Structure–Function Relationship of a Novel MTX-like Peptide (MTX1) Isolated and Characterized from the Venom of the Scorpion Maurus palmatus
Abstract
1. Introduction
2. Results
2.1. MTX1 Toxin Purification
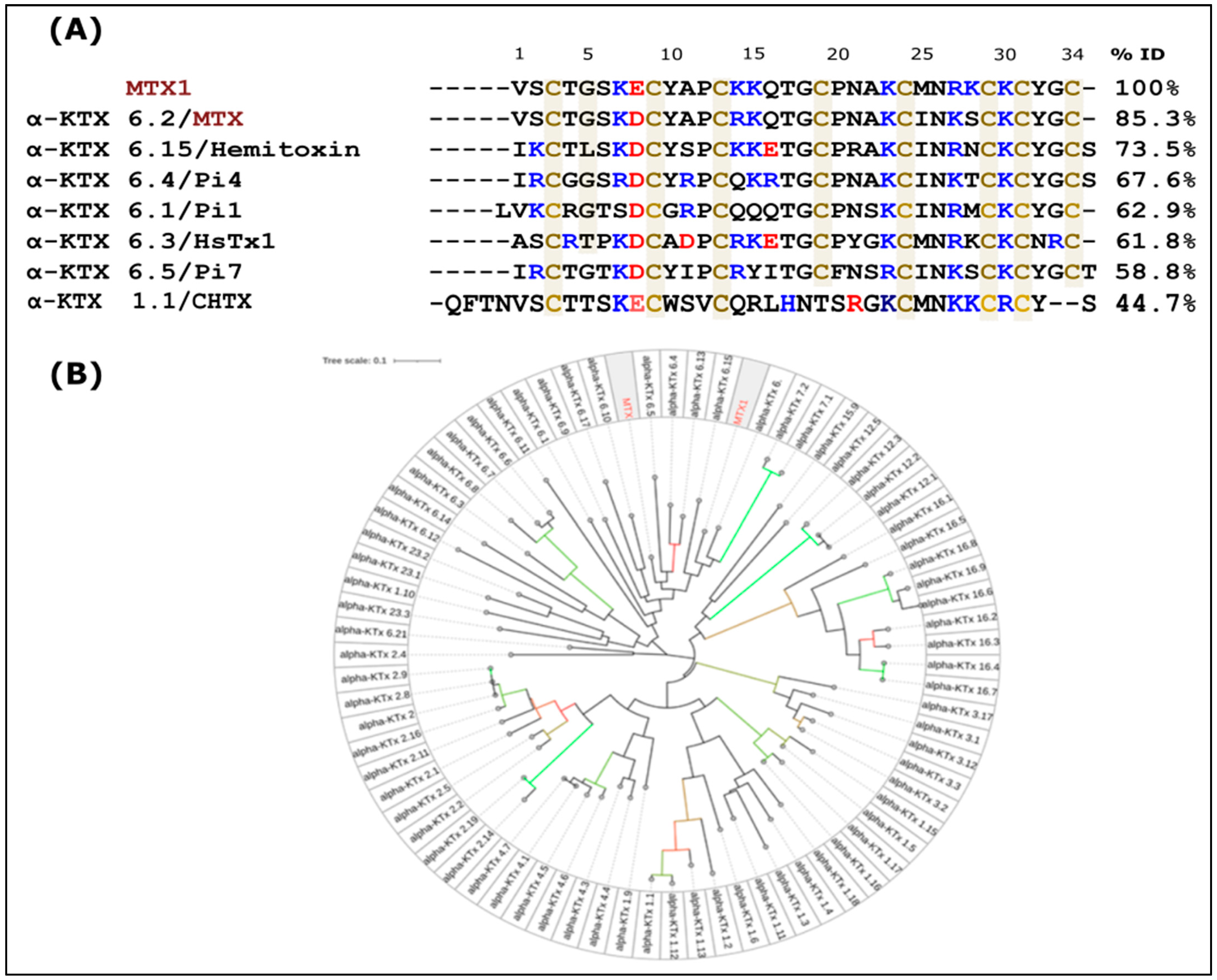
2.2. Amino Acid Sequence of MTX1 and Sequence Analysis
2.3. Neurotoxic Activity of MTX1
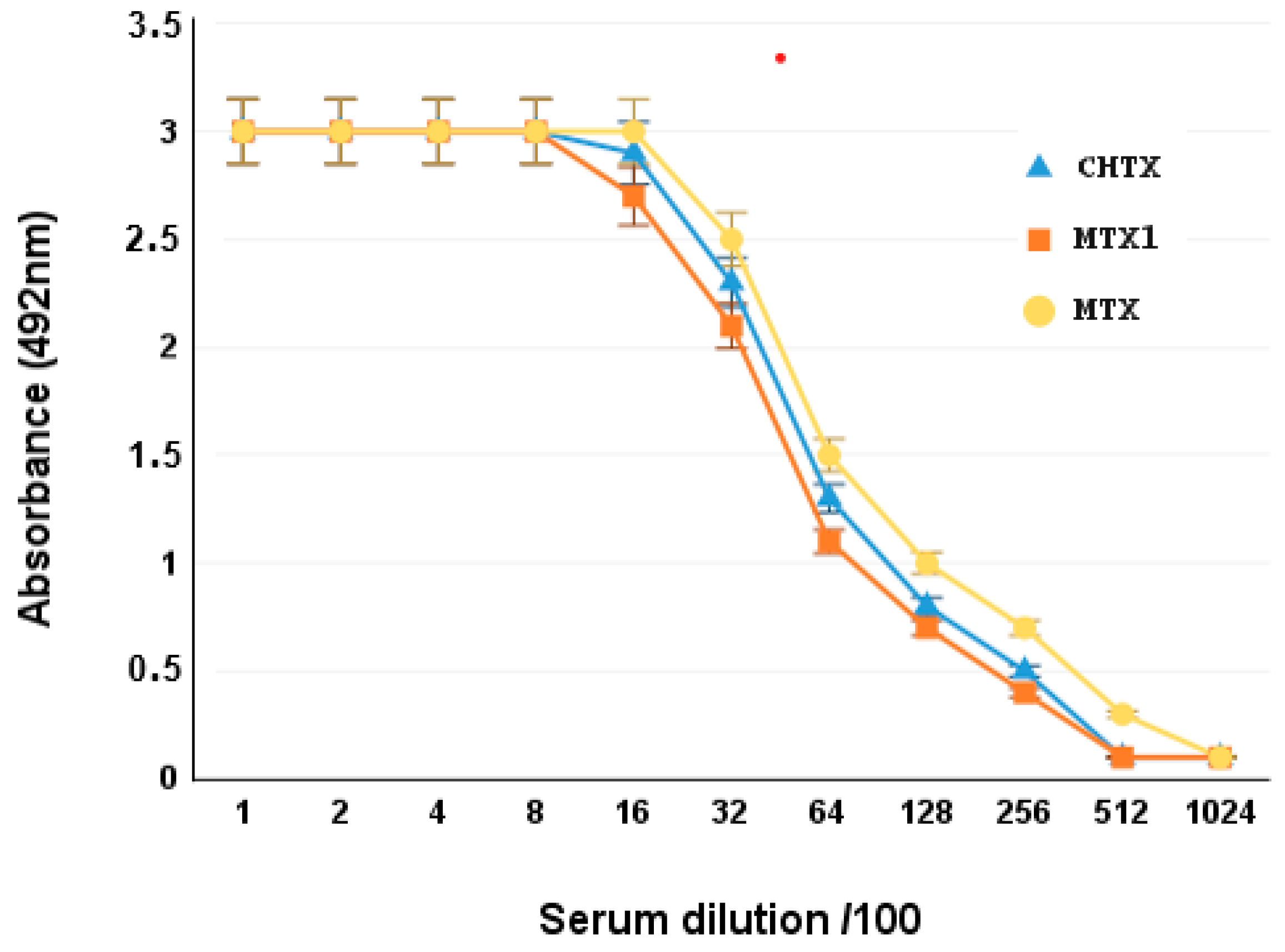
2.4. Cross-Antigenicity of MTX1 with MTX Toxin
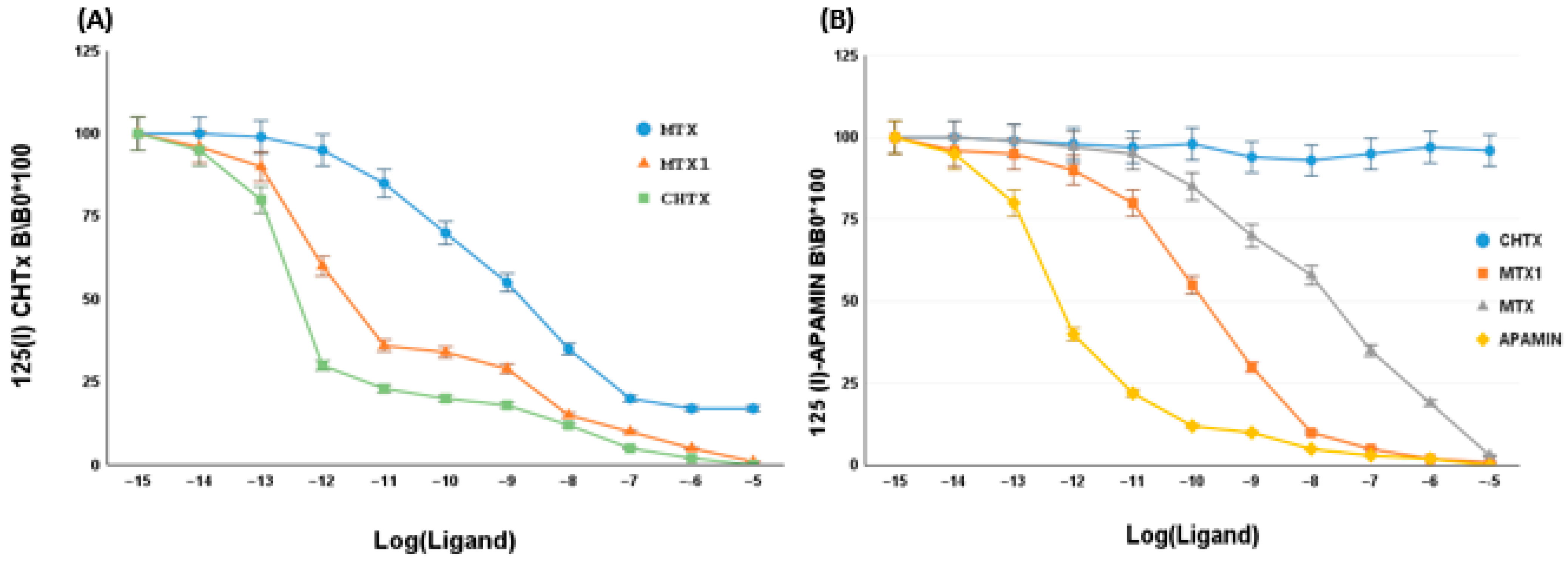
2.5. Effect of MTX1 on Rat Brain Synaptosomes
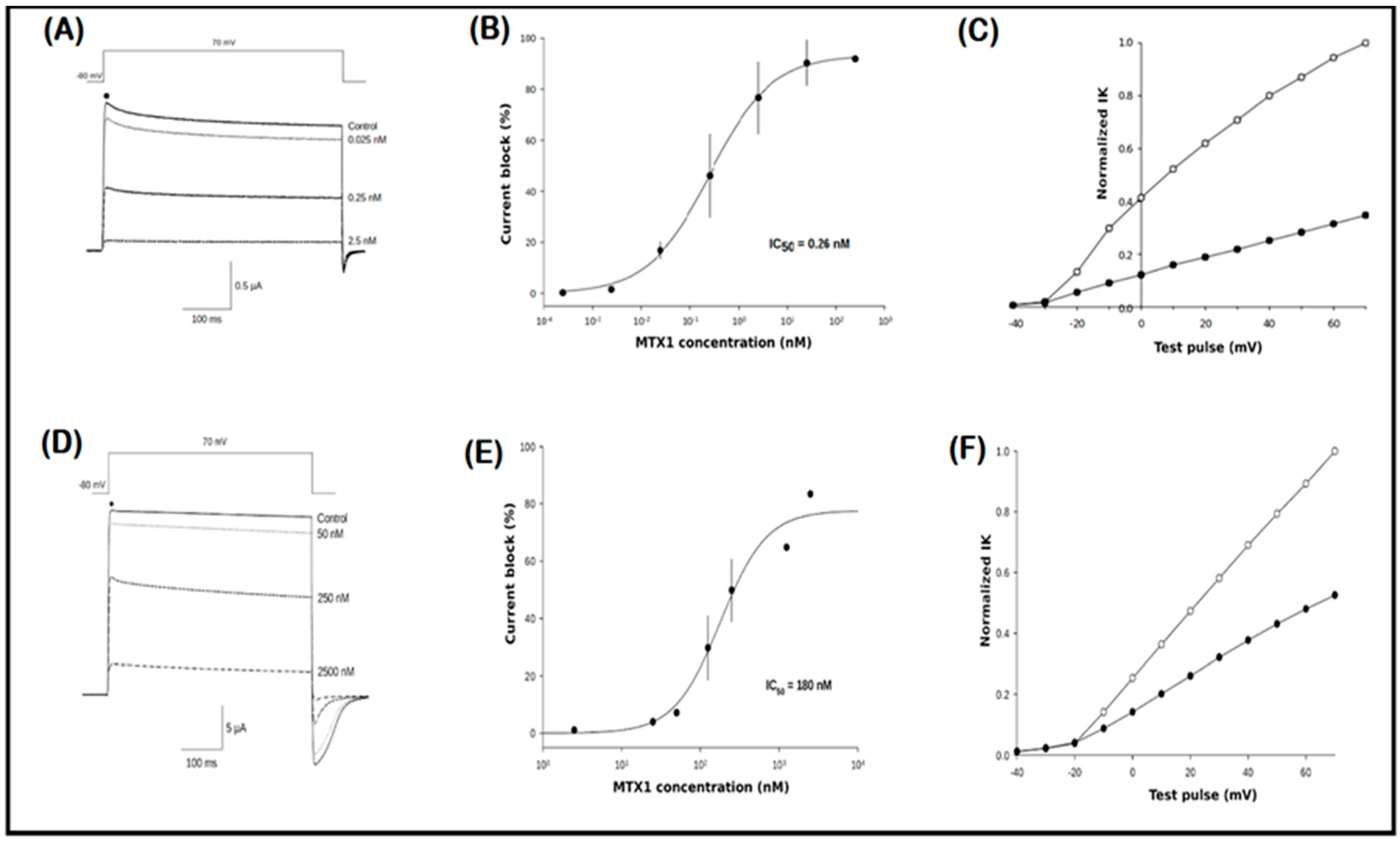
2.6. Electrophysiological Recordings
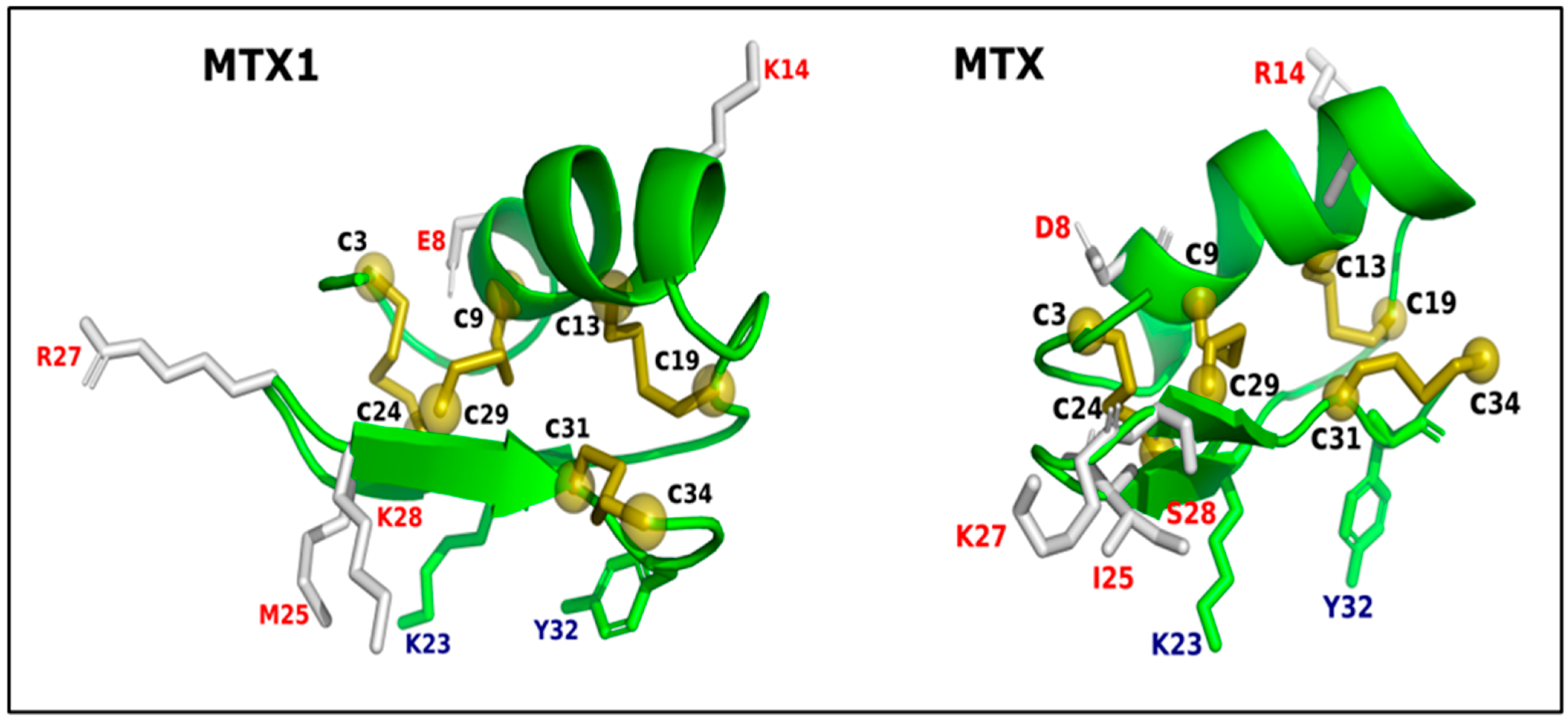
2.7. Molecular Modeling of MTX1, Kv1.2, Kv1.3 and SK2 Potassium Channels
2.8. Toxin–Channel Docking Study
3. Discussion
4. Materials and Methods
4.1. Scorpion Venom
4.2. Purification of MTX1
4.3. Amino Acid Analysis and Sequence of MTX1
4.4. Mass Spectrometry Analysis
4.5. The Neurotoxic Activity of MTX1 in Mice
4.6. Mice Immunization
4.7. ELISA Assays
4.8. Competition Binding Assay on Rat Brain Synaptosomes and Iodination of 125I-MTX1
4.9. Binding Assay for Kv Channels
4.10. Binding Assay for SKCa Channels
4.11. Oocyte Preparation and Electrophysiological Recordings
4.12. Computational Study
4.12.1. Phylogenetic Tree Determination
4.12.2. Molecular Modeling of MTX1, Kv1.2, Kv1.3 and SKCa2.2 Potassium Channel
