IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes
Abstract
1. Introduction
2. Results
2.1. Clinical Data and Disease Severity
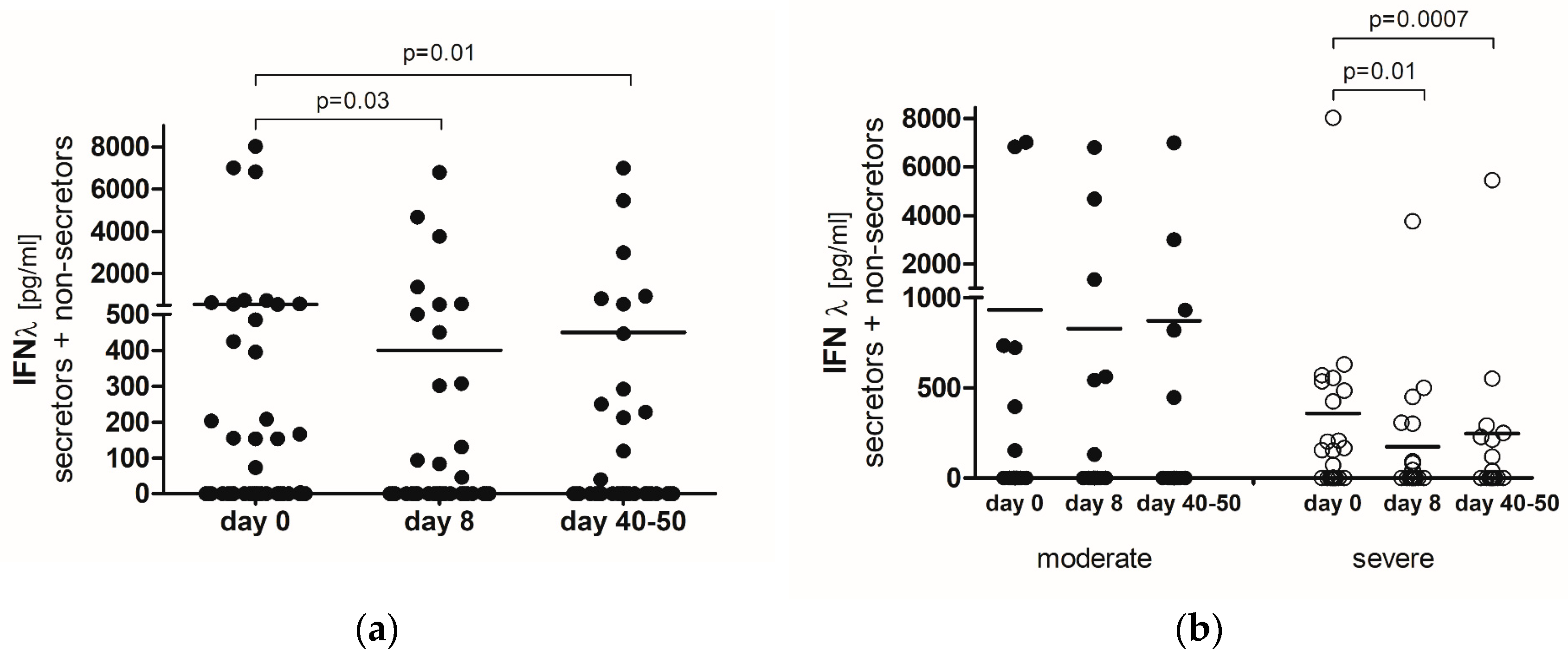
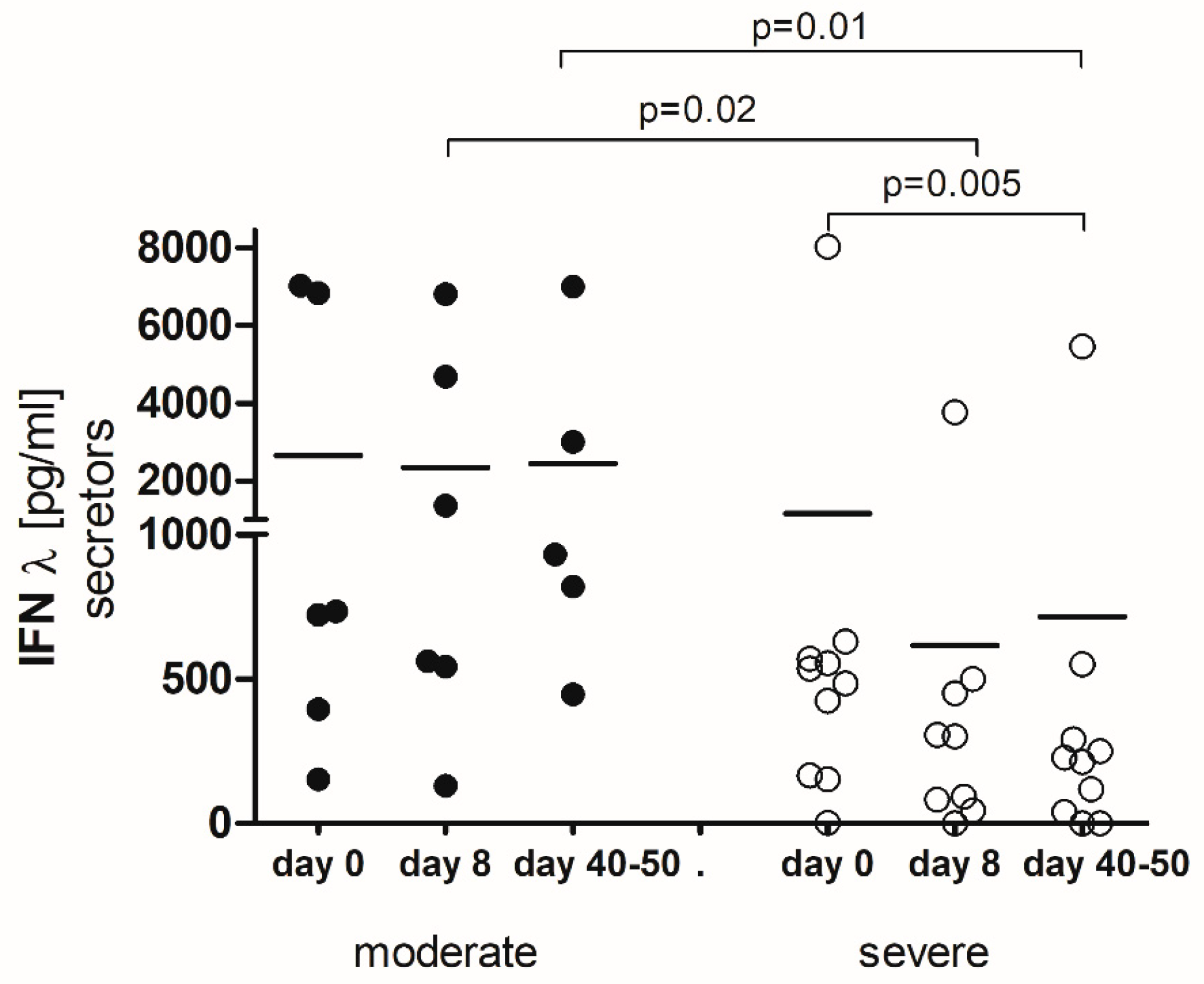
2.2. IFN-λ
2.3. IFN-λ-Secretors
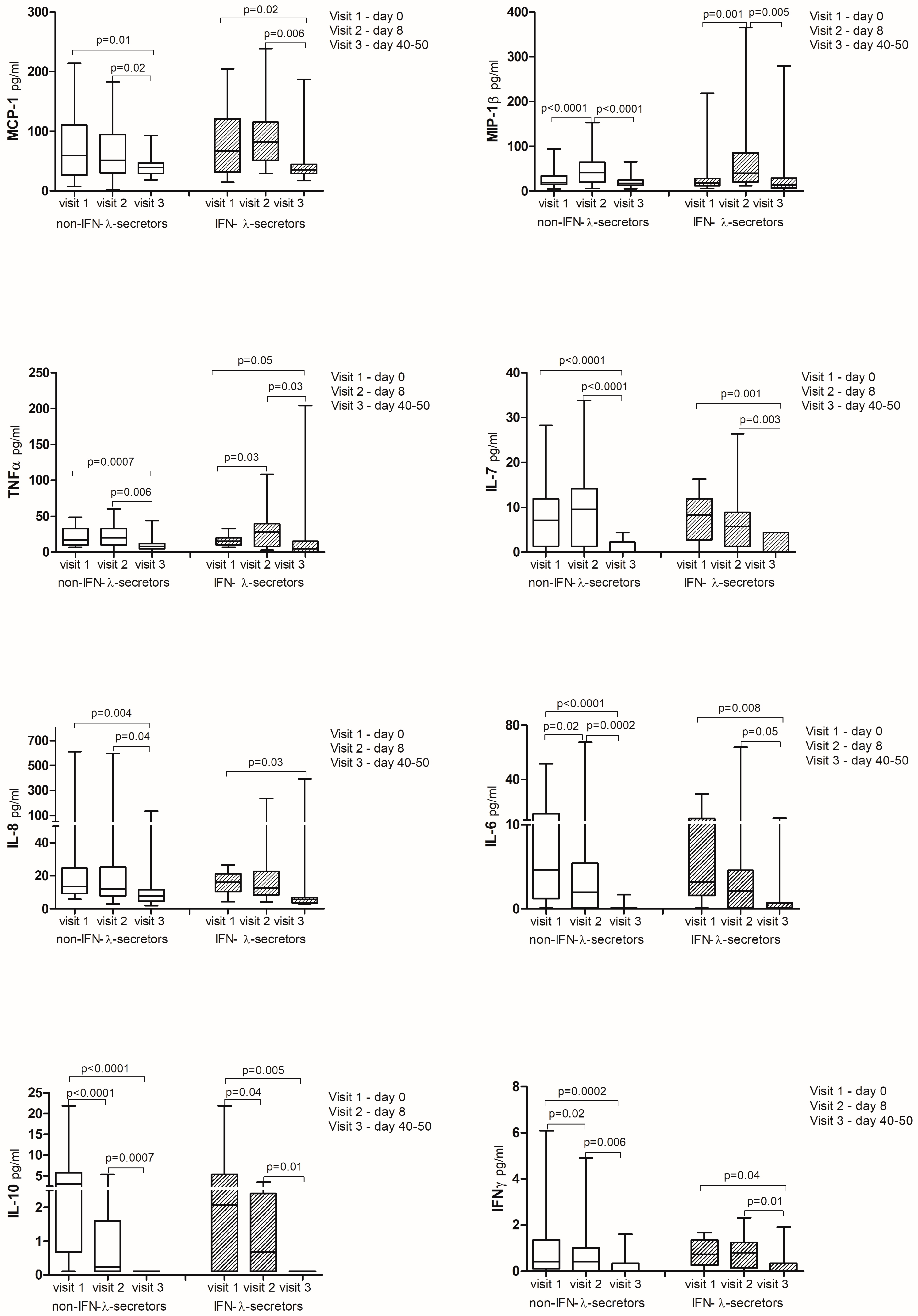
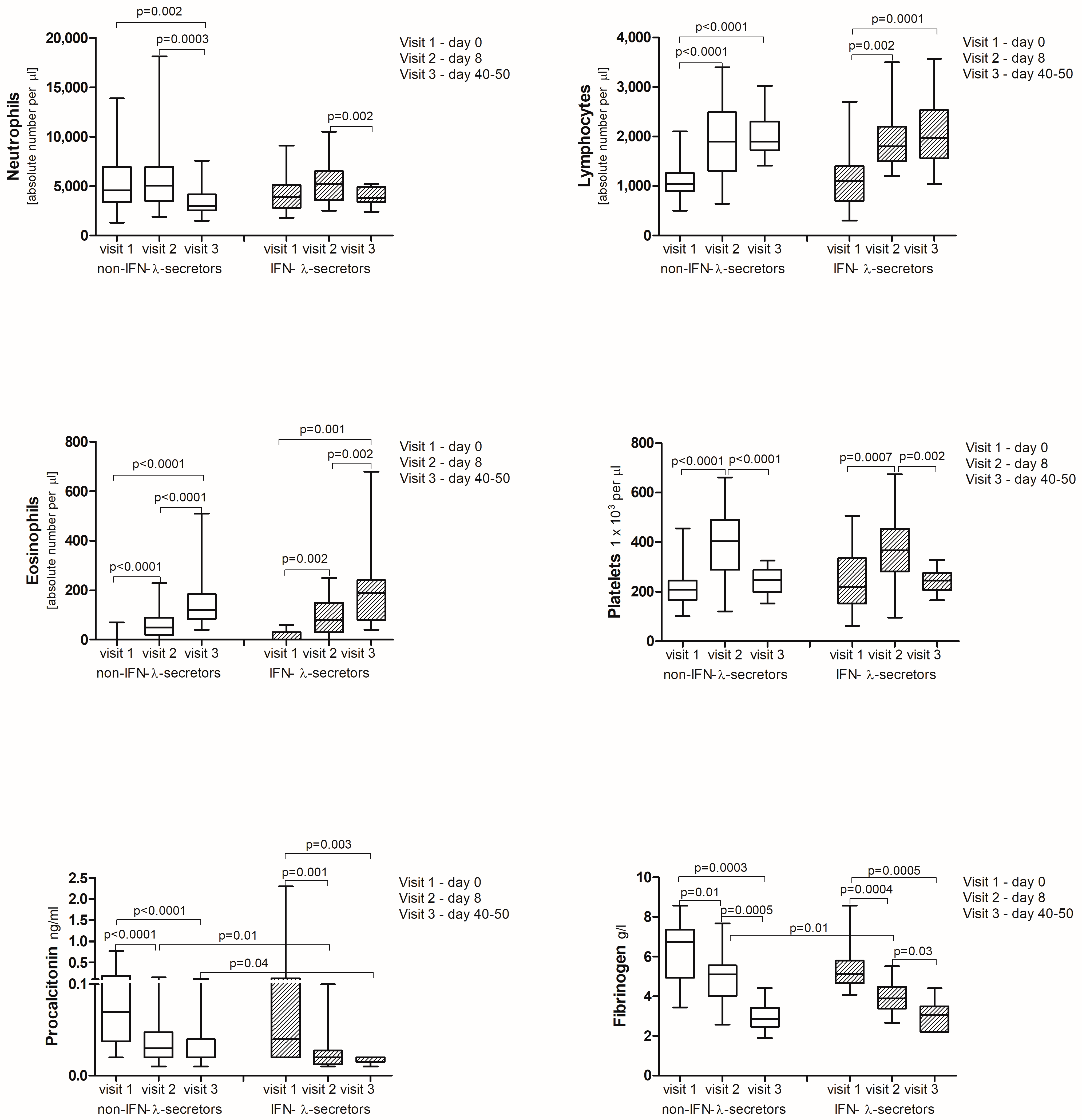
2.4. Cytokine Analysis
3. Discussion
4. Materials and Methods
4.1. Examined Group and Biochemical Analysis
4.2. Computed Tomography
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-D.; Ding, M.; Dong, X.; Zhang, J.-J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- An, P.-J.; Zhu, Y.Z.; Yang, L.-P. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol. Res. 2020, 159, 104946. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kanneganti, T.-D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Pfaender, S.; Mar, K.B.; Michailidis, E.; Kratzel, A.; Boys, I.N.; V’kovski, P.; Fan, W.; Kelly, J.N.; Hirt, D.; Ebert, N.; et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat. Microbiol. 2020, 5, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sancho, L.; Lewinski, M.K.; Pache, L.; Stoneham, C.A.; Yin, X.; Becker, M.E.; Pratt, D.; Churas, C.; Rosenthal, S.B.; Liu, S.; et al. Functional landscape of SARS-CoV-2 cellular restriction. Mol. Cell 2021, 81, 2656–2668.e8. [Google Scholar] [CrossRef]
