NLRP3 Inflammasome in the Pathogenesis of Miscarriages
Abstract
1. Introduction
2. Results
2.1. Measurement of Selected Protein Concentrations by Means of the ELISA Method
2.2. Measurement of Ca, K, Mg, and Na Concentrations by Means of Spectroscopy
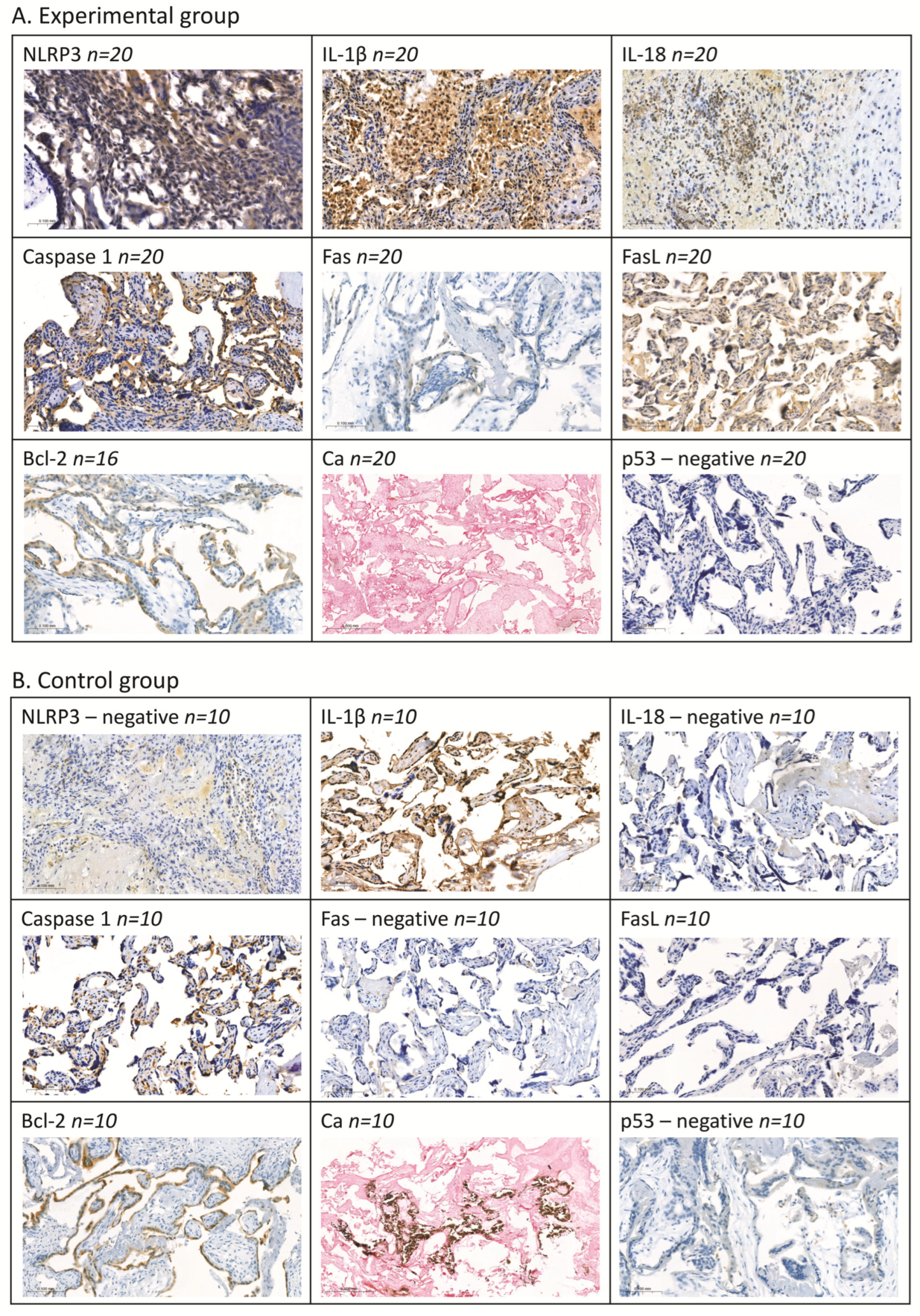
2.3. Assessment of Selected Proteins by Means of Immunohistochemical Methods
2.4. Correlations
2.5. ROC Curves
3. Discussion
4. Materials and Methods
4.1. Study and Control Group
4.2. Materials
4.3. Methods
4.3.1. Determination of Selected Proteins by Means of the Enzyme-Linked Immunosorbent Assay (ELISA) Method
4.3.2. Determination of Ca, K, Mg, and Na by Means of the ICP-OES Method
4.3.3. Analysis of Selected Proteins by Means of Immunohistochemical Methods
4.3.4. Determination of Calcium Deposits Using the Von Kossa Method
4.3.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weeks, A.; Gemzell, D.K. Spontaneous miscarriage in the first trimester. BMJ 2006, 332, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins Gynecology. ACOG practice bulletin No. 200: Early pregnancy loss. Obstet. Gynecol. 2018, 132, e197–e207. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. WHO: Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a New certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet. Gynecol. Scand. 1977, 56, 247–253. [Google Scholar]
- Wilcox, A.J.; Morken, N.-H.; Weinberg, C.R.; Håberg, S.E. Role of maternal age and pregnancy history in risk of miscarriage: Prospective register based study. BMJ 2019, 364, l869. [Google Scholar] [CrossRef]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef]
- Srinivas, S.K.; Ma, Y.; Sammel, M.D.; Chou, D.; McGrath, C.; Parry, S.; Elovitz, M.A. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am. J. Obstet. Gynecol. 2006, 195, 797–802. [Google Scholar] [CrossRef]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef]
- Yamada, H.; Morikawa, M.; Furuta, I.; Kato, E.H.; Shimada, S.; Sata, F.; Kishi, R.; Minakami, H. Circulating cytokines during early pregnancy in women with recurrent spontaneous abortion: Decreased TNF-alpha levels in abortion with normal chromosome karyotype. Hokkaido Igaku Zasshi 2004, 79, 237–241. [Google Scholar]
- Kasap, E.; Karaarslan, S.; Gene, M.; Gur, E.B.; Sahin, N.; Guclu, S. The role of cytokines in first trimester pregnancy losses with fetal chromosomal anomaly. Ginekol. Pol. 2015, 86, 827–832. [Google Scholar] [CrossRef]
- Goddijn, M.; Leschot, N.J. Genetic aspects of miscarriage. Baillieres Best. Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 855–865. [Google Scholar] [CrossRef]
