Untargeted Metabolomics Approach for the Discovery of Salinity-Related Alkaloids in a Stony Coral-Derived Fungus Aspergillus terreus
Abstract
1. Introduction
2. Results
2.1. Morphological Comparison
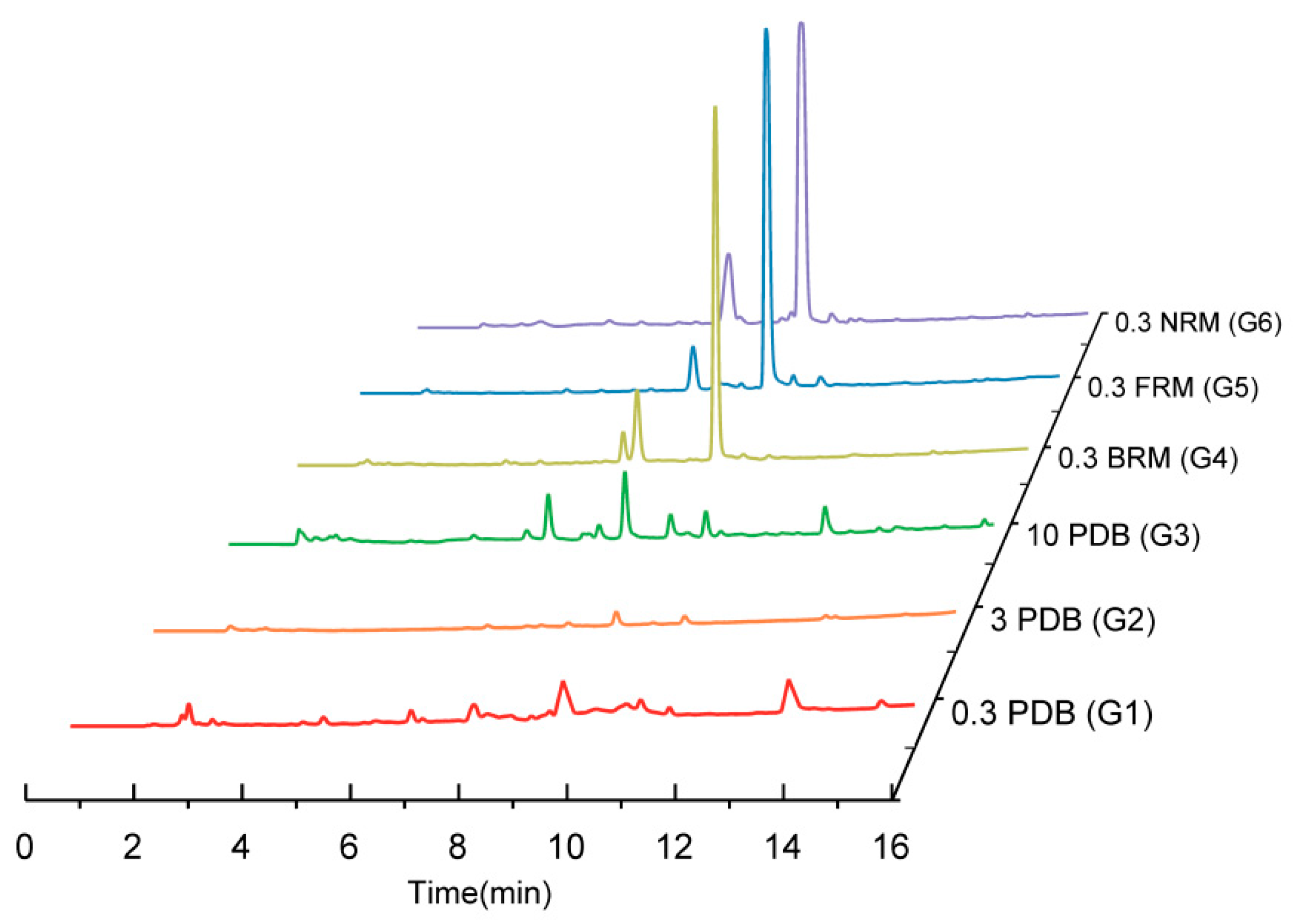
2.2. Comparison of TLC Fingerprints and HPLC
2.3. Biological Activities of Crude Extracts
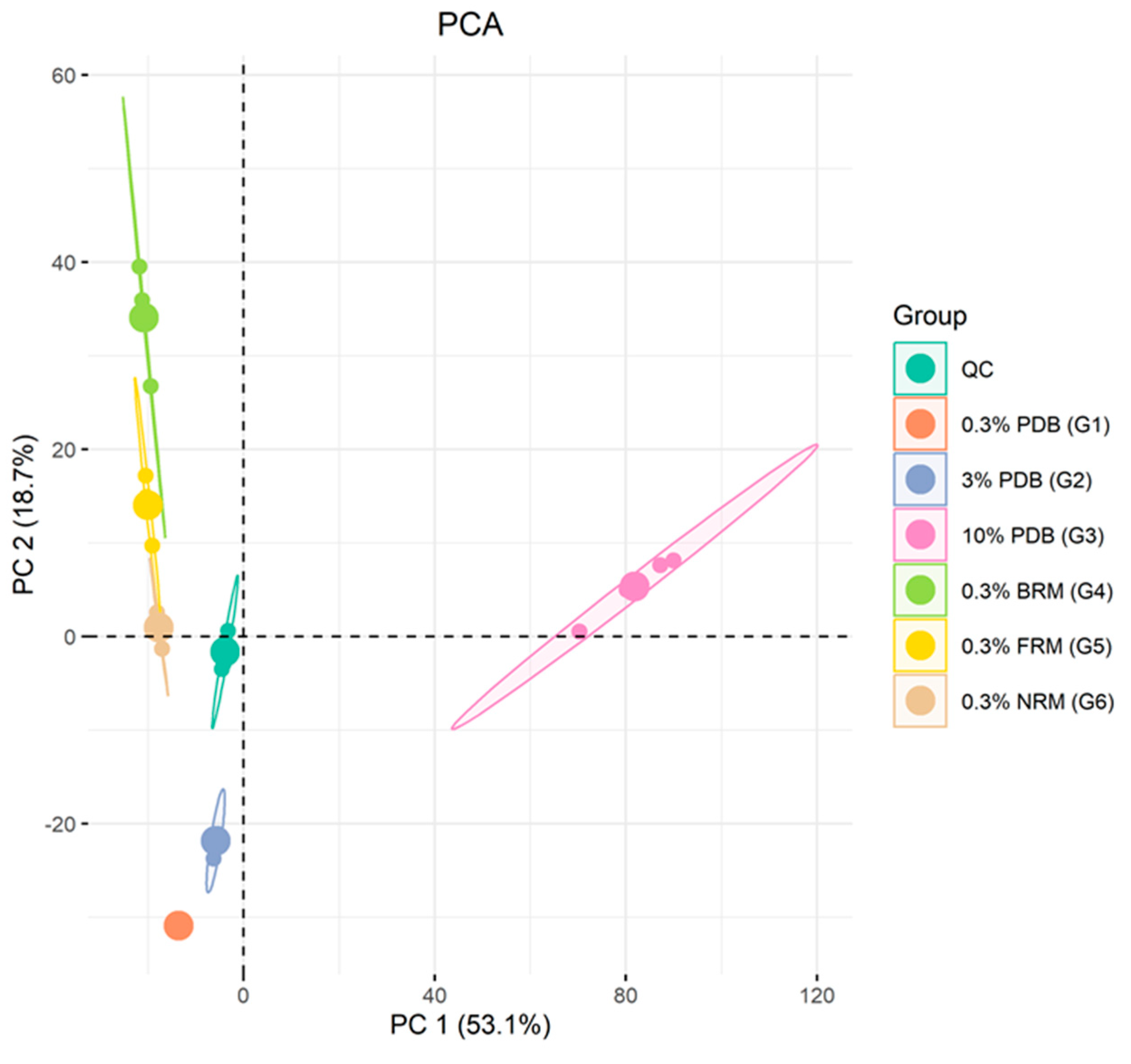
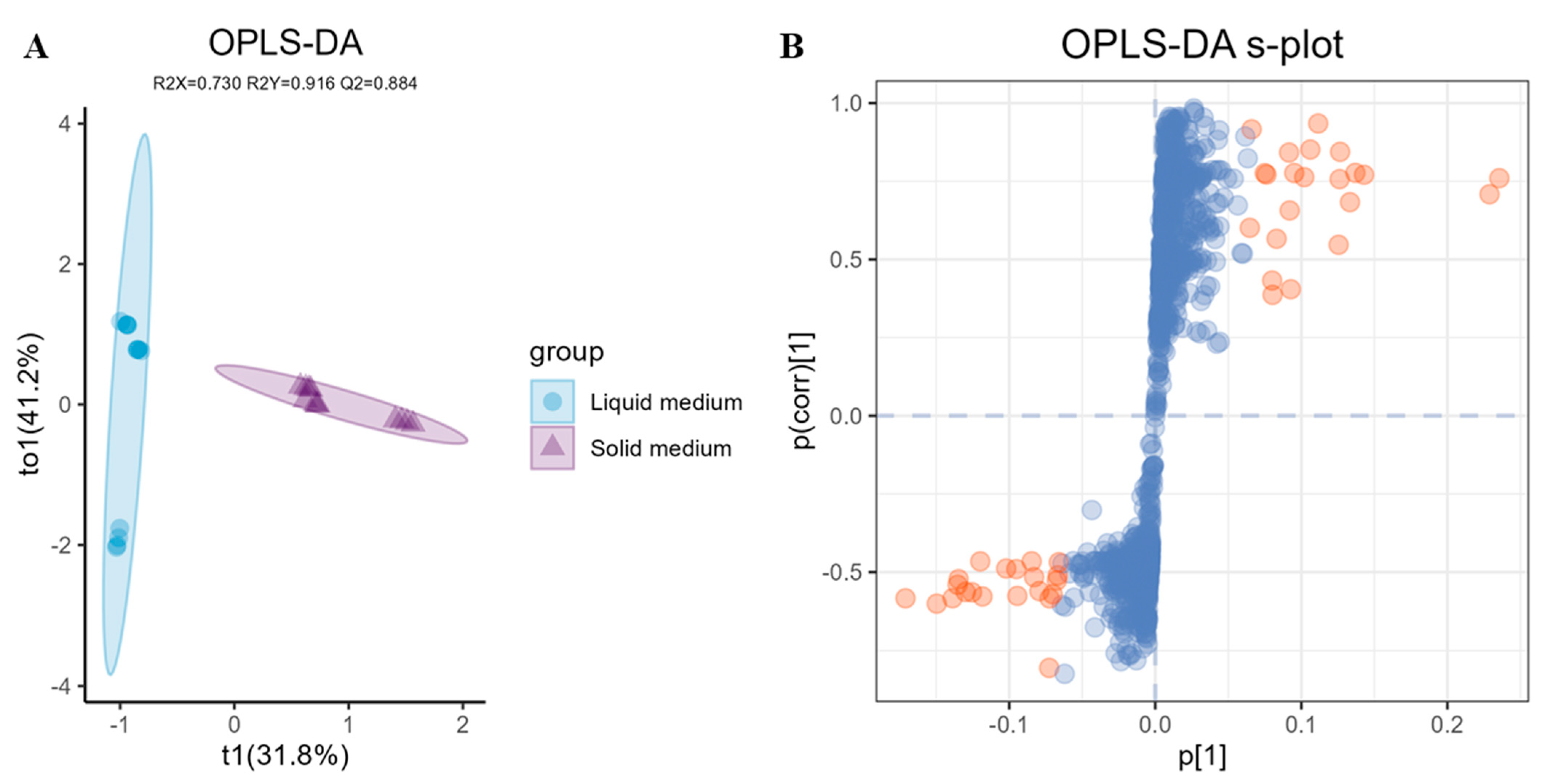
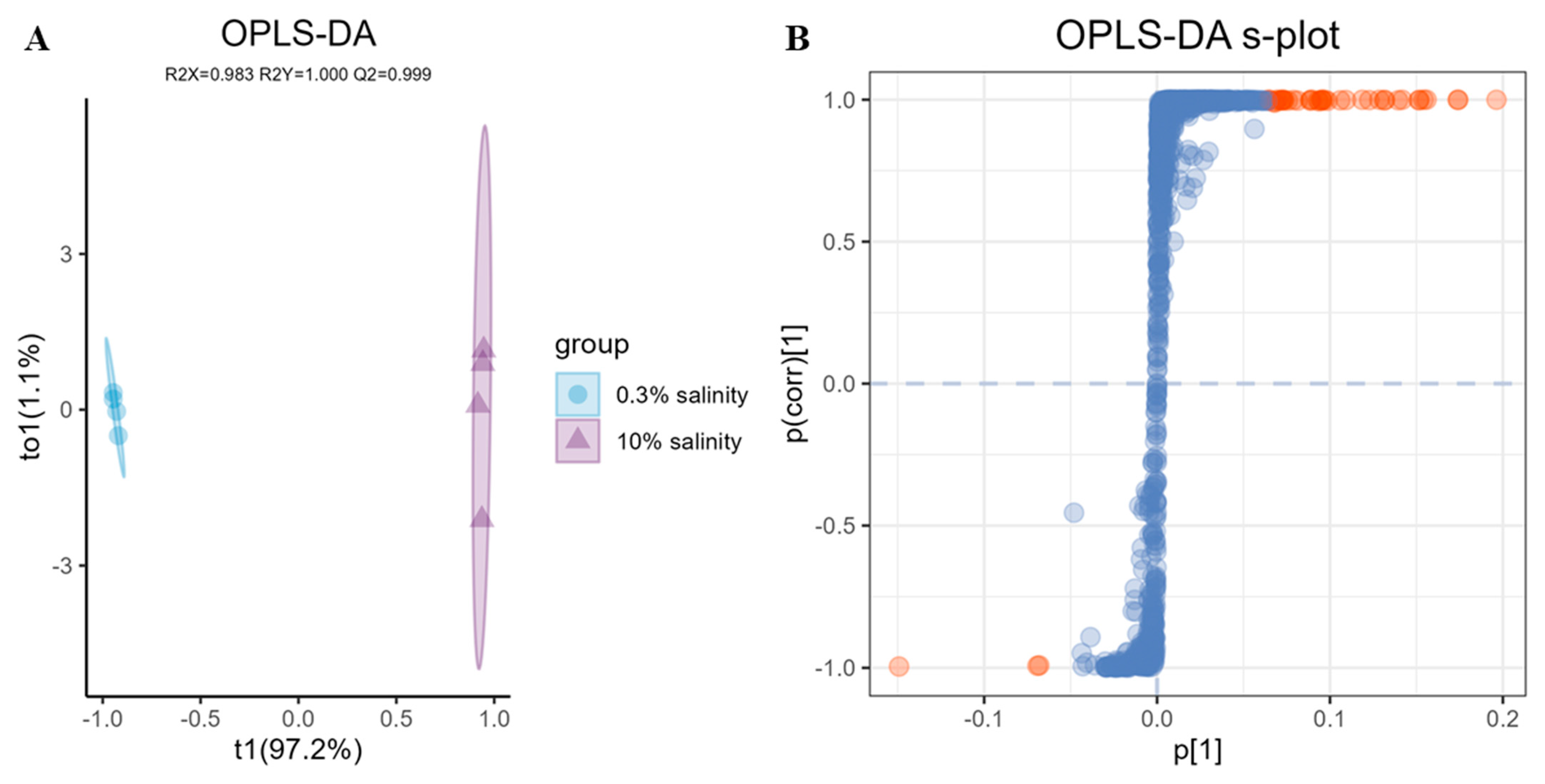
2.4. LC-MS/MS Based Data Statistical Analysis
2.5. LC-MS/MS-Based MSDIAL Analysis and FBMN Networking
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Fermentation and Extraction for OSMAC Approach
4.3. TLC and HPLC Analyses
4.4. AChE Inhibitory Activity Assay
4.5. DPPH Free Radical Scavenging Assay
4.6. Antibacterial Assay
4.7. LC-MS Analyses
4.8. Data Processing and Statistical Analysis
4.9. FBMN Analysis
4.10. Annotation of MS Features
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Pang, X.; He, Y.; Lin, X.; Zhou, X.; Liu, Y.; Yang, B. Secondary metabolites from coral-associated fungi: Source, chemistry and bioactivities. J. Fungi 2022, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Liu, L.T.; Yang, L.; Li, J.; Dong, X. Chemistry and bioactivity of marine-derived bisabolane sesquiterpenoids: A review. Front. Chem. 2022, 10, 881767. [Google Scholar] [CrossRef]
- Orlova, T.I.; Bulgakova, V.G.; Polin, A.N. Secondary metabolites from marine microorganisms. II. marine fungi and their habitats. Antibiot Khimioter. 2016, 61, 52–63. [Google Scholar] [PubMed]
- Nie, Y.; Yang, J.; Zhou, L.; Yang, Z.; Liang, J.; Liu, Y.; Ma, X.; Qian, Z.; Hong, P.; Kalueff, A.V.; et al. Marine fungal metabolite butyrolactone I prevents cognitive deficits by relieving inflammation and intestinal microbiota imbalance on aluminum trichloride-injured zebrafish. J. Neuroinflammation 2022, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Shao, C.; Wang, K.; Zhao, D.; Wang, Y.; Wang, C. Secondary metabolites and their bioactivities of a soft coral-derived fungus Aspergillus versicolor (ZJ-2008015). Chin. J. Mar. Drugs 2012, 31, 7–13. [Google Scholar]
