Study on the Thermal Condensation Mechanism of Dehydrogenated Polymer (DHP) and Glucuronic Acid
Abstract
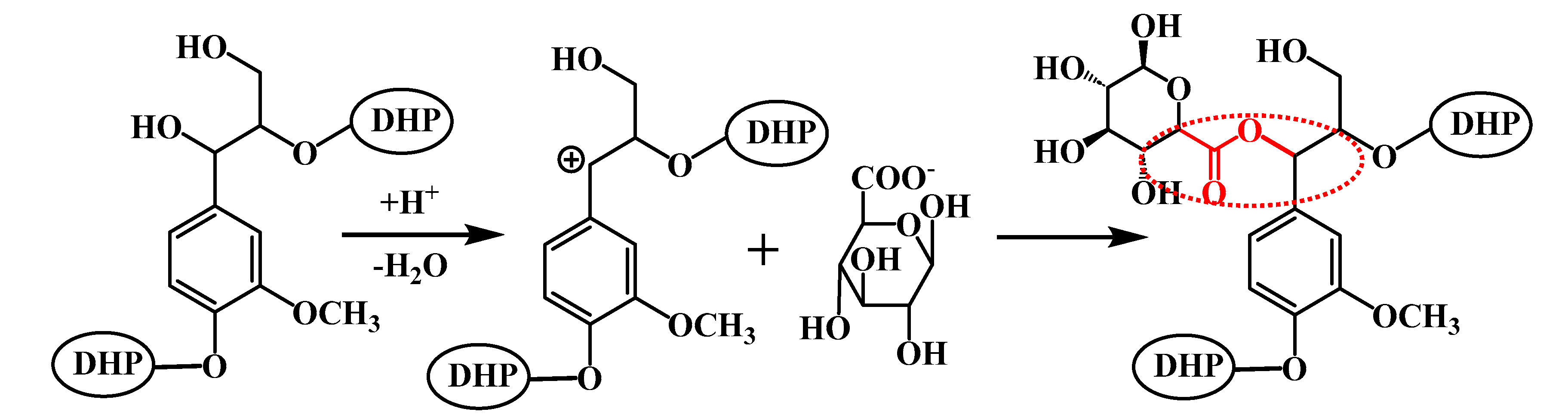
:1. Introduction
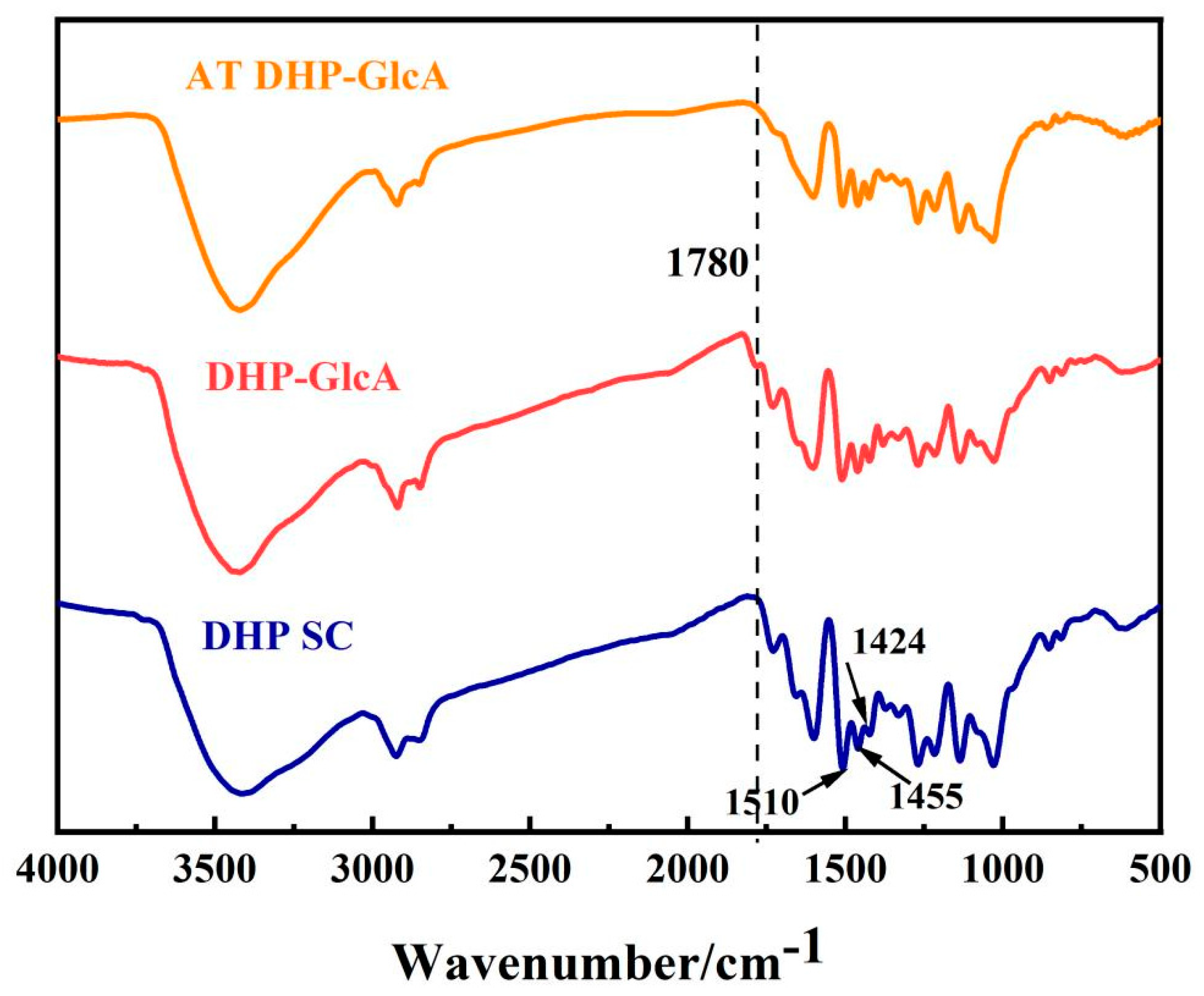
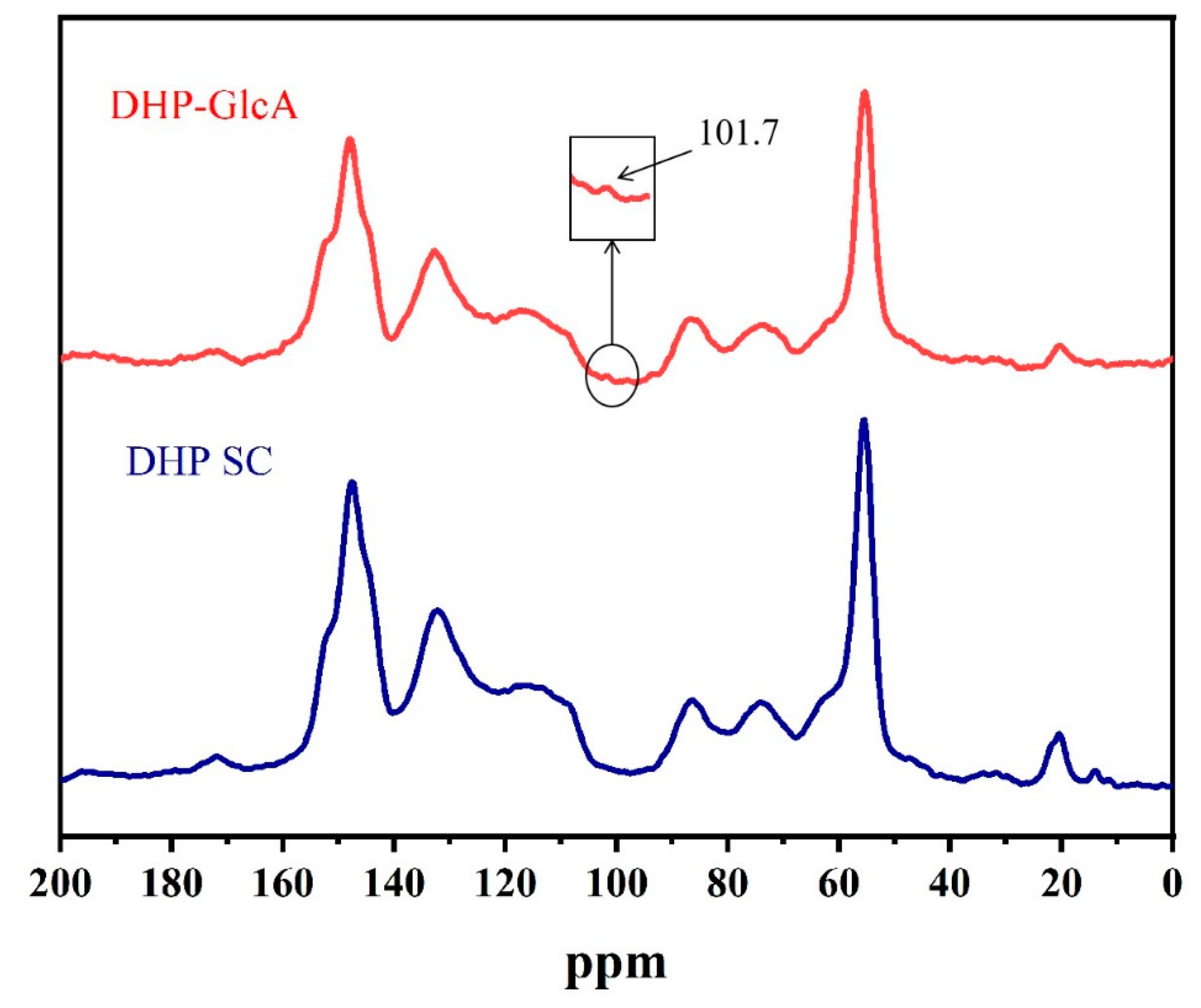
2. Results and Discussion
3. Methods and Materials
3.1. Materials and Reagents
3.2. Thermal Condensation of DHP with Glucuronic Acid
3.3. Ball Milling Treatment of DHP–Glucuronic Acid Complex
3.4. Alkali Treatment of the DHP–Glucuronic Acid Complex Powder after Ball Milling
3.5. Structural Characterization of DHP–Glucuronic Acid Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Batiancela, M.A.; Acda, M.N.; Cabangon, R.J. Particleboard from waste tea leaves and wood particles. J. Compos. Mater. 2013, 48, 911–916. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Q.; Qin, Z.; Luo, J.; Li, J. Research and Development of Wood Adhesive in China. China Wood-Based Panels 2014, 3, 8–12. [Google Scholar]
- Myers, G.E. Advances in methods to reduce formaldehyde emission. In Composite Board Products for Furniture and Cabinets-Innovationsin Manufacture and Utilization; Forest Products Research Society: New York, NY, USA, 1989; pp. 58–64. [Google Scholar]
