27-Hydroxycholesterol Negatively Affects the Function of Bone Marrow Endothelial Cells in the Bone Marrow
Abstract
:1. Introduction
2. Results
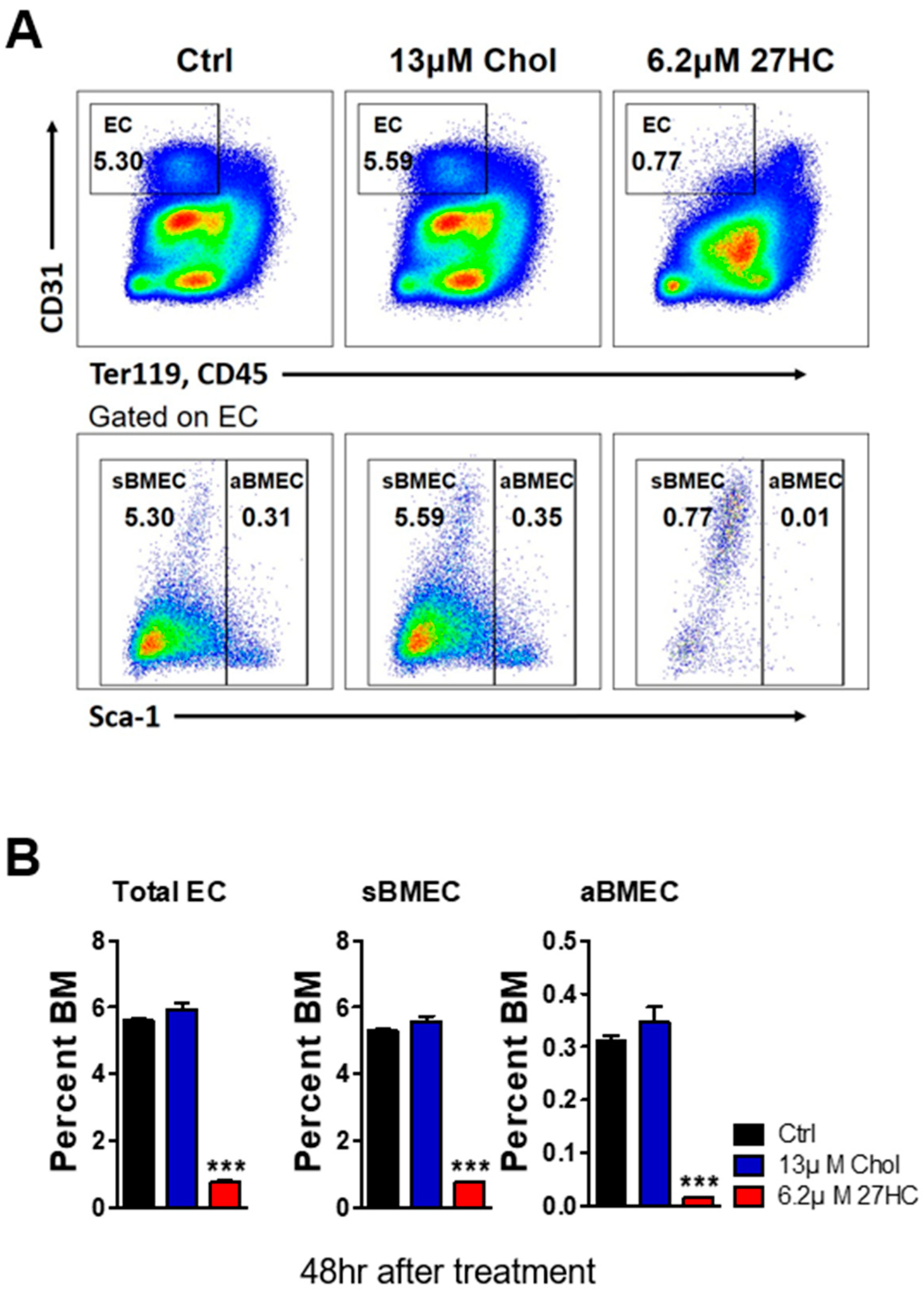
2.1. 27HC Has a Greater Impact on BMECs than on HSCs
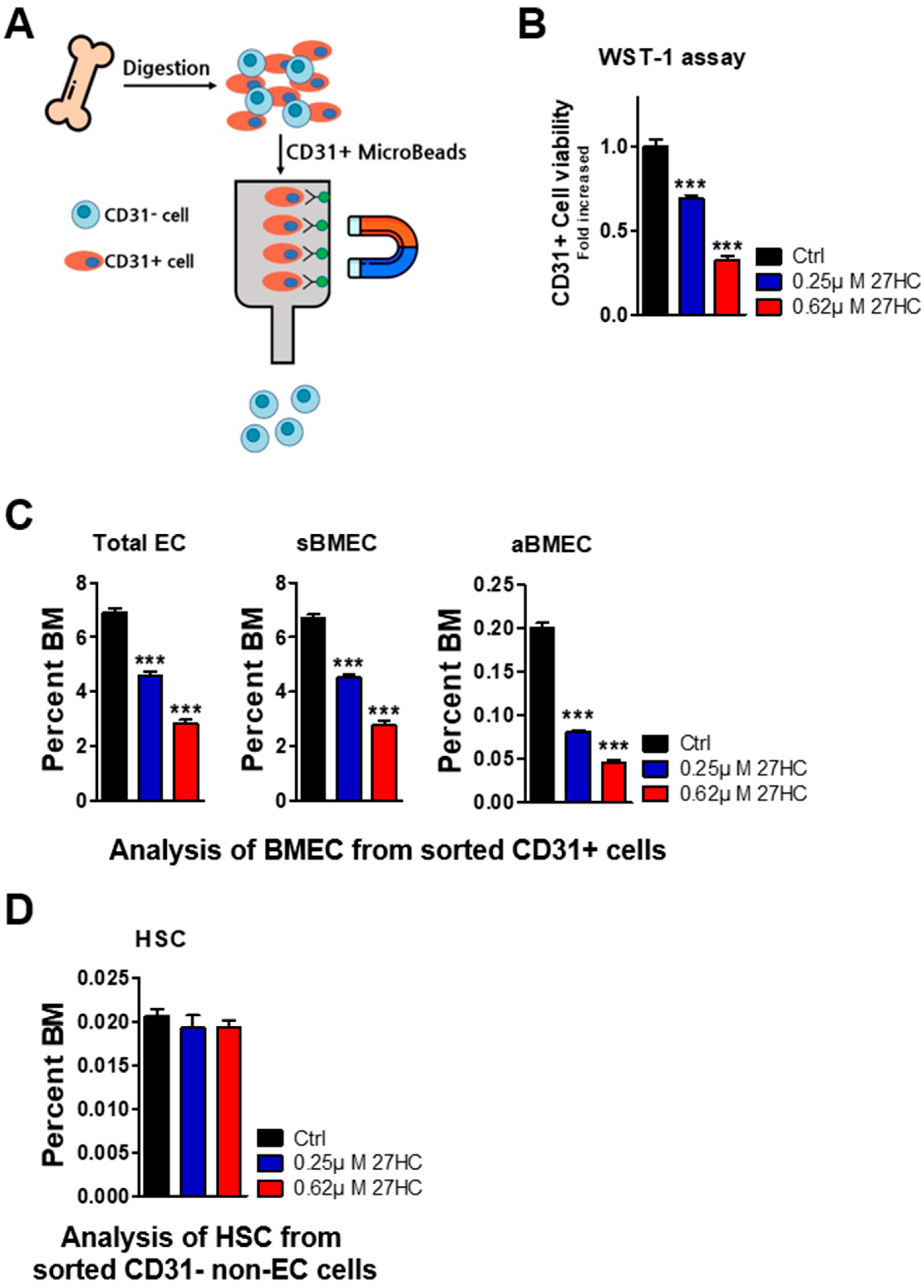
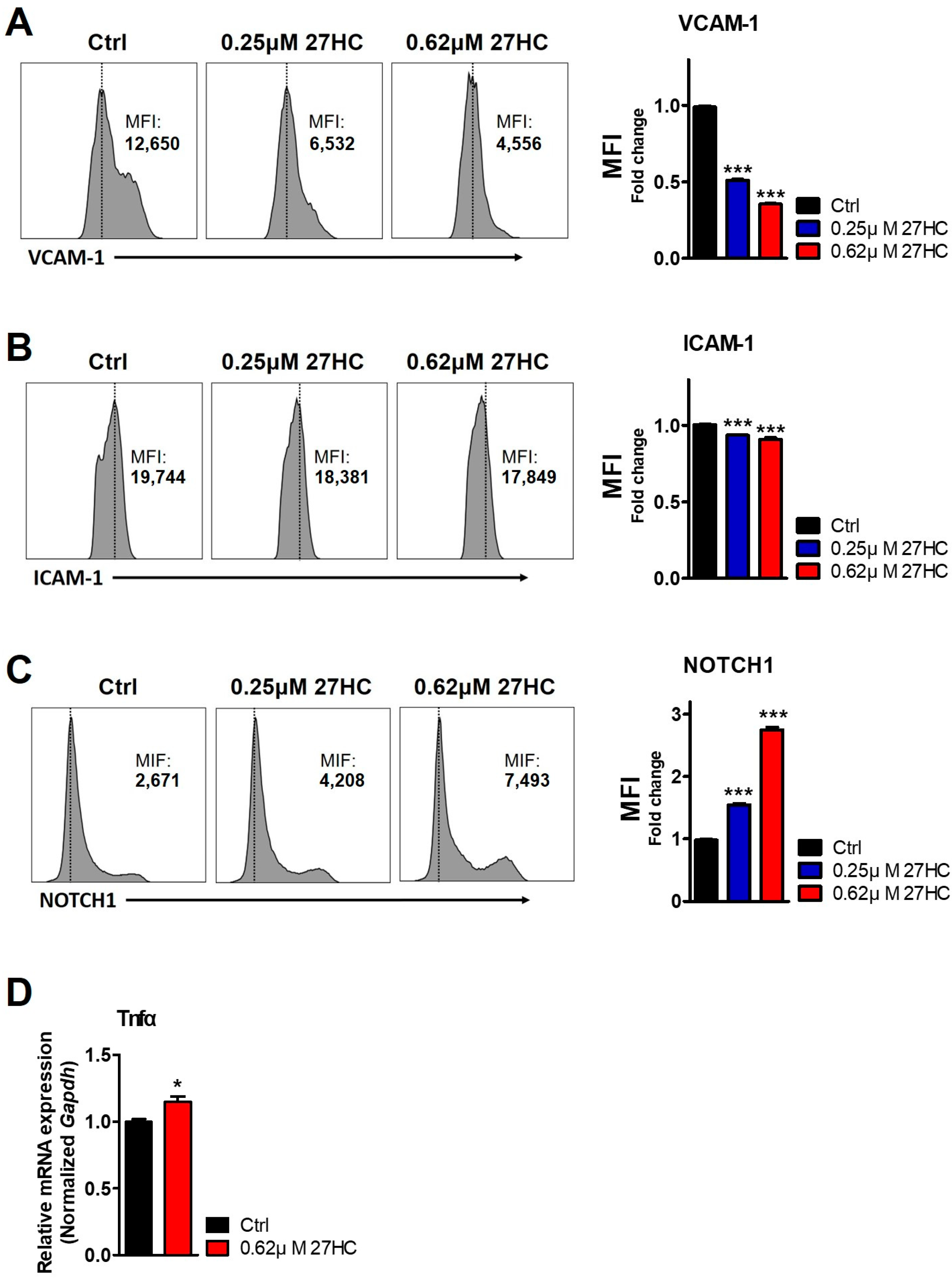
2.2. CD31+ BMECs but Not HSCs Are Impacted by 27HC
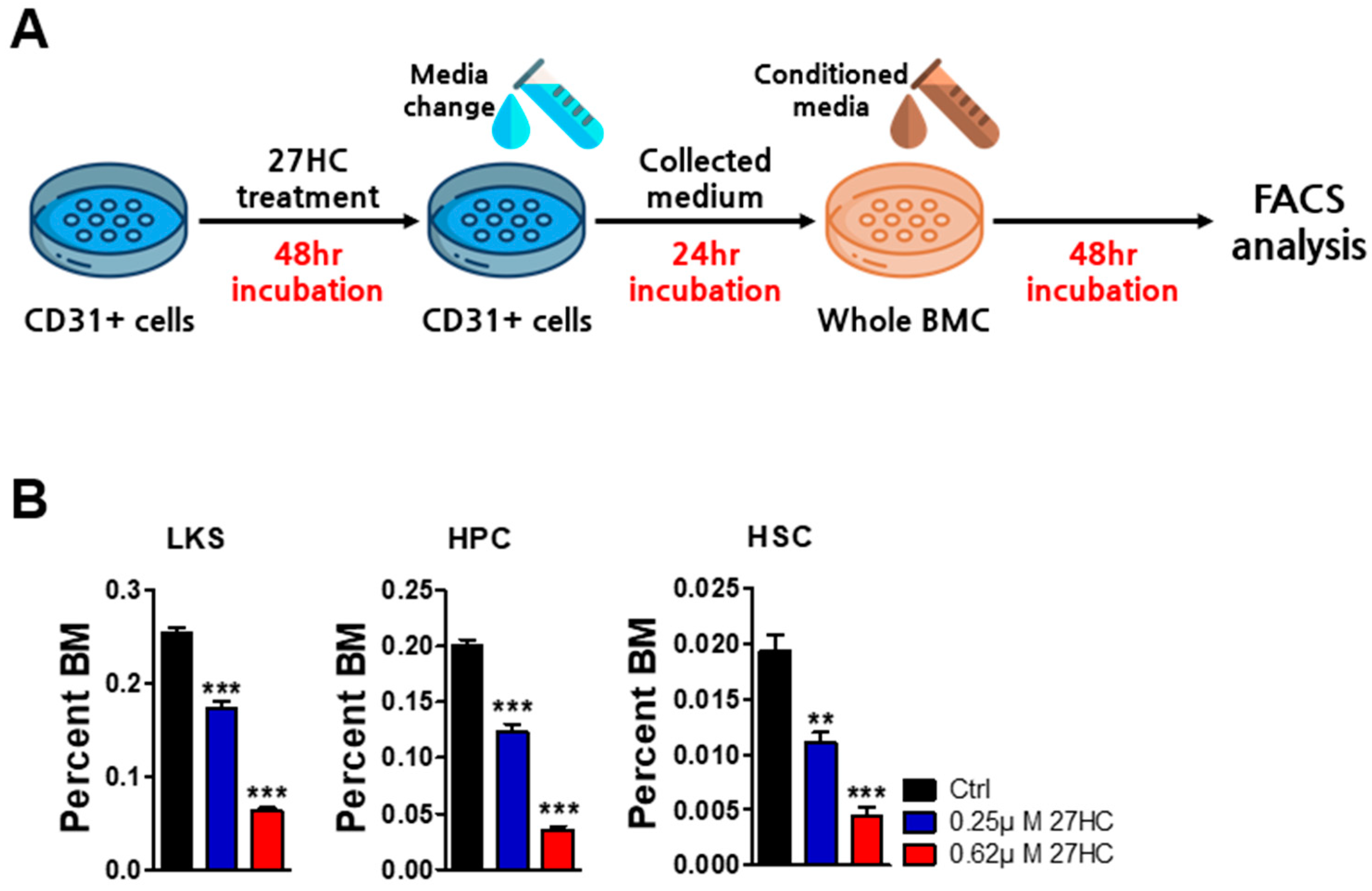
2.3. BMEC Dysfunction by Exogenous 27HC Treatment Affects HSCs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Mouse BM Cell Culture and 27HC Treatment
4.3. Flow Cytometric Analysis
4.4. WST-1 Assay
4.5. Magnetic Cell Sorting Analysis
4.6. Quantitative RT-PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scharf, P.; Broering, M.F.; Oliveira da Rocha, G.H.; Farsky, S.H.P. Cellular and molecular mechanisms of environmental pollutants on hematopoiesis. Int. J. Mol. Sci. 2020, 21, 6996. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Doan, P.; Chute, J. The vascular niche: Home for normal and malignant hematopoietic stem cells. Leukemia 2012, 26, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Butler, J.M.; O’donnell, R.; Kobayashi, M.; Ding, B.S.; Bonner, B.; Chiu, V.K.; Nolan, D.J.; Shido, K.; Benjamin, L.; et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 2010, 12, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Hsu, Y.M.S.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Frenette, P.S. The secrets of the bone marrow niche: Enigmatic niche brings challenge for HSC expansion. Nat. Med. 2012, 18, 864–865. [Google Scholar] [CrossRef]