- Luo, X.W.; Gao, C.H.; Lu, H.M.; Wang, J.M.; Su, Z.Q.; Tao, H.M.; Zhou, X.F.; Yang, B.; Liu, Y.H. HPLC-DAD-guided isolation of diversified chaetoglobosins from the coral-associated fungus Chaetomium globosum C2F17. Molecules 2020, 25, 1237. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, J.; Wang, L.; Li, F.; Wang, Y.; Jiang, Y.; Song, X.; Qin, S.; Zheng, K.; Ye, J.; et al. Anti-HSV-1 activity of Aspergillipeptide D, a cyclic pentapepetide isolated from fungus Aspergillus sp. SCSIO 41501. Virol. J. 2020, 17, 41. [Google Scholar] [CrossRef]
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020, 11, 572. [Google Scholar] [CrossRef]
- Hou, X.-M.; Hai, Y.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. Chemical and bioactive marine natural products of coral-derived microorganisms (2015–2017). Curr. Med. Chem. 2019, 26, 6930–6941. [Google Scholar] [CrossRef]
- Hou, X.M.; Xu, R.F.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Biological and chemical diversity of coral-derived microorganisms. Curr. Med. Chem. 2015, 22, 3707–3762. [Google Scholar] [CrossRef]
- Wei, X.; Feng, C.; Wang, S.-Y.; Zhang, D.-M.; Li, X.-H.; Zhang, C.-X. New indole diketopiperazine alkaloids from soft coral-associated epiphytic fungus Aspergillus sp. EGF 15-0-3. Chem. Biodivers. 2020, 17, e2000106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, D.; Cheng, W.; Proksch, P.; Lin, W. Versiquinazolines L-Q, new polycyclic alkaloids from the marine-derived fungus Aspergillus versicolor. RCS Adv. 2018, 8, 31427–31439. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Teles, A.P.C.; de Almeida Pinto Bracarense, A.; Gomes, D.C. Classical and epigenetic approaches to metabolite diversification in filamentous fungi. Phytochem. Rev. 2013, 12, 773–789. [Google Scholar] [CrossRef]
- Song, W.; Ji, L.; Zhang, Y.; Cao, L. New cytotoxic indole derivatives with anti-FADU potential produced by the endophytic fungus Penicillium oxalicum 2021CDF-3 through the OSMAC strategy. Front. Microbiol. 2024, 15, 1400803. [Google Scholar] [CrossRef]