4.12.3. Toxin–Channel Docking Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Kamata, S.; Kimura, M.; Ohyama, S.; Yamashita, S.; Shibukawa, Y. Large-Conductance Calcium-Activated Potassium Channels and Voltage-Dependent Sodium Channels in Human Cementoblasts. Front. Physiol. 2021, 12, 634846. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chung, S.H. Structural basis of the selective block of Kv1.2 by maurotoxin from computer simulations. PLoS ONE 2012, 7, e47253. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Perez, G.J.; Bonev, A.D.; Eckman, D.M.; Kosek, J.C.; Wiler, S.W.; Patterson, A.J.; Nelson, M.T.; Aldrich, R.W. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 2000, 407, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef]
- Choi, C.R.; Kim, E.J.; Choi, T.H.; Han, J.; Kang, D. Enhancing Human Cutaneous Wound Healing through Targeted Suppression of Large Conductance Ca2+-Activated K+ Channels. Int. J. Mol. Sci. 2024, 25, 803. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Q.; Zhu, Y.; Qi, H.; Qu, D.; Yao, Y.; Jia, Y.; Guo, J.; Cheng, J.; Ji, Y.; et al. Glycosylation of β1 subunit plays a pivotal role in the toxin sensitivity and activation of BK channels. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200182. [Google Scholar] [CrossRef]
- Peigneur, S.; Tytgat, J. Toxins in Drug Discovery and Pharmacology. Toxins 2018, 10, 126. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef]