- Younesi Kordkheili, H.; Pizzi, A.; Niyatzade, G. Reduction of formaldehyde emission from particleboard by phenolated kraft lignin. J. Adhes. 2016, 92, 485–497. [Google Scholar] [CrossRef]
- Peng, Y.; Zhen, X.; Lu, H. Purification and Application of Lignin in Urea-formaldehyde Resin. Guizhou Chem. Ind. 2006, 35, 377–379. [Google Scholar]
- Alonso, M.V.; Oliet, M.; Rodríguez, F.; Astarloa, G.; Echeverría, J.M. Use of a methylolated softwood ammonium lignosulfonate as partial substitute of phenol in resol resins manufacture. J. Appl. Polym. Sci. 2004, 94, 643–650. [Google Scholar] [CrossRef]
- Alonso, M.V.; Oliet, M.; Rodrıguez, F.; Garcıa, J.; Gilarranz, M.A.; Rodrıguez, J.J. Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour. Technol. 2005, 96, 1013–1018. [Google Scholar] [CrossRef]
- Khan, M.A.; Ashraf, S.M. Studies on thermal characterization of lignin. J. Therm. Anal. Calorim. 2007, 89, 993–1000. [Google Scholar] [CrossRef]
- Khan, M.A.; Ashraf, S.M.; Malhotra, V.P. Development and characterization of a wood adhesive using bagasse lignin. Int. J. Adhes. Adhes. 2004, 24, 485–493. [Google Scholar] [CrossRef]