- Felgenhauer, U.; Schoen, A.; Gad, H.H.; Hartmann, R.; Schaubmar, A.R.; Failing, K.; Drosten, C.; Weber, F. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020, 295, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Quarleri, J.; Delpino, M.V. Type I and III IFN-mediated antiviral actions counteracted by SARS-CoV-2 proteins and host inherited factors. Cytokine Growth Factor Rev. 2021, 58, 55–65. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Shin, E.-C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon lambda for the treatment of outpatients with COVID-19: A phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Dinnon, K.H.; Leist, S.R.; Schäfer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L.; Hou, Y.J.; Adams, L.E.; et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 2020, 586, 560–566. [Google Scholar] [CrossRef]
- Reis, G.; Moreira Silva, E.A.S.; Medeiros Silva, D.C.; Thabane, L.; Campos, V.H.S.; Ferreira, T.S.; Santos, C.V.Q.; Nogueira, A.M.R.; Almeida, A.P.F.G.; Savassi, L.C.M.; et al. Early Treatment with Pegylated Interferon Lambda for Covid-19. N. Engl. J. Med. 2023, 388, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Broggi, A.; Ghosh, S.; Sposito, B.; Spreafico, R.; Balzarini, F.; Lo Cascio, A.; Clementi, N.; de Santis, M.; Mancini, N.; Granucci, F.; et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020, 369, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Hatton, C.F.; Botting, R.A.; Dueñas, M.E.; Haq, I.J.; Verdon, B.; Thompson, B.J.; Spegarova, J.S.; Gothe, F.; Stephenson, E.; Gardner, A.I.; et al. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat. Commun. 2021, 12, 7092. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Gloss, B.S.; Liddle, C.; George, J.; Ahlenstiel, G. Interferon-λ3 Exacerbates the Inflammatory Response to Microbial Ligands: Implications for SARS-CoV-2 Pathogenesis. J. Inflamm. Res. 2021, 14, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, Y.; Sekiya, M.; Nojiri, S.; Hayakawa, E.; Masui, Y.; Tajima, M.; Nishino, K.; Nishizaki, Y.; Takahashi, K. IFN-λ3 and CCL17 as predictors of disease progression in patients with mild to moderate COVID-19: A cohort study in a real-world setting. Respir. Investig. 2023, 61, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Iwamoto, N.; Tsuzuki, S.; Kakumoto, Y.; Suzuki, M.; Ashida, S.; Oshiro, Y.; Nemoto, T.; Kanda, K.; Okuhama, A.; et al. Interferon lambda 3 in the early phase of coronavirus disease-19 can predict oxygen requirement. Eur. J. Clin. Investig. 