- Borkunov, G.V.; Leshchenko, E.V.; Berdyshev, D.V.; Popov, R.S.; Chingizova, E.A.; Shlyk, N.P.; Gerasimenko, A.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Chausova, V.E.; et al. New piperazine derivatives helvamides B-C from the marine-derived fungus Penicillium velutinum ZK-14 uncovered by OSMAC (one strain many compounds) strategy. Nat. Prod. Bioprospecting 2024, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Q.; Feng, S.; Xie, R.K.; Zhu, Z.T.; Lou, Y.H.; Zhou, X.M.; Song, X.M. New indole alkaloids from the fungus Talaromyces assiutensis JTY2. Chem. Biodivers. 2024, 21, e202400937. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, W.; Yang, Y.; Shah, M.; Peng, J.; Zhou, L.; Zhang, G.; Che, Q.; Li, J.; Zhu, T.; et al. Phenylhydrazone alkaloids from the deep-sea coldseep derived fungus Talaromyces amestolkiae HDN21-0307. J. Nat. Prod. 2024, 87, 1407–1415. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Tan, Q.; Chen, Y.; Chen, T.; Zou, G.; Sun, B.; Wang, B.; Yuan, J.; She, Z. 4-Hydroxy-2-pyridone derivatives with antitumor activity produced by mangrove endophytic fungus Talaromyces sp. CY-3. Eur. J. Med. Chem. 2024, 269, 116314. [Google Scholar] [CrossRef] [PubMed]
- Simons, V.E.; Mándi, A.; Frank, M.; van Geelen, L.; Tran-Cong, N.; Albrecht, D.; Coort, A.; Gebhard, C.; Kurtán, T.; Kalscheuer, R. Colletodiol derivatives of the endophytic fungus Trichocladium sp. Fitoterapia 2024, 175, 105914. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, S.; Guo, H.; Lu, X.; Shen, H.; Liu, L.; Wang, L.; Chen, B.; Zhang, Y.; Liu, Y. Bioactive alkaloids from the mangrove-derived fungus Nigrospora oryzae SYSU-MS0024. Mar. Drugs 2024, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, X.; Zhou, L.; Yang, W.; She, Z.; Liao, Q.; Feng, Y.; Chen, X.; Zhang, Y. Quinolinones alkaloids with AChE inhibitory activity from mangrove endophytic fungus Penicillium citrinum YX-002. Chem. Biodivers. 2023, 20, e202300735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, W.; Xie, B.; Chen, T.; Chen, S.; Liu, Z.; Sun, B.; She, Z. First discovery of sulfonamides derivatives with acetylcholinesterase inhibitory activity in fungus Pestalotiopsis sp. HNY36-1D. Tetrahedron 2023, 142, 133524. [Google Scholar] [CrossRef]