- Kharazipour, A.; Mai, C.; Hüttermann, A. Polyphenoles for compounded materials. Polym. Degrad. Stab. 1998, 59, 237–243. [Google Scholar] [CrossRef]
- Vázquez, G.; González, J.; Freire, S.; Antorrena, G. Effect of chemical modification of lignin on the gluebond performance of lig-nin-phenolic resins. Bioresour. Technol. 1997, 60, 191–198. [Google Scholar] [CrossRef]
- Çetin, N.S.; Özmen, N. Use of organosolv lignin in phenol–formaldehyde resins for particleboard production: I. Organosolv lignin modified resins. Int. J. Adhes. Adhes. 2002, 22, 477–480. [Google Scholar] [CrossRef]
- Vázquez, G.; Antorrena, G.; González, J.; Mayor, J. Lignin-phenol-formaldehyde adhesives for exterior grade plywoods. Bioresour. Technol. 1995, 51, 187–192. [Google Scholar] [CrossRef]
- Haars, A.; Kharazipour, A.; Zanker, H.; Huttermann, A. Room-Temperature Curing Adhesives Based on Lignin and Phenoloxidases. In Adhesives from Renewable Resources; American Chemical Society: Washington, DC, USA, 1989; pp. 126–134. [Google Scholar]
- Hüttermann, A.; Milstein, O.; Nicklas, B.; Trojanowski, J.; Haars, A.; Kharazipour, A. Enzymatic Modification of Lignin for Technical Use. In Lignin; American Chemical Society: Washington, DC, USA, 1989; pp. 361–370. [Google Scholar]