- Baryawno, N.; Przybylski, D.; Kowalczyk, M.S.; Kfoury, Y.; Severe, N.; Gustafsson, K.; Kokkaliaris, K.D.; Mercier, F.; Tabaka, M.; Hofree, M.; et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell 2019, 177, 1915–1932.e1916. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Kunisaki, Y.; Pierce, H.; Wang, Z.; Fernandez, N.F.; Birbrair, A.; Ma’ayan, A.; Frenette, P.S. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017, 19, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Himburg, H.A.; Termini, C.M.; Schlussel, L.; Kan, J.; Li, M.; Zhao, L.; Fang, T.; Sasine, J.P.; Chang, V.Y.; Chute, J.P. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell 2018, 23, 370–381.e375. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Jeong, H.-W.; Stehling, M.; Zhou, B.; Adams, R.H. Apelin+ endothelial niche cells control hematopoiesis and mediate vascular regeneration after myeloablative injury. Cell Stem Cell 2019, 25, 768–783.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Zhang, S.Y.; Chen, Y.Y.; Shi, K.; Zou, B.; Liu, J.; Yang, Q.; Jiang, H.; Wei, L.; Li, C.Z.; et al. ICAM-1 deficiency in the bone marrow niche impairs quiescence and repopulation of hematopoietic stem cells. Stem Cell Rep. 2018, 11, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Kollet, O.; Ponomaryov, T.; Petit, I.; Franitza, S.; Grabovsky, V.; Slav, M.M.; Nagler, A.; Lider, O.; Alon, R.; et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: Role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood J. Am. Soc. Hematol. 2000, 95, 3289–3296. [Google Scholar]
- Rettig, M.P.; Ansstas, G.; DiPersio, J.F. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia 2012, 26, 34–53. [Google Scholar] [CrossRef]