- Jlassi, A.; Mekni-Toujani, M.; Ferchichi, A.; Gharsallah, C.; Malosse, C.; Chamot-Rooke, J.; ElAyeb, M.; Ghram, A.; Srairi-Abid, N.; Daoud, S. BotCl, the First Chlorotoxin-like Peptide Inhibiting Newcastle Disease Virus: The Emergence of a New Scorpion Venom AMPs Family. Molecules 2023, 28, 4355. [Google Scholar] [CrossRef]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion venom peptides: Molecular diversity, structural characteristics, and therapeutic use from channelopathies to viral infections and cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Cuellar, R.A.; Santana, C.J.C.; Magalhães, A.C.M.; Pires, O.R.; Fontes, W., Jr.; Castro, M.S. Scorpion Toxins and Ion Channels: Potential Applications in Cancer Therapy. Toxins 2020, 12, 326. [Google Scholar] [CrossRef]
- Gómez, R.; Lyz, J.; Muñoz, B.; Adriana, X.; Sierra, C.; Jhoalmis, R.M.; Laura, M.; Corredor, P.C. Scorpion venom: New promise in the treatment of cancer. Acta Biol. Colomb. 2019, 24, 213–223. [Google Scholar] [CrossRef]
- Khamessi, O.; Ben Mabrouk, H.; ElFessi-Magouri, R.; Kharrat, R. RK1, the first very short peptide from Buthus occitanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem. Biophys. Res. Commun. 2018, 499, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Choi, S.Y.; Ryu, P.D.; Lee, S.Y. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur. J. Pharmacol. 2011, 651, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Orlov, N.A.; Kryukova, E.V.; Efremenko, A.V.; Yakimov, S.A.; Toporova, V.