- Philipp, T.; Philipp, K.; Reiner, A.; Beer, F.; Kalousek, D.K. Embryoscopic and cytogenetic analysis of 233 missed abortions: Factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum. Reprod. 2003, 18, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Tasadduq, R.; Ajmal, L.; Batool, F.; Zafar, T.; Babar, A.; Riasat, A.; Shakoori, A.R. Interplay of immune components and their association with recurrent pregnancy loss. Hum. Immunol. 2021, 82, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Omeljaniuk, W.J.; Jabłońska, E.; Garley, M.; Pryczynicz, A.; Ratajczak-Wrona, W.; Socha, K.; Borawska, M.H.; Charkiewicz, A.E. Biomarkers of neutrophil extracellular traps (NETs) and nitric oxide-(NO)-dependent oxidative stress in women who miscarried. Sci. Rep. 2020, 10, 13088. [Google Scholar] [CrossRef] [PubMed]
- Omeljaniuk, W.J.; Borawska, M.H.; Socha, K.; Charkiewicz, A.E.; Laudański, T.; Kulikowski, M.; Kobylec, E. Antioxidant status in women who had a miscarriage. Adv. Med. Sci. 2015, 60, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Omeljaniuk, W.J.; Charkiewicz, A.E.; Garley, M.; Ratajczak-Wrona, W.; Czerniecki, J.; Jabłońska, E.; Cechowska-Pasko, M.; Miltyk, W. Bisphenol A: Potential Factor of Miscarriage in Women in the Context of the Phenomenon of Neutrophil Extracellular Traps. Arch. Immunol. Ther. Exp. 2022, 70, 24. [Google Scholar] [CrossRef]
- Niedźwiedzka-Rystwej, P.; Ratajczak, W.; Tokarz-Deptuła, B.; Deptuła, W. Role and characteristics of inflammasome. Post. Biol. Kom. 2016, 43, 237–254. (In Polish) [Google Scholar]
- Iwaniuk, A.; Jablonska, E. Neutrophils in Health and Disease: From Receptor Sensing to Inflammasome Activation. Int. J. Mol. Sci. 2023, 24, 6340. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Cassel, S.L.; Joly, S.; Sutterwala, F.S. The NLRP3 inflammasome: A sensor of immune danger signals. Semin. Immunol. 2009, 21, 194–198. [Google Scholar] [CrossRef]
- Duez, H.; Pourcet, B. Nuclear Receptors in the Control of the NLRP3 Inflammasome Pathway. Front. Endocrinol. 2021, 12, 630536. [Google Scholar] [CrossRef] [PubMed]
- Storek, K.M.; Monack, D.M. Bacterial recognition pathways that lead to inflammasome activation. Immunol. Rev. 2015, 265, 112–129. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a026716. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Ma, F.; Xiao, J.; Yang, L.; Li, N.; Chen, D. NLRP3 inflammasome as the potential target mechanism and therapy in recurrent spontaneous abortions. Mol. Med. Rep. 2019, 19, 1935–1941. [Google Scholar] [CrossRef]
- Gao, P.; Zha, Y.; Gong, X.; Qiao, F.; Liu, H. The role of maternal-foetal interface inflammation mediated by NLRP3inflammasome in the pathogenesis of recurrent spontaneous abortion. Placenta 2020, 101, 221–229. [Google Scholar] [CrossRef]
