendran, A.; Grant, M.; Lillywhite, R. The current status, challenges, and future perspectives for managing diseases of brassicas. Front. Microbiol. 2023, 14, 1209258. [Google Scholar] [CrossRef] [PubMed]
- Klueken, A.M.; Mahfoud, Y.; Rößler, S.; Ludwig-Müller, J. Testing Effects of Seed Treatments against Clubroot Disease in Various Oilseed Rape Hybrids. Pathogens 2023, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Liang, Y.; Zhan, Z.; Li, X.; Piao, Z. Development of a Sinitic Clubroot Differential Set for the Pathotype Classification of Plasmodiophora brassicae. Front. Plant Sci. 2020, 11, 568771. [Google Scholar] [CrossRef]
- Piao, Z.; Lim, R.Y.P. Genetics of Clubroot Resistance in Brassica Species. J. Plant Growth Regul. 2009, 28, 252–264. [Google Scholar] [CrossRef]
- Shen, S.; Xu, S.; Wang, M.; Ma, T.; Chen, N.; Wang, J.; Zheng, H.; Yang, L.; Zou, D.; Xin, W.; et al. BSA-Seq for the Identification of Major Genes for EPN in Rice. Int. J. Mol. Sci. 2023, 24, 14838. [Google Scholar] [CrossRef]
- Dai, D.; Huang, L.; Zhang, X.; Zhang, S.; Yuan, Y.; Wu, G.; Hou, Y.; Yuan, X.; Chen, X.; Xue, C. Identification of a Branch Number Locus in Soybean Using BSA-Seq and GWAS Approaches. Int. J. Mol. Sci. 2024, 25, 873. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhao, L.; Chen, C.; Wang, J.; Lu, D. Conjunctive Analysis of BSA-Seq and SSR Markers Unveil the Candidate Genes for Resistance to Rice False Smut. Biomolecules 2024, 14, 79. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Z.; Sun, Z.; Qi, F.; Wang, J.; Wang, M.; Dong, W.; Cui, K.; Zhao, M.; Wang, X.; et al. Identification of two major QTLs for pod shell thickness in peanut (Arachis hypogaea L.) using BSA-seq analysis. BMC Genom. 2024, 25, 65. [Google Scholar] [CrossRef]
- Zheng, R.; Deng, M.; Lv, D.; Tong, B.; Liu, Y.; Luo, H. Combined BSA-Seq and RNA-Seq Reveal Genes Associated with the Visual Stay-Green of Maize (Zea mays L.). Int. J. Mol. Sci. 2023, 24, 17617. [Google Scholar] [CrossRef]
- Hasan, J.; Megha, S.; Rahman, H. Clubroot in Brassica: Recent advances in genomics, breeding, and disease management. Genome 2021, 64, 735–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, X.; Huang, F.; Yu, W.; Zhou, X.; Li, B.; Zhang, Y.; Chen, P.; Zhang, C. Fine Mapping of Clubroot Resistance Loci CRA8.1 and Candidate Gene Analysis in Chinese Cabbage (Brassica rapa L.). Front. Plant Sci. 2022, 13, 898108. [Google Scholar] [CrossRef]
- Gan, C.; Yan, C.; Pang, W.; Cui, L.; Fu, P.; Yu, X.; Qiu, Z.; Zhu, M.; Piao, Z.; Deng, X. Identification of Novel Locus RsCr6 Related to Clubroot Resistance in Radish (Raphanus sativus L.). Front. Plant Sci. 2022, 13, 866211. [Google Scholar] [CrossRef]
- Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Hill, J.T.; Demarest, B.L.; Bisgrove, B.W.; Gorsi, B.; Su, Y.C.; Yost, H.J. MMAPPR: Mutation mapping analysis pipeline for pooled RNA-seq. Genome Res. 2013, 23, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wan, J.M. SSRHunter: Development of a local searching software for SSR sites. Yi Chuan 2005, 27, 808–810. [Google Scholar]
- Ge, Y.; Ramchiary, N.; Wang, T.; Liang, C.; Wang, N.; Wang, Z.; Choi, S.R.; Lim, Y.P.; Piao, Z.Y. Development and linkage mapping of unigene-derived microsatellite markers in Brassica rapa L. Jpn. J. Breed. 2011, 61, 160–167. [Google Scholar] [CrossRef]