2022, 52, e13808. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Homma, T.; Inoue, H.; Onitsuka, C.; Ikeda, H.; Goto, Y.; Sato, Y.; Kimura, T.; Hirai, K.; Ohta, S.; et al. Downregulation of type III interferons in patients with severe COVID-19. J. Med. Virol. 2021, 93, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Homma, T.; Inoue, H.; Goto, Y.; Sato, Y.; Ikeda, H.; Onitsuka, C.; Sato, H.; Akimoto, K.; Ebato, T.; et al. Serum IL-28A/IFN-λ2 is linked to disease severity of COVID-19. Sci. Rep. 2022, 12, 5458. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Amri Maleh, P.; Bagherzadeh, M.; Moulana, Z.; Sepidarkish, M.; Rezanejad, M.; Mirzakhani, M.; Ebrahimpour, S.; Ghorbani, H.; Ahmadnia, Z.; et al. Linkage of Lambda Interferons in Protection Against Severe COVID-19. J. Interferon Cytokine Res. 2021, 41, 149–152. [Google Scholar] [CrossRef]
- Kim, M.-H.; Salloum, S.; Wang, J.Y.; Wong, L.P.; Regan, J.; Lefteri, K.; Manickas-Hill, Z.; Gao, C.; Li, J.Z.; Sadreyev, R.I.; et al. Type I, II, and III Interferon Signatures Correspond to Coronavirus Disease 2019 Severity. J. Infect. Dis. 2021, 224, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, J.; Schnepf, D.; Ye, L.; Schwaderlapp, M.; Gad, H.H.; Hartmann, R.; Garcin, D.; Mahlakõiv, T.; Staeheli, P. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife 2018, 7, e33354. [Google Scholar] [CrossRef]