- Li, M.; Sun, T.; Wu, X.; An, P.; Wu, X.; Dang, H. Autophagy in the HTR-8/SVneo cell oxidative stress model is associated with the NLRP1 inflammasome. Oxidative Med. Cell Longev. 2021, 2021, 2353504. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, C.; Zhao, Y.; Yang, L.; Wang, R.; Shen, D.; Ren, N.; Zhang, Q. Inflammasomes in humanreproductivediseases. Mol. Hum. Reprod. 2023, 29, gaad035. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Tersigni, C.; Marana, R.; Di Nicuolo, F.; Gaglione, R.; Rossi, E.D.; Castellani, R.; Scambia, G.; Di Simone, N. Inflammosome in the human endometrium: Further step in the evaluation of the “maternal side”. Fertil. Steril. 2016, 105, 111–118.e4. [Google Scholar] [CrossRef]
- Balci, C.N.; Acar, N. NLRP3 inflammasome pathway, the hidden balance in pregnancy: A comprehensive review. J. Reprod. Immunol. 2024, 161, 104173. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Taylor, H.S. The role of growth factors and cytokines during implantation: Endocrine and paracrine interactions. Semin. Reprod. Med. 2009, 27, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Motomura, K.; Miller, D.; Garcia-Flores, V.; Galaz, J.; Romero, R. Inflammasomes: Their role in normal and complicated pregnancies. J. Immunol. 2019, 203, 2757–2769. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Jana, S.K.; Pasricha, P.; Ghosh, S.; Chakravarty, B.; Chaudhury, K. Proinflammatory cytokines induced altered expression of cyclooxygenase-2 gene results in unreceptive endometrium in women with idiopathic recurrent spontaneous miscarriage. Fertil. Steril. 2013, 99, 179–187. [Google Scholar] [CrossRef]
- Mulla, M.J.; Salmon, J.E.; Chamley, L.W.; Brosens, J.J.; Boeras, C.M.; Kavathas, P.B.; Abrahams, V.M. A role for uricacid and the Nalp3inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS ONE 2013, 8, e65237. [Google Scholar] [CrossRef]
- Petrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Koumangoye, R. The role of Cl− and K+ efflux in NLRP3 inflammasome and innate immune response activation. Am. J. Physiol. Cell Physiol. 2022, 322, C645–C652. [Google Scholar] [CrossRef]
- Kot, K.; Łanocha-Arendarczyk, N.; Kupnicka, P.; Szymański, S.; Malinowski, W.; Kalisińska, E.; Chlubek, D.; Kosik-Bogacka, D. Selected Metal Concentration in Maternal and Cord Blood. Int. J. Environ. Res. Public Health 2021, 18, 12407. [Google Scholar] [CrossRef]
- Mazurek, D.; Łoźna, K.; Bronkowska, M. The Concentration of Selected Elements in the Placenta According to Selected Sociodemographic Factors and Their Effect on Birth Mass and Birth Length of Newborns. J. Trace Elem. Med. Biol. 2020, 58, 126425. [Google Scholar] [CrossRef]
- Guenther, S.; Vrekoussis, T.; Heublein, S.; Bayer, B.; Anz, D.; Knabl, J.; Navrozoglou, I.; Dian, D.; Friese, K.; Makrigiannakis, A.; et al. Decidualmacrophages are significantly increased in spontaneousmiscarriages and over-express FasL: A potential role for macrophages in trophoblast apoptosis. Int. J. Mol. Sci. 2012, 13, 9069–9080. [Google Scholar] [CrossRef]