- Yamaguchi, H.; Maeda, Y.; Sakata, I. Application of the dehydrogenative polymerization of vanillic acid to bonding of woody fibers. Mokuzai Gakkaishi 1991, 37, 220–226. [Google Scholar]
- Lund, M.; Felby, C. Wet strength improvement of unbleached kraft pulp through laccase catalyzed oxidation. Enzym. Microb. Technol. 2001, 28, 760–765. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Maeda, Y.; Sakata, I. Applications of phenol dehydrogenative polymerization by laccase to bonding among woody-fibers. Mokuzai Gakkaishi 1992, 38, 931–937. [Google Scholar]
- Yamaguchi, H.; Maeda, Y.; Sakata, I. Bonding among woody fibers by use of enzymatic phenol dehydrogenative polymerization. Mokuzai Gakkaishi 1994, 40, 185–190. [Google Scholar]
- Pei, J. Lignocellulosic Chemistry; China Light Industry Press: Beijing, China, 2014. [Google Scholar]
- Bolker, H.I. A Lignin Carbohydrate Bond as revealed by Infra-red Spectroscopy. Nature 1963, 197, 489–490. [Google Scholar] [CrossRef]
- Yang, G.; Gong, Z.; Luo, X.; Chen, L.; Shuai, L. Bonding wood with uncondensed lignins as adhesives. Nature 2023, 621, 511–515. [Google Scholar] [CrossRef]