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; Feofanov, A.V. Interactions of the Kv1.1 Channel with Peptide Pore Blockers: A Fluorescent Analysis on Mammalian Cells. Membranes 2023, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.U.; Carcamo-Noriega, E.; Beltran-Vidal, J.; Borrego, J.; Szanto, T.G.; Zamudio, F.Z.; Delgado-Prudencio, G.; Possani, L.D.; Panyi, G. Cm28, a scorpion toxin having a unique primary structure, inhibits KV1.2 and KV1.3 with high affinity. J. Gen. Physiol. 2022, 8, 154. [Google Scholar] [CrossRef]
- Diego-García, E.; Peigneur, S.; Debaveye, S.; Gheldof, E.; Tytgat, J.; Caliskan, F. Novel potassium channel blocker venom peptides from Mesobuthus gibbosus (Scorpiones: Buthidae). Toxicon 2013, 61, 72–82. [Google Scholar] [CrossRef]
- ElFessi, R.; Khamessi, O.; Srairi-Abid, N.; Sabatier, J.M.; Tytgat, J.; Peigneur, S.; Kharrat, R. Purification and Characterization of Bot33: A Non-Toxic Peptide from the Venom of Buthus occitanus tunetanus Scorpion. Molecules 2022, 27, 7278. [Google Scholar] [CrossRef]
- Bergeron, Z.L.; Bingham, J.P. Scorpion toxins specific for potassium (K+) channels: A historical overview of peptide bioengineering. Toxins 2012, 4, 1082–1119. [Google Scholar] [CrossRef]
- Fajloun, Z.; Mosbah, A.; Carlier, E.; Mansuelle, P.; Sandoz, G.; Fathallah, M.; di Luccio, E.; Devaux, C.; Rochat, H.; Darbon, H.; et al. Maurotoxin versus Pi1/HsTx1 scorpion toxins. Toward new insights in the under-standing of their distinct disulfide bridge patterns. J. Biol. Chem. 2000, 275, 50. [Google Scholar] [CrossRef]