- Atia, T.A. Placental apoptosis in recurrent miscarriage. Kaohsiung J. Med. Sci. 2017, 33, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Klatka, M.; Klatka, J. Types of cell death. Pomeranian J. Life Sci. 2015, 61, 411–418. (In Polish) [Google Scholar] [PubMed]
- Yampolsky, M.; Salafia, C.M.; Shlakhter, O.; Haas, D.; Eucker, B.; Thorp, J. Modeling the Variability of Shapes of a Human Placenta. Placenta 2008, 29, 790–797. [Google Scholar] [CrossRef]
- Belkacemi, L.; Simoneau, L.; Lafond, J. Calcium-binding proteins: Distribution and implication in mammalian placenta. Endocrine 2002, 19, 57–64. [Google Scholar] [CrossRef]
- Rossi, C.; Gerosa, C.; Pampaloni, P.; Puddu, M.; Ravarino, A.; Angioni, S.; Fanni, D.; Faa, G. Placental Calcification Score: A new semiquantitative method to assess pattern and grading of placental calcifications. JPNIM 2019, 8, e080206. [Google Scholar] [CrossRef]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Criticalrole for calciummobilization in activation of the NLRP3inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Ponneri Babuharisankar, A.; Lin, Y.C.; Lien, H.W.; Lo, Y.K.; Chou, H.Y.; Tangeda, V.; Cheng, L.C.; Cheng, A.N.; Lee, A.Y. Mitochondrialoxidativestress in the tumormicroenvironment and cancerimmunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Papierkowski, A. Importance of magnesium in clinical practice. Part II. Diagnosis and therapy of magnesium disorders. Med. Rodz. 2002, 2, 84–88. (In Polish) [Google Scholar]
- Gong, L.; Yang, Q.; Liu, C.W.; Wang, X.; Zeng, H.L. Assessment of 12Essential and ToxicElements in Whole Blood of Pregnant and Non-Pregnant Women Living in Wuhan of China. Biol. Trace Elem. Res. 2021, 199, 2121–2130. [Google Scholar] [CrossRef]
- Thomas, L. Clinical Laboratory Diagnostics Use and Assessment of Clinical Laboratory Results, 1st ed.; TH-Books Verlags Gesellschaft: Frankfurt/Main, Germany, 1998; pp. 231–241. [Google Scholar]
- Grzeszczak, K.; Kapczuk, P.; Kupnicka, P.; Cecerska-Heryć, E.; Kwiatkowski, S.; Chlubek, D.; Kosik-Bogacka, D. Calcium, Potassium, Sodium, and MagnesiumConcentrations in the Placenta, Umbilical Cord, and Fetal Membrane from Women with Multiple Pregnancies. Life 2023, 13, 153. [Google Scholar] [CrossRef]
- Vladimer, I.G.; Marty-Roix, R.; Ggosh, S.; Weng, D.; Lien, E. Inflammasomes and host defenses against infections. Curr. Opin. Microbiol. 2013, 16, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1—Activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ciraci, C.; Janczy, J.R.; Sutterwala, F.S.; Cassel, S.L. Control of innate adaptive immunity by the inflammasome. Microbes Infect. 2012, 14, 1263–1270. [Google Scholar] [CrossRef]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Flavell, R.A. The missing link: How the inflammasome senses oxidative stress. Immunol. Cell Biol. 2010, 88, 510–512. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–226. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]