- Wang, X. Study on Thermal Condensation Reaction Mechanism of Dehydrogenation Polymer Catalyzed with Acid. Master’s Thesis, Hubei University of Technology, Wuhan, China, 2022. [Google Scholar]
- Jiang, B.; Shen, F.; Jiang, Y.; Huang, M.; Zhao, L.; Lei, Y.; Hu, J.; Tian, D.; Shen, F. Extraction of super high-yield lignin-carbohydrate complexes from rice straw without compromising cellulose hydrolysis. Carbohydr. Polym. 2024, 323, 121452. [Google Scholar] [CrossRef]
- Richel, A.; Nicks, F.; Laurent, P.; Wathelet, B.; Wathelet, J.-P.; Paquot, M. Efficient microwave-promoted synthesis of glucuronic and galacturonic acid derivatives using sulfuric acid impregnated on silica. Green Chem. Lett. Rev. 2012, 5, 179–186. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Han, J.; You, X.; Wang, S.; Chen, C.; Yao, S.; Meng, C.; Liang, C.; Zhao, J. Chlorine dioxide oxidation of hemicellulose from alkaline hydrolysate bagasse to remove lignin unit in lignin-carbohydrate complex. Carbohydr. Polym. 2022, 277, 118817. [Google Scholar] [CrossRef] [PubMed]

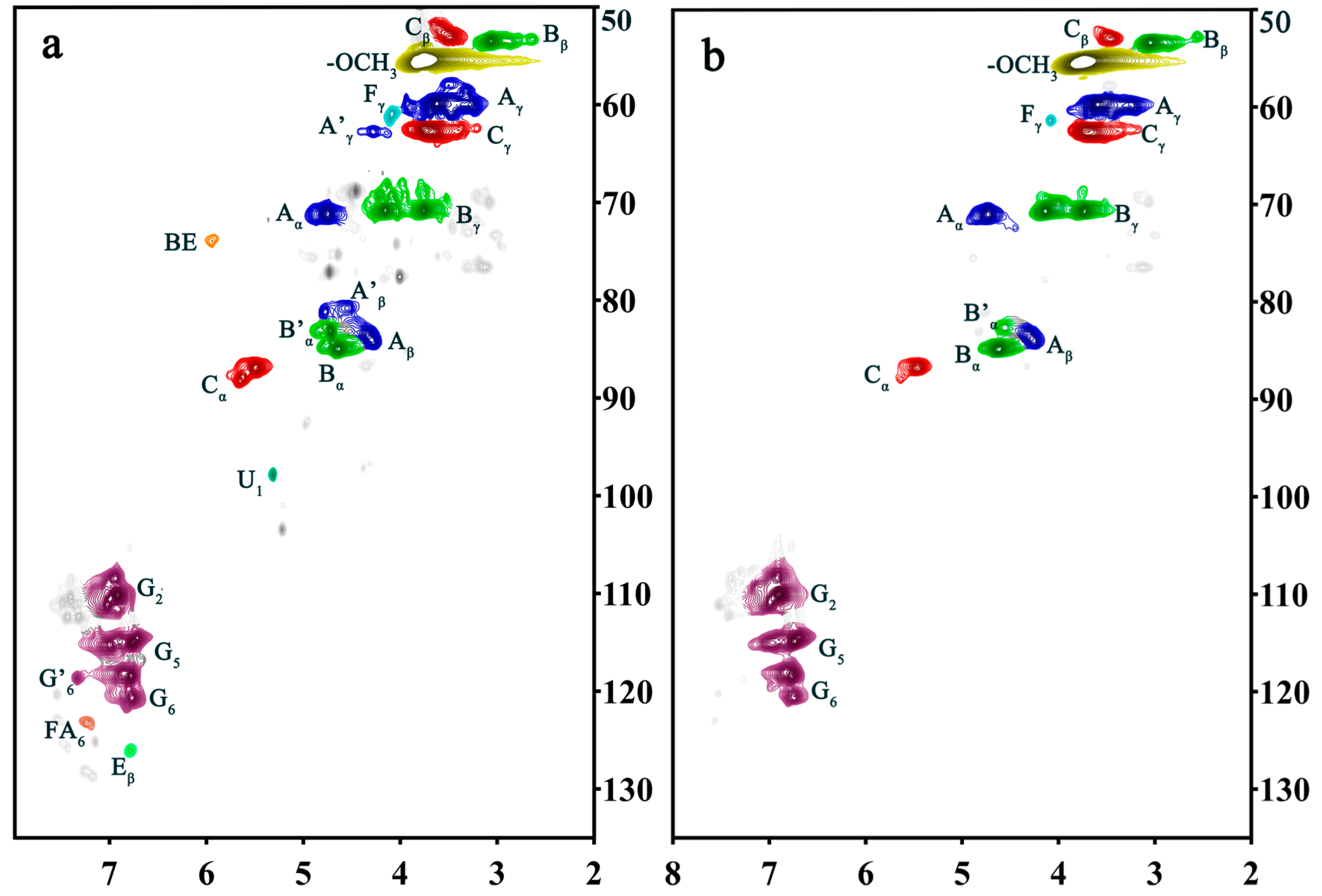
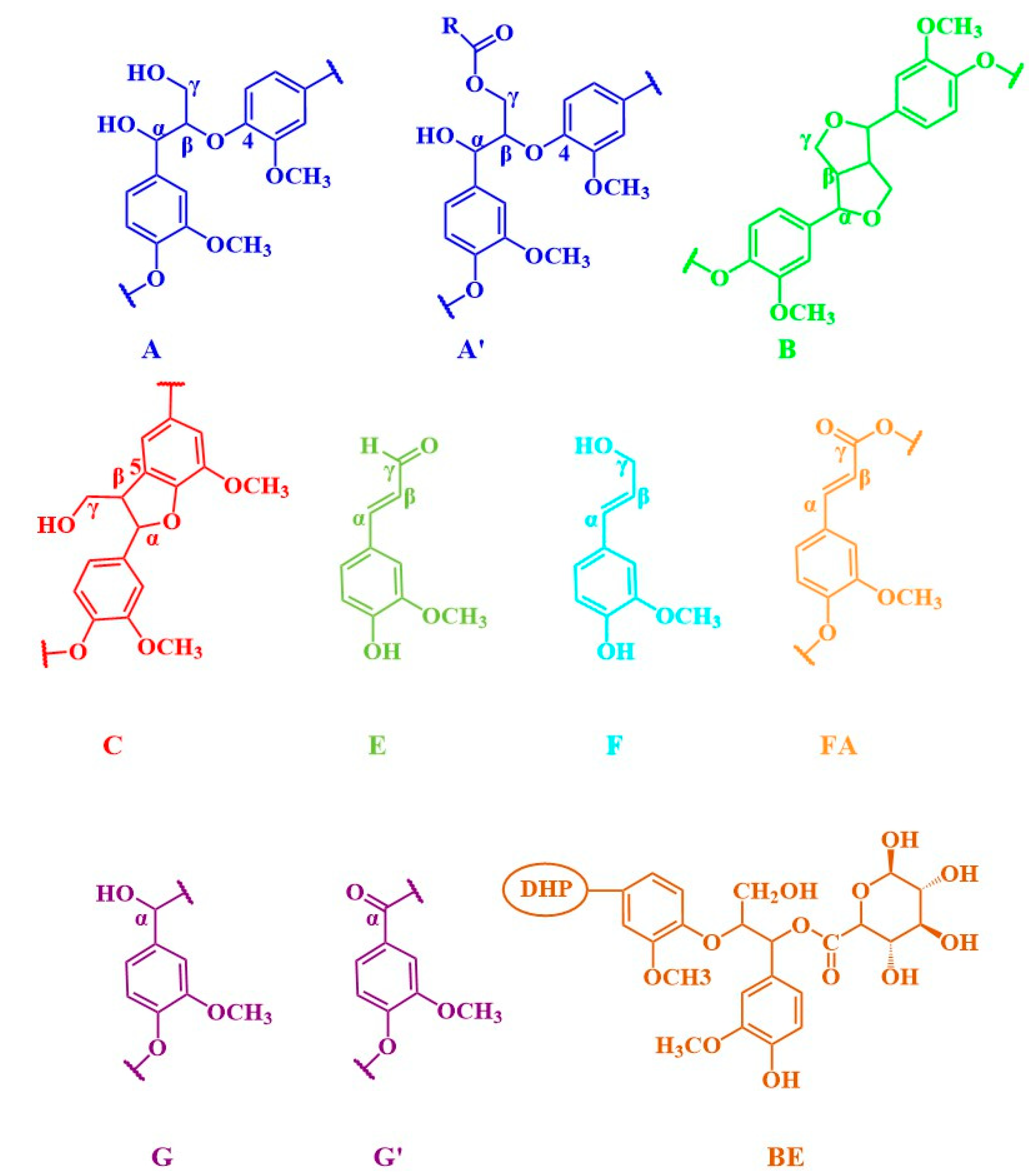
| Label | pH = 4 | pH = 4 (Alkali Treatment) | Assignments |
|---|---|---|---|
| δC/δH (ppm) | δC/δH (ppm) | ||