- Scadden, D.T. The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Nakada, D.; Oguro, H.; Levi, B.P.; Ryan, N.; Kitano, A.; Saitoh, Y.; Takeichi, M.; Wendt, G.R.; Morrison, S.J. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 2014, 505, 555–558. [Google Scholar] [CrossRef]
- Oguro, H.; McDonald, J.G.; Zhao, Z.; Umetani, M.; Shaul, P.W.; Morrison, S.J. 27-Hydroxycholesterol induces hematopoietic stem cell mobilization and extramedullary hematopoiesis during pregnancy. J. Clin. Investig. 2017, 127, 3392–3401. [Google Scholar] [CrossRef]
- Umetani, M.; Domoto, H.; Gormley, A.K.; Yuhanna, I.S.; Cummins, C.L.; Javitt, N.B.; Korach, K.S.; Shaul, P.W.; Mangelsdorf, D.J. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007, 13, 1185–1192. [Google Scholar] [CrossRef]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [PubMed]
- Vurusaner, B.; Gargiulo, S.; Testa, G.; Gamba, P.; Leonarduzzi, G.; Poli, G.; Basaga, H. The role of autophagy in survival response induced by 27-hydroxycholesterol in human promonocytic cells. Redox Biol. 2018, 17, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Grogan, M.J.; Jones, S.A.; Wisely, G.B.; Kliewer, S.A.; Corey, E.J.; Mangelsdorf, D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. USA 1999, 96, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014, 20, 172–182. [Google Scholar] [CrossRef]
- Raza, S.; Meyer, M.; Schommer, J.; Hammer, K.D.; Guo, B.; Ghribi, O. 27-Hydroxycholesterol stimulates cell proliferation and resistance to docetaxel-induced apoptosis in prostate epithelial cells. Med. Oncol. 2016, 33, 12. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.Y.; Xing, Y.; Wang, X.Y.; Wang, Y. The oncogenic roles of 27-hydroxycholesterol in glioblastoma. Oncol. Lett. 2019, 18, 3623–3629. [Google Scholar] [CrossRef]