| Non-parametric tests | Parametric tests | |||||||||
| Experimental group | ||||||||||
| Parameter | Serum | Parameter | Serum | Parameter | Whole blood | |||||
| CYC S [ng/mL] n = 32 | IL-1β [pg/mL] n = 33 | IL-18 [pg/mL] n = 31 | NLRP3 [ng/mL] n = 44 | Na [mmol/L] n = 66 | K [mmol/L] n = 59 | Mg [mmol/L] n = 65 | Ca [mmol/L] n = 73 | |||
| Median | 1.485 | 15.23 | 19.15 * | Mean | 0.133 * | Mean | 3.622 | 3.125 * | 1.569 | 2.120 * |
| Q1 | 1.382 | 13.39 | 14.05 | SD | 0.068 | SD | 0.220 | 0.280 | 0.130 | 0.187 |
| Q3 | 1.562 | 46.00 | 37.19 | - | - | - | - | - | - | - |
| Min. | 1.331 | 9.122 | 7.929 | Min. | 0.038 | Min. | 3.027 | 2.175 | 1.280 | 1.754 |
| Max. | 1.946 | 95.24 | 75.62 | Max. | 0.295 | Max. | 4.035 | 3.485 | 1.761 | 2.483 |
| “NET-negative” group | ||||||||||
| Parameter | Serum | Parameter | Serum | Parameter | Whole blood | |||||
| CYC S [ng/mL] n = 23 | IL-1β [pg/mL] n = 22 | IL-18 [pg/mL] n = 18 | NLRP3 [ng/mL] n = 30 | Na [mmol/L] n = 41 | K [mmol/L] n = 37 | Mg [mmol/L] n = 41 | Ca [mmol/L] n = 45 | |||
| Median | 1.510 | 34.01 ac | 23.93 a | Mean | 0.137 | Mean | 3.633 | 3.107 a | 1.563 | 2.114 a |
| Q1 | 1.421 | 14.09 | 15.46 | SD | 0.064 | SD | 0.226 | 0.268 | 0.125 | 0.193 |
| Q3 | 1.562 | 50.26 | 57.04 | - | - | - | - | - | - | - |
| Min. | 1.331 | 10.28 | 9.554 | Min. | 0.043 | Min. | 3.027 | 2.367 | 1.280 | 1.808 |
| Max. | 1.690 | 91.40 | 75.62 | Max. | 0.163 | Max. | 4.019 | 3.485 | 1.761 | 2.483 |
| “NET-positive” group | ||||||||||
| Parameter | Serum | Parameter | Serum | Parameter | Whole blood | |||||
| CYC S [ng/mL] n = 9 | IL-1β [pg/mL] n = 11 | IL-18 [pg/mL] n = 13 | NLRP3 [ng/mL] n = 14 | Na [mmol/L] n = 25 | K [mmol/L] n = 22 | Mg [mmol/L] n = 24 | Ca [mmol/L] n = 28 | |||
| Median | 1.408 | 13.58 | 16.86 | Mean | 0.123 | Mean | 3.605 | 3.155 | 1.581 | 2.130 b |
| Q1 | 1.357 | 11.06 | 10.86 | SD | 0.076 | SD | 0.213 | 0.304 | 0.140 | 0.180 |
| Q3 | 1.536 | 15.13 | 32.29 | - | - | - | - | - | - | - |
| Min. | 1.357 | 9.122 | 7.929 | Min. | 0.038 | Min. | 3.245 | 2.175 | 1.291 | 1.754 |
| Max. | 1.946 | 95.24 | 43.57 | Max. | 0.295 | Max. | 4.035 | 3.477 | 1.760 | 2.480 |
| Control group | ||||||||||
| Parameter | Serum | Parameter | Serum | Parameter | Whole blood | |||||
| CYC S [ng/mL] n = 5 | IL-1β [pg/mL] n = 10 | IL-18 [pg/mL] n = 10 | NLRP3 [ng/mL] n = 5 | Na [mmol/L] n = 9 | K [mmol/L] n = 11 | Mg [mmol/L] n = 11 | Ca [mmol/L] n = 10 | |||
| Median | 1.536 | 13.15 | 13.40 | Mean | 0.069 | Mean | 3.776 | 3.359 | 1.644 | 2.300 |
| Q1 | 1.421 | 11.93 | 9.230 | SD | 0.067 | SD | 0.304 | 0.387 | 0.139 | 0.308 |
| Q3 | 1.728 | 18.36 | 22.59 | - | - | - | - | - | - | - |