- Sorrentino, L.; Fracella, M.; Frasca, F.; D’Auria, A.; Santinelli, L.; Maddaloni, L.; Bugani, G.; Bitossi, C.; Gentile, M.; Ceccarelli, G.; et al. Alterations in the Expression of IFN Lambda, IFN Gamma and Toll-like Receptors in Severe COVID-19 Patients. Microorganisms 2023, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Busnadiego, I.; Fernbach, S.; Pohl, M.O.; Karakus, U.; Huber, M.; Trkola, A.; Stertz, S.; Hale, B.G. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio 2020, 11, e01928-20. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.-E.; Rovina, N.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021, 22, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.-A.; Garazzino, S.; Daprà, V.; Pruccoli, G.; Calvi, C.; Mignone, F.; Alliaudi, C.; Denina, M.; Scolfaro, C.; Zoppo, M.; et al. COVID-19 in Children: Expressions of Type I/II/III Interferons, TRIM28, SETDB1, and Endogenous Retroviruses in Mild and Severe Cases. Int. J. Mol. Sci. 2021, 22, 7481. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef]
- Huang, C.; Hu, X.; Wang, D.; Gong, R.; Wang, Q.; Ren, F.; Wu, Y.; Chen, J.; Xiong, X.; Li, H.; et al. Multi-cohort study on cytokine and chemokine profiles in the progression of COVID-19. Sci. Rep. 2024, 14, 10324. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, O.; Golovkin, A.; Zaikova, E.; Aquino, A.; Bezrukikh, V.; Melnik, O.; Vasilieva, E.; Karonova, T.; Kudryavtsev, I.; Shlyakhto, E. Cytokine Storm Signature in Patients with Moderate and Severe COVID-19. Int. J. Mol. Sci. 2022, 23, 8879. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Krishnan, V.; Chang, C.-Y.; Engle, S.M.; Casalini, G.; Rodgers, G.H.; Bivi, N.; Nickoloff, B.J.; Konrad, R.J.; de Bono, S.; et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2021, 147, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park-Min, K.-H.; Chen, J.; Hu, X.; Ivashkiv, L.B. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat. Immunol. 2011, 12, 607–615. [Google Scholar] [CrossRef]
- Dattola, A.; Tolone, M.; Amore, E.; Bennardo, L.; Trovato, F.; Amato, S.; Grieco, T.; Richetta, A.G.; Pellacani, G.; Skroza, N.; et al. Interleukin-13 Inhibitors in the Treatment of Atopic Dermatitis: The role of Tralokinumab. Dermatol. Pract. Concept. 2024, 14, e2024204. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. National Institutes of Health (US): Bethesda, MD, USA, 2021. 21 April–29 February 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 27 July 2024).