- Miller, C. The charybdotoxin family of K+ channel-blocking peptides. Neuron 1995, 15, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, R.; Mabrouk, K.; Crest, M.; Darbon, H.; Oughideni, R.; Martin-Eauclaire, M.F.; Jacquet, G.; el Ayeb, M.; Van Rietschoten, J.; Rochat, H.; et al. Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur. J. Biochem. 1996, 242, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Srairi-Abid, N.; Shahbazzadeh, D.; Chatti, I.; Mlayah-Bellalouna, S.; Mejdoub, H.; Borchani, L.; Benkhalifa, R.; Akbari, A.; El Ayeb, M. Hemitoxin, the first potassium channel toxin from the venom of the Iranian scorpion Hemiscorpius lepturus. FEBS J. 2008, 275, 4641–4650. [Google Scholar] [CrossRef] [PubMed]
- Castle, N.A.; London, D.O.; Creech, C.; Fajloun, Z.; Stocker, J.W.; Sabatier, J.M. Maurotoxin: A potent inhibitor of intermediate conductance Ca2+-activated potassium channels. Mol. Pharmacol. 2003, 63, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, R.; Mansuelle, P.; Sampieri, F.; Crest, M.; Oughideni, R.; Van Rietschoten, J.; Martin-Eauclaire, M.F.; Rochat, H.; El Ayeb, M. Maurotoxin, a four disulfide bridge toxin from Scorpio maurus venom: Purification, structure and action on potassium channels. FEBS Lett. 1997, 406, 284–290. [Google Scholar] [CrossRef]
- Avdonin, V.; Nolan, B.; Sabatier, J.M.; De Waard, M.; Hoshi, T. Mechanisms of maurotoxin action on Shaker potassium channels. Biophys. J. 2000, 79, 776–787. [Google Scholar] [CrossRef]