- Baek, A.E.; Yu, Y.R.A.; He, S.; Wardell, S.E.; Chang, C.Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, L.; Chu, H.; Peng, J.; Sacharidou, A.; Hsieh, H.H.; Weinstock, A.; Khan, S.; Ma, L.; Durán, J.G.B.; et al. Macrophage-to-endothelial cell crosstalk by the cholesterol metabolite 27HC promotes atherosclerosis in male mice. Nat. Commun. 2023, 14, 4101. [Google Scholar] [CrossRef]
- Woo, S.Y.; Lee, H.; Park, S.M.; Choi, H.S.; Kim, J.; Kwon, M.; Sohn, J.; Nam, J.H.; Kim, H.S.; Song, P.; et al. Role of reactive oxygen species in regulating 27-hydroxycholesterol-induced apoptosis of hematopoietic progenitor cells and myeloid cell lines. Cell Death Dis. 2022, 13, 916. [Google Scholar] [CrossRef]
- Poulos, M.G.; Crowley, M.J.; Gutkin, M.C.; Ramalingam, P.; Schachterle, W.; Thomas, J.L.; Elemento, O.; Butler, J.M. Vascular platform to define hematopoietic stem cell factors and enhance regenerative hematopoiesis. Stem Cell Rep. 2015, 5, 881–894. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Ramalingam, P.; Poulos, M.G.; Lazzari, E.; Gutkin, M.C.; Lopez, D.; Kloss, C.C.; Crowley, M.J.; Katsnelson, L.; Freire, A.G.; Greenblatt, M.B.; et al. Chronic activation of endothelial MAPK disrupts hematopoiesis via NFKB dependent inflammatory stress reversible by SCGF. Nat. Commun. 2020, 11, 666. [Google Scholar] [CrossRef]
- Ashok, D.; Polcik, L.; Dannewitz Prosseda, S.; Hartmann, T.N. Insights into bone marrow niche stability: An adhesion and metabolism route. Front. Cell Dev. Biol. 2022, 9, 798604. [Google Scholar] [CrossRef]
- Ulyanova, T.; Scott, L.M.; Priestley, G.V.; Jiang, Y.; Nakamoto, B.; Koni, P.A.; Papayannopoulou, T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 2005, 106, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Kobayashi, H.; Rafii, S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 2010, 10, 138–146. [Google Scholar] [CrossRef]