| Min. | 1.305 | 10.67 | 6.084 | Min. | 0.008 | Min. | 3.440 | 2.824 | 1.379 | 1.937 |
| Max. | 1.921 | 31.10 | 33.59 | Max. | 0.137 | Max. | 4.336 | 4.083 | 1.885 | 2.957 |
| p-value | ||||||||||
| a 0.0207 c 0.0409 | * 0.0457 a 0.0141 | * 0.0427 | * 0.0196 a 0.0169 | * 0.0108 a 0.0114 b 0.0278 | ||||||
| Group | Correlated Parameters | p | r |
|---|---|---|---|
| Experimental | NLRP3 vs. Ca | 0.0198 | 0.3501 |
| NLRP3 vs. Na | 0.0081 | 0.394 | |
| Na vs. Ca | 0.0078 | 0.3298 | |
| “NET-negative” | NLRP3 vs. Ca | 0.022 | 0.456 |
| IL-18 vs. Ca | 0.028 | −0.517 | |
| “NET-positive” | K vs. Ca | 0.0227 | 0.5192 |
| Na vs. Ca | 0.0301 | 0.4342 |
| DeLong’s Method | Serum | Whole Blood | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | CYC S | IL-1β | IL-18 | NLRP3 | Na | K | Mg | Ca |
| Women with miscarriage vs. control | ||||||||
| AUC | 0.541 | 0.680 | 0.712 | 0.646 | 0.641 | 0.636 | 0.639 | 0.708 |
| SE (AUC) | 0.287 | 0.086 | 0.095 | 0.093 | 0.112 | 0.100 | 0.090 | 0.097 |
| −95% CI | 0 | 0.511 | 0.525 | 0.462 | 0.420 | 0.438 | 0.461 | 0.517 |
| +95% CI | 1 | 0.849 | 0.900 | 0.830 | 0.862 | 0.833 | 0.816 | 0.899 |
| Z | 0.235 | 1.710 | 2.003 | 1.436 | 1.369 | 1.428 | 1.468 | 2.126 |
| p | 0.813 | 0.087 | 0.045 | 0.150 | 0.170 | 0.153 | 0.141 | 0.033 |
| NET negative vs. control | ||||||||
| AUC | 0.543 | 0.772 | 0.766 | 0.728 | 0.626 | 0.720 | 0.674 | 0.702 |
| SE (AUC) | 0.293 | 0.083 | 0.095 | 0.089 | 0.119 | 0.098 | 0.094 | 0.097 |
| −95% CI | 0 | 0.609 | 0.579 | 0.552 | 0.391 | 0.526 | 0.488 | 0.510 |
| +95% CI | 1 | 0.936 | 0.953 | 0.903 | 0.860 | 0.913 | 0.859 | 0.893 |
| Z | 0.240 | 2.439 | 2.301 | 2.081 | 1.174 | 2.008 | 1.758 | 1.985 |
| p | 0.809 | 0.014 | 0.021 | 0.037 | 0.240 | 0.044 | 0.078 | 0.047 |
| NET positive vs. control | ||||||||
| AUC | 0.537 | 0.504 | 0.638 | 0.696 | 0.666 | 0.590 | 0.579 | 0.717 |
| SE (AUC) | 0.283 | 0.135 | 0.121 | 0.187 | 0.112 | 0.116 | 0.105 | 0.106 |
| −95% CI | 0 | 0.239 | 0.400 | 0.328 | 0.445 | 0.362 | 0.372 | 0.508 |
| +95% CI | 1 | 0.769 | 0.876 | 1 | 0.888 | 0.819 | 0.786 | 0.927 |
| Z | 0.184 | 0.035 | 1.116 | 1.168 | 1.463 | 0.840 | 0.746 | 2.022 |
| p | 0.853 | 0.971 | 0.264 | 0.242 | 0.143 | 0.400 | 0.455 | 0.043 |
| NET negative vs. NET positive | ||||||||
| AUC | 0.642 | 0.766 | 0.668 | 0.577 | 0.538 | 0.582 | 0.549 | 0.523 |
| SE (AUC) | 0.129 | 0.081 | 0.099 | 0.101 | 0.075 | 0.078 | 0.079 | 0.070 |
| −95% CI | 0.389 | 0.605 | 0.474 | 0.378 | 0.390 | 0.427 | 0.394 | 0.385 |
| +95% CI | 0.895 | 0.926 | 0.863 | 0.776 | 0.686 | 0.736 | 0.705 | 0.660 |
| Z | 1.236 | 2.606 | 1.581 | 0.818 | 0.522 | 1.050 | 0.666 | 0.328 |
| p | 0.216 | 0.009 | 0.113 | 0.412 | 0.601 | 0.293 | 0.505 | 0.742 |
| Parameter | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Women with miscarriage vs. control | ||||
| IL-18 | 64.52% | 70.00% | 86.96% | 38.89% |