- Wu, J.; Chen, D.; Li, Q.; Feng, T.; Xu, J. Metabolomics-guided discovery of new dimeric xanthones from co-cultures of mangrove endophytic fungi Phomopsis asparagi DHS-48 and Phomopsis sp. DHS-11. Mar. Drugs 2024, 22, 102. [Google Scholar] [CrossRef]
- Stewart, S.; Ivy, M.A.; Anslyn, E.V. The use of principal component analysis and discriminant analysis in differential sensing routines. Chem. Soc. Rev. 2014, 43, 70–84. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Remy, S.; Solis, D.; Silland, P.; Neyts, J.; Roussi, F.; Touboul, D.; Litaudon, M. Isolation of phenanthrenes and identification of phorbol ester derivatives as potential anti-CHIKV agents using FBMN and NAP from Sagotia racemosa. Phytochemistry 2019, 167, 112101. [Google Scholar] [CrossRef]
- Phelan, V.V. Feature-based molecular networking for metabolite annotation. Methods Mol. Biol. 2020, 2104, 227–243. [Google Scholar]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vander Gheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen rearrangement rules: Computational MS/MS fragmentation and structure elucidation using MS-FINDER software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Satoh, A.; Uchino, H.; Cajka, T.; Arita, M.; Arita, M. Mass spectrometry data repository enhances novel metabolite discoveries with advances in computational metabolomics. Metabolites 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Liu, Y.; Zhou, L.; Liao, Q.; Nie, Y.; Wang, X.; Lei, X.; Hong, P.; Feng, Y.; Hu, X.; et al. The screening for marine fungal strains with high potential in alkaloids production by in situ colony assay and LC-MS/MS based secondary metabolic profiling. Front. Microbiol. 2023, 14, 1144328. [Google Scholar] [CrossRef]
- Mallik, A. Challenges and opportunities in allelopathy research: A brief overview. J. Chem. Ecol. 2000, 26, 2007–2009. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, R.; Luo, S.; Gu, W.; Nie, C.; Cao, M.; Li, X. Antifungus activity of DIMBOA from maize seedlings compared with several phenolic acids. Nat. Prod. Res. Dev. 2007, 19, 572. [Google Scholar]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M. Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Nat. Prod. Rep. 2009, 26, 478–489. [Google Scholar] [CrossRef]
