Preliminary Investigation of Potential Early Biomarkers for Gestational Diabetes Mellitus: Insights from PTRPG and IGKV2D-28 Expression Analysis
Abstract
:1. Introduction
2. Results
2.1. Increased PTPRG and IGKV2D-28 Expression Levels in the GDM and Non-GDM Groups
2.2. Correlations between Gene Expressions with Clinical Variables and GDM Status
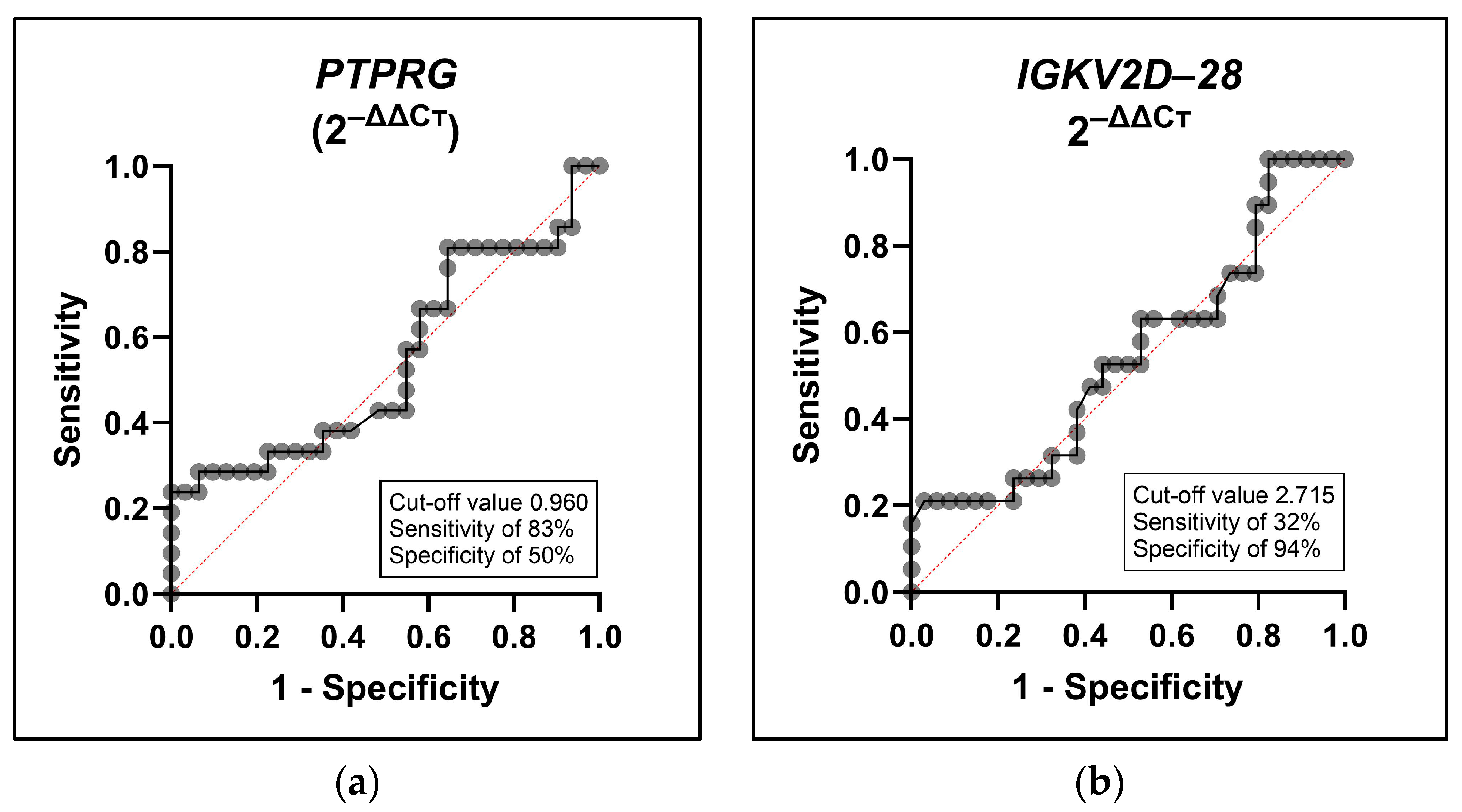
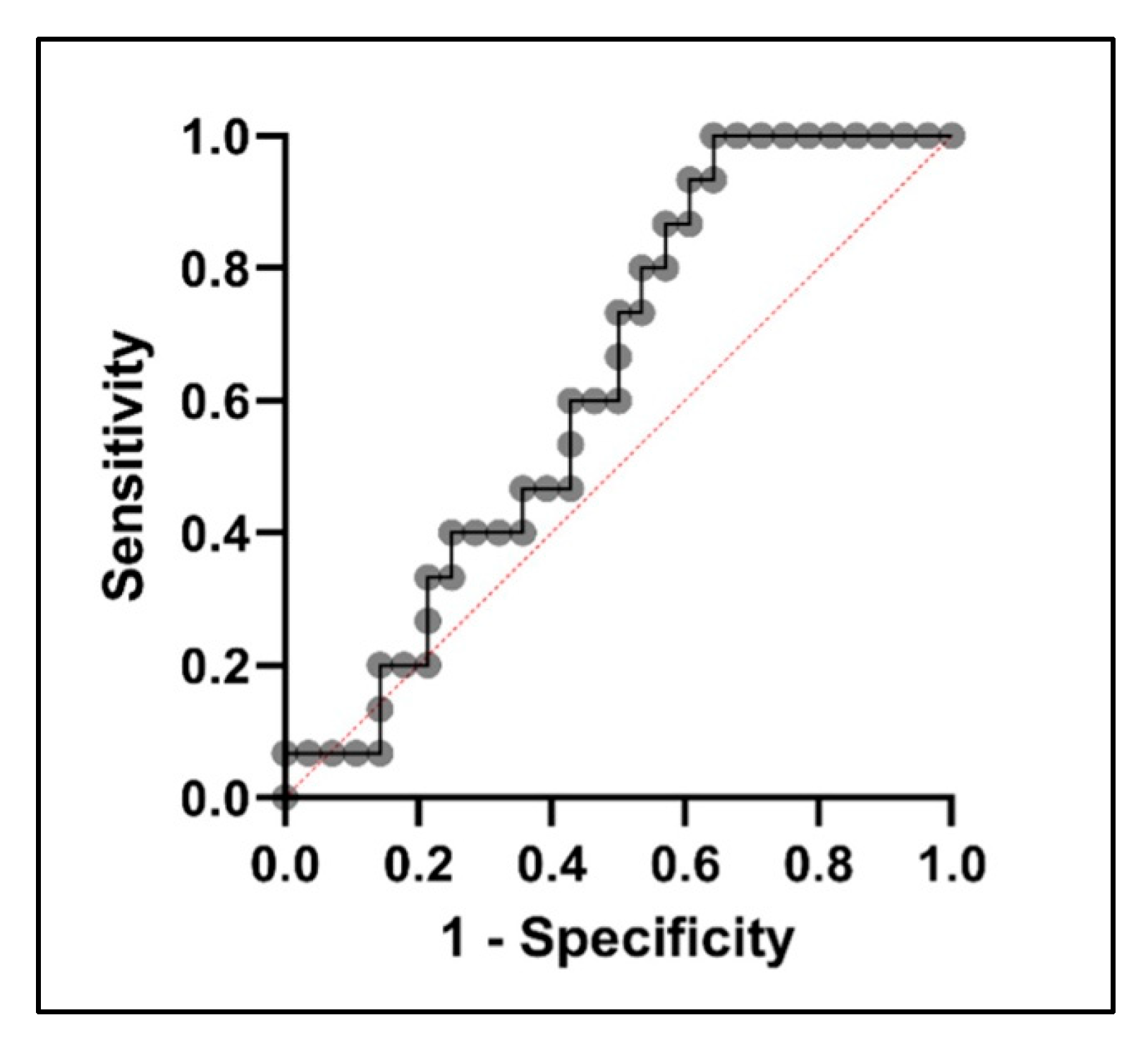
2.3. Diagnostic Potential of PTPRG and IGKV2D-28 in GDM Prediction
3. Discussion
4. Materials and Methods
4.1. First-Trimester RNA Samples
4.2. Reverse Transcription and cDNA Synthesis
4.3. Real-Time Quantitative RT-PCR
4.4. Computation of Gene Expression
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2019, 48, 479–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, K.; Jin, L.; Zhou, Y.; Shang, X.; Wang, X.; Yu, H. MTNR1B gene variations and high pre-pregnancy BMI increase gestational diabetes mellitus risk in Chinese women. Gene 2024, 894, 148023. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. Gestational diabetes mellitus in southeast Asia: A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 1272. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Huang, L.; Tobias, D.K.; Zhang, C. Gestational Diabetes Mellitus among Asians—A Systematic Review from a Population Health Perspective. Front. Endocrinol. 2022, 13, 840331. [Google Scholar] [CrossRef] [PubMed]
- Pineda Cortel, M.R.B.; Manalo, M.E.M.; Canivel, R.R.C.; Matias, R.S.; Dizon, A.J.B. Screening and Diagnosis of Gestational Diabetes Mellitus Using 75-g Oral Glucose Tolerance Test Following the WHO, ADA, and IADPSG Criteria. J. Diabetes Metab. 2018, 09, 7. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Kapur, A.; Divakar, H.; Hod, M. Gestational Diabetes Mellitus—Innovative Approach to Prediction, Diagnosis, Management, and Prevention of Future NCD—Mother and Offspring. Front. Endocrinol. 2020, 11, 614533. [Google Scholar] [CrossRef]
- Huhn, E.A.; Rossi, S.W.; Hoesli, I.; Göbl, C.S. Controversies in Screening and Diagnostic Criteria for Gestational Diabetes in Early and Late Pregnancy. Front. Endocrinol. 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Tenenbaum-Gavish, K.; Sharabi-Nov, A.; Binyamin, D.; Møller, H.J.; Danon, D.; Rothman, L.; Hadar, E.; Idelson, A.; Vogel, I.; Koren, O.; et al. First trimester biomarkers for prediction of gestational diabetes mellitus. Placenta 2020, 101, 80–89. [Google Scholar] [CrossRef]