| IFN-λ-Secretors | Non-IFN-λ-Secretors | p | |
|---|---|---|---|
| (n = 16) | (n = 35) | ||
| Age, mean ± SD | 51.6 ± 11.3 | 54.1 ± 11.7 | p = 0.434 |
| Female, n | 6 (38%) | 9 (26%) | p = 0.510 |
| Male, n | 10 (62%) | 26 (74%) | p = 0.510 |
| BMI kg/m2, mean ± SD | 30.7 ± 5.1 | 29.0 ± 4.0 | p = 0.272 |
| % lung infiltrates, mean ± SD | 55.9 ± 21.5 | 54.3 ± 22.6 | p = 0.775 |
| Symptoms, n: | |||
| - fever | 16 (100%) | 34 (97%) | p = 0.161 |
| - dry cough | 13(81%) | 33 (94%) | p =0.309 |
| - dyspnea | 15 (94%) | 32 (91%) | p = 1.000 |
| - muscle pain | 7 (44%) | 17 (49%) | p = 0.772 |
| - headache | 5 (31%) | 14 (40%) | p = 0.756 |
| - diarrhea | 6 (38%) | 10 (29%) | p = 0.534 |
| - loss of taste | 5 (31%) | 12 (34%) | p = 1.000 |
| - loss of smell | 6 (38%) | 9 (26%) | p =0.510 |
| - weakness | 0 | 5 (14%) | p = 0.167 |
| - sore throat | 5 (31%) | 2 (6%) | p = 0.025 * |
| - nausea | 2 (13%) | 4 (11%) | p = 1.000 |
| - vomiting | 1 (6%) | 2 (6%) | p = 1.000 |
| - rash | 1 (6%) | 1 (3%) | p = 0.533 |
| - sleep disturbance | 0 | 2 (6%) | p = 1.000 |
| - bronchospasm | 0 | 1 (3%) | p = 1.000 |
| Comorbidities, n: | |||
| - hypertension | 3 (19%) | 16 (46%) | p = 0.117 |
| - diabetes | 2 (13%) | 4 (11%) | p = 1.000 |
| - hypothyroidism | 1 (6%) | 5 (14%) | p = 0.651 |
| - asthma | 3 (19%) | 3 (9%) | p = 0.362 |
| - allergic rhinitis | 1 (6%) | 4 (11%) | p = 1.000 |
| - food allergy | 1 (6%) | 0 | p = 0.313 |
| - ischemic heart disease | 1 (6%) | 3 (9%) | p = 1.000 |
| - gout | 0 | 5 (14%) | p = 0.167 |
| - psoriasis | 2 (13%) | 1 (3%) | p = 0.228 |
| - arrhythmia | 2 (13%) | 1 (3%) | p = 0.228 |
| - prostate hyperplasia | 2 (13%) | 1 (3%) | p = 0.228 |
| - varicose disease | 1 (6%) | 1 (3%) | p = 0.533 |
| - nephrolithiasis | 1 (6%) | 1 (3%) | p = 0.533 |
| - reflux disease | 1 (6%) | 1 (3%) | p = 0.533 |
| - heart failure | 0 | 1 (3%) | p = 1.000 |
| - sleep apnea | 0 | 1 (3%) | p = 1.000 |
| - chronic sinusitis | 0 | 1 (3%) | p = 1.000 |
| - COPD | 0 | 1 (3%) | p = 1.000 |
| - chronic kidney disease | 0 | 1 (3%) | p = 1.000 |
| - adrenal adenoma | 0 | 1 (3%) | p = 1.000 |
| - osteoporosis | 1 (6%) | 0 | p = 0.313 |
| Disease severity: | |||
| Moderate COVID-19, n | 6 (38%) | 11 (31%) | p = 0.753 |
| Severe COVID-19, n | 6 (38%) | 20 (57%) | p = 0.236 |
| Critical COVID-19, n | 4 (25%) | 4 (11%) | p = 0.239 |
| Treatment: | |||
| Systemic GKS, n | 10 (62%) | 26 (74%) | p = 0.510 |
| Remdesivir, n | 7 (43%) | 17 (48%) | p = 0.772 |
| Tocilizumab, n | 1 (6%) | 1 (3%) | p = 0.533 |
| Oxygen, n | 15 (94%) | 34 (97%) | p = 0.533 |
| Day 0 | IFN-λ | Day 8 | IFN-λ | Δ | IFN-λ | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||||