- Wu, M.; Kwon, H.Y.; Rattis, F.; Blum, J.; Zhao, C.; Ashkenazi, R.; Jackson, T.L.; Gaiano, N.; Oliver, T.; Reya, T. Imaging hematopoietic precursor division in real time. Cell Stem Cell 2007, 1, 541–554. [Google Scholar] [CrossRef]
- Varnum-Finney, B.; Xu, L.; Brashem-Stein, C.; Nourigat, C.; Flowers, D.; Bakkour, S.; Pear, W.S.; Bernstein, I.D. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 2000, 6, 1278–1281. [Google Scholar] [CrossRef]
- Butler, J.M.; Nolan, D.J.; Vertes, E.L.; Varnum-Finney, B.; Kobayashi, H.; Hooper, A.T.; Seandel, M.; Shido, K.; White, I.A.; Kobayashi, M.; et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 2010, 6, 251–264. [Google Scholar] [CrossRef]
- Shao, L.; Sottoriva, K.; Palasiewicz, K.; Zhang, J.; Hyun, J.; Soni, S.S.; Paik, N.Y.; Gao, X.; Cuervo, H.; Malik, A.B.; et al. A Tie2-Notch1 signaling axis regulates regeneration of the endothelial bone marrow niche. Haematologica 2019, 104, 2164. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Passegué, E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell 2019, 25, 357–372.e357. [Google Scholar] [CrossRef]
- Pronk, C.J.; Veiby, O.P.; Bryder, D.; Jacobsen, S.E.W. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: Involvement of two distinct receptors. J. Exp. Med. 2011, 208, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wang, J.; Gao, B.; Li, J.; Wu, F.; Zou, J.X.; Xu, J.; Jiang, Y.; Zou, H.; Huang, Z.; et al. RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat. Commun. 2019, 10, 4621. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, X.; Lv, J.; Zhang, J.; Xia, B.O.; Kim, J.D.; Wang, R.; Xiong, F.; Meng, S.; Clements, T.P.; et al. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science 2019, 363, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Priem, B.; van Leent, M.M.; Teunissen, A.J.; Sofias, A.M.; Mourits, V.P.; Willemsen, L.; Klein, E.D.; Oosterwijk, R.S.; Meerwaldt, A.E.; Munitz, J.; et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell 2020, 183, 786–801.e719. [Google Scholar] [CrossRef]
- Kloudova, A.; Guengerich, F.P.; Soucek, P. The role of oxysterols in human cancer. Trends Endocrinol. Metab. 2017, 28, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Tie, G.; Messina, K.E.; Yan, J.; Messina, J.A.; Messina, L.M. Hypercholesterolemia induces oxidant stress that accelerates the ageing of hematopoietic stem cells. J. Am. Heart Assoc. 2014, 3, e000241. [Google Scholar] [CrossRef]
- Adler, B.J.; Kaushansky, K.; Rubin, C.T. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat. Rev. Endocrinol. 2014, 10, 737–748. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L. Dissecting the bone marrow HSC niches. Cell Res. 2016, 26, 975–976. [Google Scholar] [CrossRef]
- Reglero-Real, N.; Colom, B.; Bodkin, J.V.; Nourshargh, S. Endothelial cell junctional adhesion molecules: Role and regulation of expression in inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2048–2057. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, D.; Bisen, S.; Mukhopadhyay, C.S.; Gurdziel, K.; Singh, N.K. Vascular cell-adhesion molecule 1 (VCAM-1) regulates JunB-mediated IL-8/CXCL1 expression and pathological neovascularization. Commun. Biol. 2023, 6, 516. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lewallen, M.; Xie, T. Adhesion in the stem cell niche: Biological roles and regulation. Development 2013, 140, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Varnum-Finney, B.; Halasz, L.M.; Sun, M.; Gridley, T.; Radtke, F.; Bernstein, I.D. Notch2 governs the rate of generation of mouse long-and short-term repopulating stem cells. J. Clin. Investig. 2011, 121, 1207–1216. [Google Scholar] [CrossRef]
- Lee, D.; Wang, Y.H.; Kalaitzidis, D.; Ramachandran, J.; Eda, H.; Sykes, D.B.; Raje, N.; Scadden, D.T. Endogenous transmembrane protein UT2 inhibits pSTAT3 and suppresses hematological malignancy. J. Clin. Investig. 2016, 126, 1300–1310. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Hong, C.M.; Nam, J.H.; Yeo, H.J.; Cho, W.H.; Kim, H.S.; Hong, C.; Kim, Y.H.; Lee, D. endogenous endoplasmic reticulum transmembrane protein SURF4 suppresses cell death by negatively regulating the STING-STAT6 axis in myeloid leukemia. Cancer Commun. 2023, 43, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidis, D.; Lee, D.; Efeyan, A.; Kfoury, Y.; Nayyar, N.; Sykes, D.B.; Mercier, F.E.; Papazian, A.; Baryawno, N.; Victora, G.D.; et al. Amino acid–insensitive mTORC1 regulation enables nutritional stress resilience in hematopoietic stem cells. J. Clin. Investig. 2017, 127, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yokota, T.; Xia, L.; Kincade, P.W.; McEver, R.P. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood 2005, 106, 4093–4101. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef]



| Antibody | Company | Cat |
|---|---|---|
| PE-Cy5-CD3 (145-2C11) | BD, Franklin Lakes, NJ, USA | 555276 |
| PE-Cy5-CD4 (RM4-5) | BD | 553050 |
| PE-Cy5-CD8 (53-6.7) | Biolegend, San Diego, CA, USA | 100710 |