- Boni, C.; Laudanna, C.; Sorio, C. A Comprehensive Review of Receptor-Type Tyrosine-Protein Phosphatase Gamma (PTPRG) Role in Health and Non-Neoplastic Disease. Biomolecules 2022, 12, 84. [Google Scholar] [CrossRef]
- Pineda-Cortel, M.R.B.; Bunag, J.A.A.; Mamerto, T.P.; Abulencia, M.F.B. Differential gene expression and network-based analyses of the placental transcriptome reveal distinct potential biomarkers for gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2021, 180, 109046. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Pokhrel, K.M.; Lamichhane, P.; Khanal, K.; Rawal, S. Prevalence of Gestational Diabetes Mellitus in Nepal: A Systematic Review and Meta-analysis. J. Nepal Health Res. Counc. 2022, 20, 12–20. [Google Scholar] [PubMed]
- Nielsen, K.K.; de Courten, M.; Kapur, A. The urgent need for universally applicable simple screening procedures and diagnostic criteria for gestational diabetes mellitus—Lessons from projects funded by the World Diabetes Foundation. Glob. Health Action 2012, 5, 17277. [Google Scholar] [CrossRef]
- Amarra, M.S.; Chong, M.F.F.; Titapant, V.; Somprasit, C.; Rogacion, J.; Irwinda, R.; Huynh, T.N.K.; Nalliah, S. ILSI Southeast Asia symposium: Prevalence, risk factors, and actions to address gestational diabetes in selected Southeast Asian countries. Eur. J. Clin. Nutr. 2021, 75, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Gandevani, S.; Amiri, M.; Yarandi, R.B.; Tehrani, F.R. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Zhou, B.; Xie, L. Modulation of protein kinase signaling by protein phosphatases and inhibitors. Pharmacol. Ther. 2002, 93, 307–317. [Google Scholar] [CrossRef]
- Behl, T.; Gupta, A.; Sehgal, A.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bhatia, S.; Bungau, S. Exploring protein tyrosine phosphatases (PTP) and PTP-1B inhibitors in management of diabetes mellitus. Biomed. Pharmacother. 2022, 153, 113405. [Google Scholar] [CrossRef]