- Lecomte, C.; Ben Khalifa, R.; Martin-Eauclaire, M.F.; Kharrat, R.; El Ayeb, M.; Darbon, H.; Rochat, H.; Crest, M.; Sabatier, J.M. Maurotoxin and the Kv1.1 channel: Voltage-dependent binding upon enantiomerization of the scorpion toxin disulfide bridge Cys31–Cys34. J. Pept. Res. 2000, 55, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Han, S.M.; Park, K.K. Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Maruyama, Y.; Igarashi, R.; Ushiku, Y.; Mitsutake, A. Analysis of Protein Folding Simulation with Moving Root Mean Square Deviation. J. Chem. Inf. Model. 2023, 63, 1529–1541. [Google Scholar] [CrossRef]
- Lebrun, B.; Romi-Lebrun, R.; Martin-Eauclaire, M.F.; Yasuda, A.; Ishiguro, M.; Oyama, Y.; Pongs, O.; Nakajima, T. A four-disulphide-bridges toxin, with high affinity towards voltage-gated K+ channels, isolated from Heterometrus spinnifer (Scorpionidae) venom. Biochem. J. 1997, 328, 321–327. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; Gómez-Lagunas, F.; Gurrola, G.B.; Possani, L.D. A novel structural class of K+-channel blocking toxin from the scorpion Pandinus imperator. Biochem. J. 1996, 315, 977–981. [Google Scholar] [CrossRef]
- Auguste, P.; Hugues, M.; Mourre, C.; Moinier, D.; Tartar, A.; Lazdunski, M. Scyllatoxin, a blocker of Ca (2+)-activated K+ channels: Structure-function relationships and brain localization of the binding sites. Biochemistry 1992, 31, 648–654. [Google Scholar] [CrossRef]
- Srairi-Abid, N.; Mansuelle, P.; Mejri, T.; Karoui, H.; Rochat, H.; Sampieri, F.; El Ayeb, M. Purification, characterization and molecular modelling of two toxin-like proteins from the Androctonus australis Hector venom. Eur. J. Biochem. 2000, 267, 5614–5620. [Google Scholar] [CrossRef]