| PE-Cy5-CD19 (6D5) | Biolegend | 115510 |
| PE-Cy5-B220R (RA3-6B2) | eBioscience, San Diego, CA, USA | 15-0452-82 |
| PE-Cy5-Gr1 (RB6-8C5) | eBioscience | 15-5931-82 |
| PE-Cy5-Ter119 (TER119) | eBioscience | 15-5921-82 |
| PE-Sca1 (D7) | eBioscience | 12-5981-82 |
| APC-cKit (2B8) | Biolegend | 105812 |
| PE-Cy7-CD150 (Tc15-12F122) | Biolegend | 115914 |
| APC-Cy7-CD48 (HM48-1) | BD | 561242 |
| PE-CD31 (MEC 13.3) | BD | 553373 |
| APC-Cy7-Ter119 (TER119) | BD | 560509 |
| APC-Cy7-CD45 (30-F11) | Biolegend | 103116 |
| FITC-Sca1 (D7) | eBioscience | 11-5981-82 |
| Primer | Sequence | |
|---|---|---|
| mTNFα | Forward | 5′ CTG AGG TCA ATC TGC CCA AGT AC 3′ |
| Reverse | 5′ CTT CAC AGA GCA ATG ACT CCA AAG 3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.-Y.; Shim, W.-S.; Lee, H.; Baryawno, N.; Song, P.; Kim, B.S.; Yoon, S.; Oh, S.-O.; Lee, D. 27-Hydroxycholesterol Negatively Affects the Function of Bone Marrow Endothelial Cells in the Bone Marrow. Int. J. Mol. Sci. 2024, 25, 10517. https://doi.org/10.3390/ijms251910517
Woo S-Y, Shim W-S, Lee H, Baryawno N, Song P, Kim BS, Yoon S, Oh S-O, Lee D. 27-Hydroxycholesterol Negatively Affects the Function of Bone Marrow Endothelial Cells in the Bone Marrow. International Journal of Molecular Sciences. 2024; 25(19):10517. https://doi.org/10.3390/ijms251910517
Chicago/Turabian StyleWoo, Soo-Yeon, Wan-Seog Shim, Hyejin Lee, Ninib Baryawno, Parkyong Song, Byoung Soo Kim, Sik Yoon, Sae-Ock Oh, and Dongjun Lee. 2024. "27-Hydroxycholesterol Negatively Affects the Function of Bone Marrow Endothelial Cells in the Bone Marrow" International Journal of Molecular Sciences 25, no. 19: 10517. https://doi.org/10.3390/ijms251910517