| Cβ | 52.96/3.49 | 52.93/3.46 | Cβ–Hβ in phenylcoumaran (C) |
| Bβ | 53.48/3.06 | 53.38/3.04 | Cβ–Hβ in β-β (resinol) (B) |
| OCH3 | 55.49/3.77 | 55.32/3.76 | C–H in methoxyls |
| Aγ | 59.89/3.62 59.99/3.28 | 59.84/3.25 59.74/3.69 | Cγ–Hγ in β–O–4 substructures (A) |
| Fγ | 60.90/4.10 | 61.37/4.08 | Cγ–Hγ in cinnamyl alcohol end-groups (F) |
| Cγ | 62.66/3.73 | 62.56/3.71 | Cγ–Hγ in phenylcoumaran (C) |
| A′γ | 62.74/4.27 | ND | Cγ-Hγ in γ-acylated β-O-4 substructures(A’) |
| Bγ | 70.78/3.75 70.83/4.51 | 70.61/3.74 70.68/4.13 | Cγ–Hγ in β-β resinol (B) |
| Aα | 71.19/4.76 | 71.03/4.73 | Cα–Hα in β–O–4 unit (A) |
| BE | 73.74/5.94 | ND | α-ester |
| Aβ | 84.00/4.32 | 84.02/4.29 | Cβ–Hβ in β–O–4 substructures (A) |
| A′β | 80.81/4.55 | ND | Cβ–Hβ in β–O–4 linked to G (A) |
| Bα | 84.96/4.63 | 84.83/4.62 | Cα–Hα in β-β resinol (B) |
| B′α | 83.25/4.72 | 82.68/4.55 | Cα–Hα in β-β (B’, tetrahydrofuran) |
| Cα | 86.89/5.48 87.89/5.64 | 86.73/5.46 | Cα–Hα in phenylcoumaran (C) |
| U1 | 97.86/5.31 | ND | C1-H1 in 4-O-methyl-α-D-GlcUA |
| G2 | 108.51/6.94 110.23/6.93 | 108.31/6.92 110.03/6.90 | C2–H2 in guaiacyl units (G) |
| G5 | 114.65/6.76 115.25/6.99 | 114.51/6.74 115.17/7.06 | C5–H5 in guaiacyl units (G) |