| MCP-1 | V1 | 0.17 | ns | V2 | 0.16 | ns | V2 − V1 | 0.12 | ns |
| MIP-1β | V1 | 0.01 | ns | V2 | −0.03 | ns | V2 − V1 | −0.15 | ns |
| TNF-α | V1 | −0.11 | ns | V2 | −0.01 | ns | V2 − V1 | −0.13 | ns |
| IL-7 | V1 | 0.15 | ns | V2 | −0.14 | ns | V2 − V1 | 0.26 | ns |
| IL-8 | V1 | 0.08 | ns | V2 | −0.27 | ns | V2 − V1 | 0.11 | ns |
| IL-6 | V1 | −0.01 | ns | V2 | −0.08 | ns | V2 − V1 | 0.16 | ns |
| IL-10 | V1 | −0.15 | ns | V2 | −0.05 | ns | V2 − V1 | 0.04 | ns |
| IFN-γ | V1 | 0.15 | ns | V2 | 0.04 | ns | V2 − V1 | 0.02 | ns |
| CRP | V1 | −0.03 | ns | V2 | −0.21 | ns | V2 − V1 | 0.11 | ns |
| ALT | V1 | 0.14 | ns | V2 | 0.06 | ns | V2 − V1 | −0.19 | ns |
| AST | V1 | 0.08 | ns | V2 | −0.25 | ns | V2 − V1 | −0.02 | ns |
| GGT | V1 | 0.11 | ns | V2 | −0.18 | ns | V2 − V1 | 0.44 * | 0.02 |
| D-dimer | V1 | 0.21 | ns | V2 | −0.05 | ns | V2 − V1 | 0.29 * | 0.04 |
| ALDH | V1 | 0.01 | ns | V2 | −0.15 | ns | V2 − V1 | 0.23 | ns |
| Ferritin | V1 | −0.03 | ns | V2 | −0.26 | ns | V2 − V1 | 0.21 | ns |
| Leukocytes | V1 | −0.04 | ns | V2 | 0.02 | ns | V2 − V1 | 0.03 | ns |
| Lymphocytes | V1 | −0.01 | ns | V2 | 0.08 | ns | V2 − V1 | 0.16 | ns |
| Neutrophils | V1 | −0.08 | ns | V2 | −0.04 | ns | V2 − V1 | 0.01 | ns |
| Eosinophils | V1 | 0.25 | ns | V2 | 0.25 | ns | V2 − V1 | 0.01 | ns |
| Platelets | V1 | 0.06 | ns | V2 | −0.07 | ns | V2 − V1 | 0.22 | ns |
| Procalcitonin | V1 | −0.10 | ns | V2 | −0.38 * | 0.008 | V2 − V1 | 0.20 | ns |
| Fibrinogen | V1 | −0.06 | ns | V2 | −0.45 * | 0.009 | V2 − V1 | −0.07 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaleska, A.; Dor-Wojnarowska, A.; Radlińska, A.; Rorat, M.; Szymański, W.; Gajewski, A.; Chałubiński, M. IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes. Int. J. Mol. Sci. 2024, 25, 10530. https://doi.org/10.3390/ijms251910530
Zaleska A, Dor-Wojnarowska A, Radlińska A, Rorat M, Szymański W, Gajewski A, Chałubiński M. IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes. International Journal of Molecular Sciences. 2024; 25(19):10530. https://doi.org/10.3390/ijms251910530
Chicago/Turabian StyleZaleska, Anna, Anna Dor-Wojnarowska, Anna Radlińska, Marta Rorat, Wojciech Szymański, Adrian Gajewski, and Maciej Chałubiński. 2024. "IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes" International Journal of Molecular Sciences 25, no. 19: 10530. https://doi.org/10.3390/ijms251910530
APA StyleZaleska, A., Dor-Wojnarowska, A., Radlińska, A., Rorat, M., Szymański, W., Gajewski, A., & Chałubiński, M. (2024). IFN Lambda Deficiency Contributes to Severe COVID-19 Outcomes. International Journal of Molecular Sciences, 25(19), 10530. https://doi.org/10.3390/ijms251910530