- Ivanov, A.; Shamagsumova, R.; Larina, M.; Evtugyn, G. Electrochemical acetylcholinesterase sensors for anti-Alzheimer’s disease drug determination. Biosensors 2024, 14, 93. [Google Scholar] [CrossRef]
- Bhusal, C.K.; Uti, D.E.; Mukherjee, D.; Alqahtani, T.; Alqahtani, S.; Bhattacharya, A.; Akash, S. Unveiling nature’s potential: Promising natural compounds in Parkinson’s disease management. Park. Relat Disord. 2023, 115, 105799. [Google Scholar] [CrossRef]
- Igboeli, H.A.; Marchbank, D.H.; Correa, H.; Overy, D.; Kerr, R.G. Discovery of Primarolides A and B from marine fungus Asteromyces cruciatus using osmotic stress and treatment with suberoylanilide hydroxamic acid. Mar. Drugs 2019, 17, 435. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef]
- Papadimitropoulos, M.-E.P.; Vasilopoulou, C.G.; Maga-Nteve, C.; Klapa, M.I. Untargeted GC-MS Metabolomics. MMethods Mol. Biol. 2018, 1738, 133–147. [Google Scholar]
- Le, V.T.; Bertrand, S.; Robiou du Pont, T.; Fleury, F.; Caroff, N.; Bourgeade-Delmas, S.; Gentil, E.; Logé, C.; Genta-Jouve, G.; Grovel, O. Untargeted metabolomics approach for the discovery of environment-related pyran-2-ones chemodiversity in a marine-sourced Penicillium restrictum. Mar. Drugs 2021, 19, 378. [Google Scholar] [CrossRef]
- Kang, D.; Kim, J.; Choi, J.N.; Liu, K.H.; Lee, C.H. Chemotaxonomy of Trichoderma spp. using mass spectrometry-based metabolite profiling. J. Microbiol. Biotechnol. 2011, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, S.B.; Xie, M.Y.; Nie, S.P.; Liu, W.; Li, C.; Gong, X.F.; Wang, Y.X. Quality control and original discrimination of Ganoderma lucidum based on high-performance liquid chromatographic fingerprints and combined chemometrics methods. Anal. Chim. Acta 2008, 623, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Sun, K.; Zhu, W. Effects of high salt stress on secondary metabolite production in the marine-derived fungus Spicaria elegans. Mar. Drugs 2011, 9, 535–542. [Google Scholar] [CrossRef]