- Ooijen, J.; Jw, V.T.V. JoinMap 4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma BV: Wageningen, The Netherlands, 2006. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D.L. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Resistant Plant | Susceptible Plant | Theoretical Ratio | χ2 | χ20.05 | |
|---|---|---|---|---|---|
| 377 | 36 | 0 | |||
| 12A | 0 | 36 | |||
| F1 | 28 | 0 | |||
| F2 | 357 | 113 | 3:1 | 0.18 | 3.84 |
| Sample | R-Pool | S-Pool |
|---|---|---|
| Genome Length | 351,063,200 | 351,063,200 |
| Clean Reads | 72,065,694 | 67,798,184 |
| Clean Bases | 10,553,867,052 | 9,861,094,696 |
| Mapped Bases | 10,004,832,128 | 9,374,230,064 |
| Mapping Rate (%) | 94.80 | 95.06 |
| Duplication Rate (%) | 17.41 | 17.60 |
| Uniq Rate (%) | 67.00 | 67.46 |
| Mean Depth | 28.45 | 26.65 |
| Coverage Rate (%) (>=4X) | 94.88 | 94.97 |
| Genome Length | 351,063,200 | 351,063,200 |
| Gene | Homologous Genes | Functional Annotations |
|---|---|---|
| Bra020856 | AT4G22140 | Encoding a chromatin remodeling factor that regulates flowering time |
| Bra020857 | AT4G22120 | Calcium-permeable stretch-activated cation channel |
| Bra020858 | AT4G22100 | Beta glucosidase 2 |
| Bra020859 | AT4G22080 | Root hair specific 14 |
| Bra020860 | AT4G22060 | F-box protein |
| Bra020861 | AT3G25510 | Disease resistance protein (TIR-NBS-LRR class) family protein |
| Bra020862 | AT4G21910 | MATE efflux family protein |
| Bra020863 | AT4G21910 | MATE efflux family protein |
| Bra020864 | AT4G21895 | DNA binding protein |
| Bra020865 | AT4G21870 | HSP20-like chaperone |
| Bra020866 | AT4G21865 | Hypothetical protein |
| Bra020867 | AT4G32270 | Unknown |
| Bra020868 | AT4G21850 | Methionine sulfoxide reductase B9 |
| Bra020869 | AT3G18550 | Unknown |
| Bra020870 | AT4G21810 | DERLIN-2.1 |
| Bra020871 | AT4G21800 | Conserved hypothetical ATP binding protein |
| Bra020872 | AT4G21750 | Encodes a homeobox protein similar to GL2 |
| Bra020873 | AT4G21710 | Encodes the unique second-largest subunit of DNA-dependent RNA polymerase II |
| Bra020874 | AT4G21710 | Encodes the unique second-largest subunit of DNA-dependent RNA polymerase II |
| Bra020875 | AT4G20930 | Encodes a 3-hydroxyisobutyrate dehydrogenase |
| Bra020876 | AT4G20940 | Leucine-rich repeat N-terminal domain |
| Bra020877 | AT4G20960 | Cytidine and deoxycytidylate deaminase zinc-binding region |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Yang, Y.; Zhang, Y.; Zhan, Z.; Piao, Z. Genetic Mapping and Characterization of the Clubroot Resistance Gene BraPb8.3 in Brassica rapa. Int. J. Mol. Sci. 2024, 25, 10462. https://doi.org/10.3390/ijms251910462
Kong L, Yang Y, Zhang Y, Zhan Z, Piao Z. Genetic Mapping and Characterization of the Clubroot Resistance Gene BraPb8.3 in Brassica rapa. International Journal of Molecular Sciences. 2024; 25(19):10462. https://doi.org/10.3390/ijms251910462
Chicago/Turabian StyleKong, Liyan, Yi Yang, Yufei Zhang, Zongxiang Zhan, and Zhongyun Piao. 2024. "Genetic Mapping and Characterization of the Clubroot Resistance Gene BraPb8.3 in Brassica rapa" International Journal of Molecular Sciences 25, no. 19: 10462. https://doi.org/10.3390/ijms251910462
APA StyleKong, L., Yang, Y., Zhang, Y., Zhan, Z., & Piao, Z. (2024). Genetic Mapping and Characterization of the Clubroot Resistance Gene BraPb8.3 in Brassica rapa. International Journal of Molecular Sciences, 25(19), 10462. https://doi.org/10.3390/ijms251910462