- Galeotti, N.; Bartolini, A.; Ghelardini, C. Diphenhydramine-induced amnesia is mediated by Gi-protein activation. Neuroscience 2003, 122, 471–478. [Google Scholar] [CrossRef]
- Gray, E.G.; Whittaker, V.P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962, 96, 79–88. [Google Scholar]
- Leinonen, R.; Diez, F.G.; Binns, D.; Fleischmann, W.; Lopez, R.; Apweiler, R. UniProt archive. Bioinformatics 2004, 20, 3236–3237. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Hollich, V.; Milchert, L.; Arvestad, L.; Sonnhammer, E.L. Assessment of Protein Distance Measures and Tree-Building Methods for Phylogenetic Tree Reconstruction. Mol. Biol. Evol. 2005, 22, 2257–2264. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modeling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Shen, M.Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Rullmannn, J.A.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [CrossRef]
- Vincenzi, M.; Mercurio, F.A.; Leone, M. Protein Interaction Domains: Structural Features and Drug Discovery Applications (Part 2). Curr. Med. Chem. 2021, 28, 854–892. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Khamessi, O.; Ben Mabrouk, H.; Othman, H.; ElFessi-Magouri, R.; De Waard, M.; Hafedh, M.; Marrakchi, N.; Srairi-Abid, N.; Kharrat, R. RK, the first scorpion peptide with dual disintegrin activity on α1β1 and αvβ3 integrins. Int. J. Biol. Macromol. 