- Yu, J.-H.; Keller, N. Regulation of secondary metabolism in filamentous fungi. Annu. Rev. Phytopathol. 2005, 43, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Kralj Kuncic, M.; Kogej, T.; Drobne, D.; Gunde-Cimerman, N. Morphological response of the halophilic fungal genus Wallemia to high salinity. Appl. Environ. Microbiol. 2010, 76, 329–337. [Google Scholar] [CrossRef]
- Pérez-Llano, Y.; Rodríguez-Pupo, E.C.; Druzhinina, I.S.; Chenthamara, K.; Cai, F.; Gunde-Cimerman, N.; Zalar, P.; Gostinčar, C.; Kostanjšek, R.; Folch-Mallol, J.L.; et al. Stress reshapes the physiological response of halophile fungi to salinity. Cells 2020, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Plemenitas, A.; Vaupotic, T.; Lenassi, M.; Kogej, T.; Gunde-Cimerman, N. Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: A molecular perspective at a glance. Stud. Mycol. 2008, 61, 67–75. [Google Scholar] [CrossRef]
- Gostinčar, C.; Lenassi, M.; Gunde-Cimerman, N.; Plemenitaš, A. Fungal adaptation to extremely high salt concentrations. Adv. Appl. Microbiol. 2011, 77, 71–96. [Google Scholar]
- Turk, M.; Méjanelle, L.; Sentjurc, M.; Grimalt, J.O.; Gunde-Cimerman, N.; Plemenitas, A. Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi. Extremophiles 2004, 8, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, K.; Lu, Y.; Gong, G. Morphological, transcriptional, and metabolic analyses of osmotic-adapted mechanisms of the halophilic Aspergillus montevidensis ZYD4 under hypersaline conditions. Appl. Microbiol. Biotechnol. 2019, 103, 3829–3846. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, D.; Yan, J.; Zhang, N.; Li, B. Metabolomics integrated with transcriptomics: Assessing the central metabolism of marine red yeast Sporobolomyces pararoseus under salinity stress. Arch. Microbiol. 2021, 203, 889–899. [Google Scholar] [CrossRef]
- Ravishankar, J.; Suryanarayanan, T.; Muruganandam, V. Strategies for osmoregulation in the marine fungus Cirrenalia pygmea Kohl.(Hyphomycetes). Indian J. Geo-Mar. Sci. 2006, 35, 351–358. [Google Scholar]