| G6 | 118.40/6.78 120.51/6.77 | 118.25/6.16 120.55/6.74 | C6–H6 in guaiacyl units (G) |
| G′6 | 118.52/7.29 | 118.70/7.27 | α C6-H6 in G-type structural units with oxidized sites |
| Eβ | 125.99/6.79 | ND | Cβ–Hβ in cinnamyl aldehyde end-groups (E) |
| FA6 | 123.25/7.21 | ND | C6–H6 in ferulate (p-FA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhang, X.; Peng, W.; Chen, J.; An, J.; Zhang, G.; Xie, J. Study on the Thermal Condensation Mechanism of Dehydrogenated Polymer (DHP) and Glucuronic Acid. Int. J. Mol. Sci. 2024, 25, 10533. https://doi.org/10.3390/ijms251910533
Wang P, Zhang X, Peng W, Chen J, An J, Zhang G, Xie J. Study on the Thermal Condensation Mechanism of Dehydrogenated Polymer (DHP) and Glucuronic Acid. International Journal of Molecular Sciences. 2024; 25(19):10533. https://doi.org/10.3390/ijms251910533
Chicago/Turabian StyleWang, Peng, Xu Zhang, Wenyao Peng, Junjun Chen, Junjian An, Guangyan Zhang, and Junxian Xie. 2024. "Study on the Thermal Condensation Mechanism of Dehydrogenated Polymer (DHP) and Glucuronic Acid" International Journal of Molecular Sciences 25, no. 19: 10533. https://doi.org/10.3390/ijms251910533