2018, 120 (Pt B), 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; De Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElFessi, R.; Khamessi, O.; De Waard, M.; Srairi-Abid, N.; Ghedira, K.; Marrouchi, R.; Kharrat, R. Structure–Function Relationship of a Novel MTX-like Peptide (MTX1) Isolated and Characterized from the Venom of the Scorpion Maurus palmatus. Int. J. Mol. Sci. 2024, 25, 10472. https://doi.org/10.3390/ijms251910472
ElFessi R, Khamessi O, De Waard M, Srairi-Abid N, Ghedira K, Marrouchi R, Kharrat R. Structure–Function Relationship of a Novel MTX-like Peptide (MTX1) Isolated and Characterized from the Venom of the Scorpion Maurus palmatus. International Journal of Molecular Sciences. 2024; 25(19):10472. https://doi.org/10.3390/ijms251910472
Chicago/Turabian StyleElFessi, Rym, Oussema Khamessi, Michel De Waard, Najet Srairi-Abid, Kais Ghedira, Riadh Marrouchi, and Riadh Kharrat. 2024. "Structure–Function Relationship of a Novel MTX-like Peptide (MTX1) Isolated and Characterized from the Venom of the Scorpion Maurus palmatus" International Journal of Molecular Sciences 25, no. 19: 10472. https://doi.org/10.3390/ijms251910472
APA StyleElFessi, R., Khamessi, O., De Waard, M., Srairi-Abid, N., Ghedira, K., Marrouchi, R., & Kharrat, R. (2024). Structure–Function Relationship of a Novel MTX-like Peptide (MTX1) Isolated and Characterized from the Venom of the Scorpion Maurus palmatus. International Journal of Molecular Sciences, 25(19), 10472. https://doi.org/10.3390/ijms251910472