- Hachicho, N.; Birnbaum, A.; Heipieper, H.J. Osmotic stress in colony and planktonic cells of Pseudomonas putida mt-2 revealed significant differences in adaptive response mechanisms. AMB Express 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Wang, Y.; Glukhov, E.; He, Y.; Liu, Y.; Zhou, L.; Ma, X.; Hu, X.; Hong, P.; Gerwick, W.H.; Zhang, Y. Secondary metabolite variation and bioactivities of two marine Aspergillus strains in static co-culture investigated by molecular network analysis and multiple database mining based on LC-PDA-MS/MS. Antibiotics 2022, 11, 513. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, W.; Liu, Y.; Yang, J.; Lei, X.; Gerwick, W.H.; Zhang, Y. Acetylcholinesterase inhibitors and antioxidants mining from marine fungi: Bioassays, bioactivity coupled LC–MS/MS analyses and molecular networking. Mar. Life Sci. Technol. 2020, 2, 386–397. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Oppong-Danquah, E.; Parrot, D.; Blümel, M.; Labes, A.; Tasdemir, D. Molecular networking-based metabolome and bioactivity analyses of marine-adapted fungi co-cultivated with phytopathogens. Front. Microbiol. 2018, 9, 2072. [Google Scholar] [CrossRef] [PubMed]



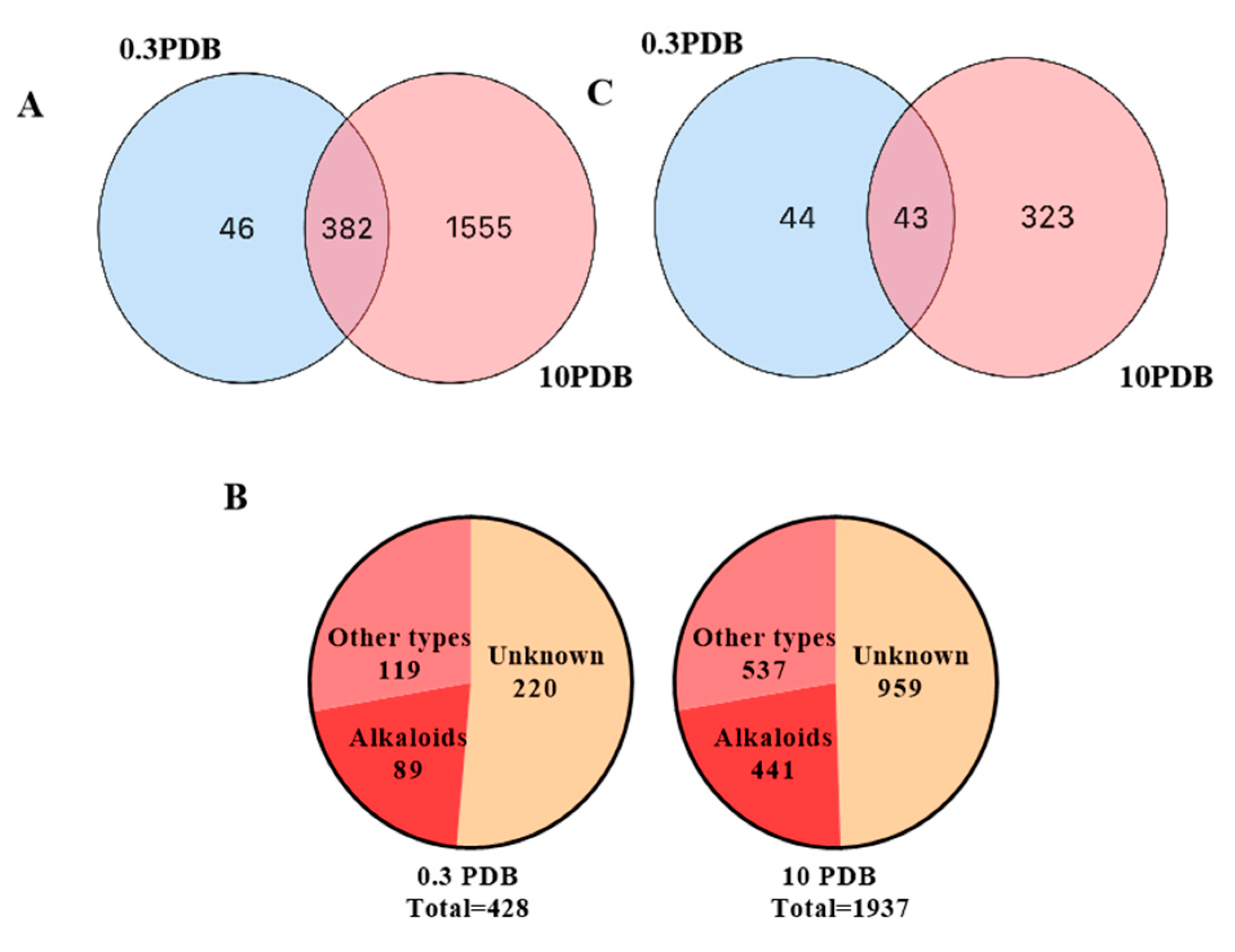
 respectively, 0.3 PDB and 10 PDB. The thickness of the connecting lines between nodes is positively correlated with the cosine value. The node size reflects the feature abundance (ion intensity). The A, B, C, D, E represent a locally enlarged view of a, b, c, d, e, respectively.
respectively, 0.3 PDB and 10 PDB. The thickness of the connecting lines between nodes is positively correlated with the cosine value. The node size reflects the feature abundance (ion intensity). The A, B, C, D, E represent a locally enlarged view of a, b, c, d, e, respectively.
 respectively, 0.3 PDB and 10 PDB. The thickness of the connecting lines between nodes is positively correlated with the cosine value. The node size reflects the feature abundance (ion intensity). The A, B, C, D, E represent a locally enlarged view of a, b, c, d, e, respectively.