| NLRP3 | 54.55% | 70.00% | 88.89% | 25.93% |
| K | 55.93% | 63.64% | 89.19% | 21.21% |
| Ca | 89.04% | 50.00% | 92.86% | 38.46% |
| NET negative vs. control | ||||
| IL-1β | 68.18% | 70.00% | 83.33% | 50.00% |
| IL-18 | 72.22% | 70.00% | 81.25% | 58.33% |
| K | 56.00% | 80.00% | 87.50% | 42.11% |
| Ca | 86.67% | 50.00% | 88.64% | 45.45% |
| NET positive vs. control | ||||
| Ca | 57.14% | 80.00% | 88.89% | 40.00% |
| NET negative vs. NET positive | ||||
| IL-1β | 92.86% | 60.00% | 61.90% | 92.31% |
| Optical System | Polichromator Eschelle |
|---|---|
| Wavelength | Ca 317.333 nm |
| Mg 285.213 nm | |
| K 766.49 nm | |
| Na 589.592 nm | |
| Detector | Semiconductor device (SCD) |
| RF generator power | 1.3 kW |
| Radio frequency | 27.12 MHz |
| Plasma observation | Radial |
| Pomp rate | 1 mL/min |
| Integration time | 5 s |
| Spray chamber | Cyclonic with concentric Mira-mist nebuliser |
| Gas flow | auxiliary 0.2 L/min |
| plasma 13 L/min | |
| nebulizer 0.55 L/min |
| Step | Power (W) | Ramp Time (s) | Hold Time (min) |
|---|---|---|---|
| 1 | 800 | 15 | 10 |
| 2 | 0 | - | 15 |
| Material | Serum | Whole Blood | Placental Tissues | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | CYC S | IL-1β | IL-18 | NLRP3 | Na | K | Mg | Ca | NLRP3 | IL-1β | IL-18 | Casp1 | Fas | FasL | Bcl-2 | Ca Deposit |
| Optimal cut-off for the diagnostic test | 1.510 | 15.23 | 16.43 | 0.126 | 3.598 | 3.118 | 1.615 | 2.302 | 1 | 2 | 1 | 2 | - | 0 | 0 | 0 |
| Optimal cut-off for the differential test | 1.433 | 31.95 | 18.53 | 0.121 | 3.671 | 3.179 | 1.649 | 2.193 | 0 | 2 | 2 | - | - | - | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omeljaniuk, W.J.; Garley, M.; Pryczynicz, A.; Motyka, J.; Charkiewicz, A.E.; Milewska, E.; Laudański, P.; Miltyk, W. NLRP3 Inflammasome in the Pathogenesis of Miscarriages. Int. J. Mol. Sci. 2024, 25, 10513. https://doi.org/10.3390/ijms251910513
Omeljaniuk WJ, Garley M, Pryczynicz A, Motyka J, Charkiewicz AE, Milewska E, Laudański P, Miltyk W. NLRP3 Inflammasome in the Pathogenesis of Miscarriages. International Journal of Molecular Sciences. 2024; 25(19):10513. https://doi.org/10.3390/ijms251910513
Chicago/Turabian StyleOmeljaniuk, Wioleta Justyna, Marzena Garley, Anna Pryczynicz, Joanna Motyka, Angelika Edyta Charkiewicz, Elżbieta Milewska, Piotr Laudański, and Wojciech Miltyk. 2024. "NLRP3 Inflammasome in the Pathogenesis of Miscarriages" International Journal of Molecular Sciences 25, no. 19: 10513. https://doi.org/10.3390/ijms251910513
APA StyleOmeljaniuk, W. J., Garley, M., Pryczynicz, A., Motyka, J., Charkiewicz, A. E., Milewska, E., Laudański, P., & Miltyk, W. (2024). NLRP3 Inflammasome in the Pathogenesis of Miscarriages. International Journal of Molecular Sciences, 25(19), 10513. https://doi.org/10.3390/ijms251910513