- Appiah, K.K.; Kostich, W.A.; Gerritz, S.W.; Huang, Y.; Hamman, B.D.; Allen, J.; Zhang, W.; Lanthorn, T.H.; Albright, C.F.; Westphal, R.; et al. A high-throughput screen for receptor protein tyrosine phosphatase-γ selective inhibitors. J. Biomol. Screen. 2011, 16, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Metabolic Disease: Protein tyrosine phosphatase inhibitor reverses diabetes. Nat. Rev. Drug Discov. 2017, 16, 312–313. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Jayabalan, N.; Salomon, C.; Lappas, M. Molecular pathways disrupted by gestational diabetes mellitus. J. Mol. Endocrinol. 2019, 63, R51–R72. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational diabetes mellitus: A harbinger of the vicious cycle of diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Moratti, E.; Vezzalini, M.; Tomasello, L.; Giavarina, D.; Sorio, C. Identification of protein tyrosine phosphatase receptor gamma extracellular domain (sPTPRG) as a natural soluble protein in plasma. PLoS ONE 2015, 10, e0119110. [Google Scholar] [CrossRef]
- Brenachot, X.; Ramadori, G.; Ioris, R.M.; Veyrat-Durebex, C.; Altirriba, J.; Aras, E.; Ljubicic, S.; Kohno, D.; Fabbiano, S.; Clement, S.; et al. Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat. Commun. 2017, 8, 1820. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, E.S.; Yoo, J.; Kim, Y. Predicting risk of type 2 diabetes mellitus in Korean adults aged 40–69 by integrating clinical and genetic factors. Prim. Care Diabetes 2019, 13, 3–10. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Sun, Q.; Yang, Y.; Gao, Y.; Ge, W.; Liu, J.; Xu, X.; Weng, D.; Wang, S.; et al. Adenine nucleotide-mediated regulation of hepatic PTP1B activity in mouse models of type 2 diabetes. Diabetologia 2019, 62, 2106–2117. [Google Scholar] [CrossRef] [PubMed]
- Esparvarinha, M.; Nickho, H.; Mohammadi, H.; Aghebati-Maleki, L.; Abdolalizadeh, J.; Majidi, J. The role of free kappa and lambda light chains in the pathogenesis and treatment of inflammatory diseases. Biomed. Pharmacother. 2017, 91, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Cells, B. B Cells, Immunoglobulin Genes, and Immunoglobulin Structure. In Immunology Guidebook; Academic Press: Cambridge, MA, USA, 2004; pp. 277–309. [Google Scholar]
- Zieleniak, A.; Zurawska-Klis, M.; Cypryk, K.; Wozniak, L.; Wojcik, M. Transcriptomic Dysregulation of Inflammation-Related Genes in Leukocytes of Patients with Gestational Diabetes Mellitus (GDM) during and after Pregnancy: Identifying Potential Biomarkers Relevant to Glycemic Abnormality. Int. J. Mol. Sci. 2022, 23, 14677. [Google Scholar] [CrossRef]
- McElwain, C.J.; McCarthy, F.P.; McCarthy, C.M. Gestational diabetes mellitus and maternal immune dysregulation: What we know so far. Int. J. Mol. Sci. 2021, 22, 4261. [Google Scholar] [CrossRef]
- Matsumori, A.; Shimada, T.; Shimada, M.; Drayson, M.T. Immunoglobulin free light chains: An inflammatory biomarker of diabetes. Inflamm. Res. 2020, 69, 715–718. [Google Scholar] [CrossRef]
- Sorvor, E.; Owiredu, W.K.B.A.; Okyere, P.; Annani-Akollor, M.E.; Donkor, S.; Bannor, R.; Sorvor, F.B.K.; Ephraim, R.K. Assessment of Serum Free Light Chains as a Marker of Diabetic Nephropathy; A Cross-Sectional Study in the Kumasi Metropolis. Front. Clin. Diabetes Healthc. 2022, 3, 881202. [Google Scholar] [CrossRef] [PubMed]

| Candidate Genes | Glucose | 1st Hour OGTT | 2nd Hour OGTT | HbA1c | GDM Status | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | r | p Value | |
| PTPRG (2−ΔΔCT) | 0.40 b | 0.01 a | −0.02 b | 0.88 | −0.21 b | 0.17 | 0.18 b | 0.23 | 0.31 b | 0.03 a |
| IGKV2D-28 (2−ΔΔCT) | 0.34 b | 0.01 a | −0.08 b | 0.57 | −0.14 b | 0.34 | 0.21 b | 0.15 | 0.32 b | 0.01 a |
| Biochemical Characteristics | Non-GDM (n = 36) | GDM (n = 24) |
|---|---|---|
| Glucose (mmol/L) | 4.49 ± 0.39 | 5.51 ± 0.78 |
| First hour OGTT (mmol/L) | 6.29 ± 0.83 | 8.55 ± 1.75 |
| Second hour OGTT (mmol/L) | 6.28 ± 0.98 | 7.69 ± 1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payot, M.D.; Villavieja, A.; Pineda-Cortel, M.R. Preliminary Investigation of Potential Early Biomarkers for Gestational Diabetes Mellitus: Insights from PTRPG and IGKV2D-28 Expression Analysis. Int. J. Mol. Sci. 2024, 25, 10527. https://doi.org/10.3390/ijms251910527
Payot MD, Villavieja A, Pineda-Cortel MR. Preliminary Investigation of Potential Early Biomarkers for Gestational Diabetes Mellitus: Insights from PTRPG and IGKV2D-28 Expression Analysis. International Journal of Molecular Sciences. 2024; 25(19):10527. https://doi.org/10.3390/ijms251910527
Chicago/Turabian StylePayot, Mariejim Diane, Adrian Villavieja, and Maria Ruth Pineda-Cortel. 2024. "Preliminary Investigation of Potential Early Biomarkers for Gestational Diabetes Mellitus: Insights from PTRPG and IGKV2D-28 Expression Analysis" International Journal of Molecular Sciences 25, no. 19: 10527. https://doi.org/10.3390/ijms251910527