respectively, 0.3 PDB and 10 PDB. The thickness of the connecting lines between nodes is positively correlated with the cosine value. The node size reflects the feature abundance (ion intensity). The A, B, C, D, E represent a locally enlarged view of a, b, c, d, e, respectively.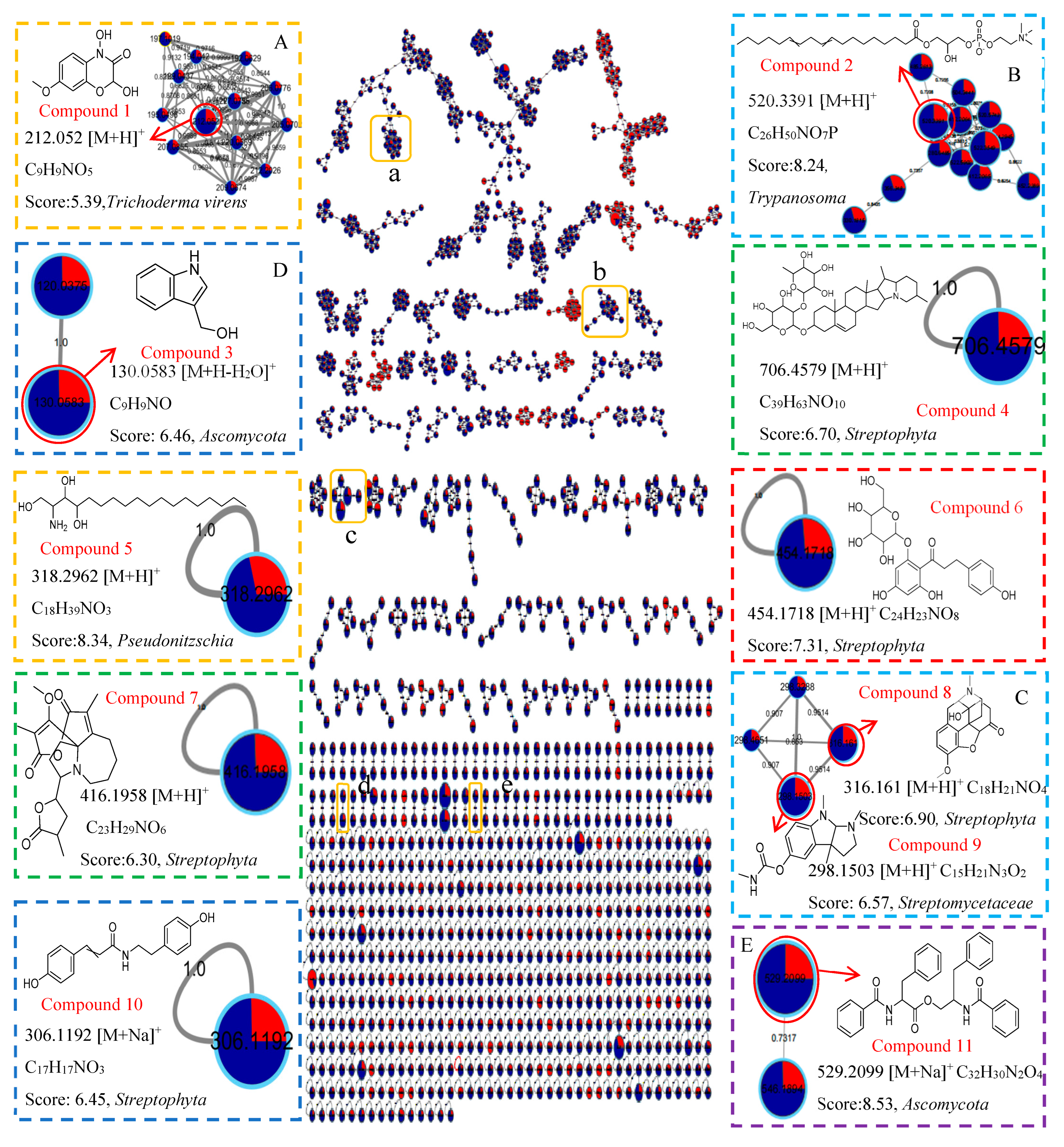
| Sample Number | IC50/(μg/mL) | Inhibition (%) b |
|---|---|---|
| G1 | 5.2 ± 0.6 | 72.6 ± 0.5 |
| G2 | 32.4 ± 1.2 | 15.0 ± 1.1 |
| G3 | 52.3 ± 0.9 | 12.3 ± 1.2 |
| G4 | 0.5 ± 0.7 | 71.9 ± 0.3 |
| G5 | 0.4 ± 0.8 | 73.2 ± 1.5 |
| G6 | 0.7 ± 0.1 | 80.6 ± 1.4 |
| Donepezil hydrochloride a | 0.2 ± 0.1 | 99.1 ± 2.0 |
| Sample Number | EC50/(μg/mL) | Inhibition (%) b |
|---|---|---|
| G1 | 57.2 ± 0.8 | 90.7 ± 1.2 |
| G2 | 183.2 ± 0.6 | 76.0 ± 0.8 |
| G3 | 352.3 ± 1.1 | 52.0 ± 1.2 |
| G4 | 36.5 ± 0.9 | 91.7 ± 1.5 |
| G5 | 31.7 ± 0.7 | 93.9 ± 0.6 |
| G6 | 88.8 ± 1.2 | 90.1 ± 1.9 |
| Ascorbic acid a | 7.2 ± 0.2 | 97.1 ± 1.1 |
| Sample Number | MRSA | Pseud omonus aeruginosa | Vibrio parahaemo lyticus | Vibrio alginol yticus | Shewanella putrefaciens | Bacillus subtilis |
|---|---|---|---|---|---|---|
| G1 | 14.0 ± 0.6 | 13.0 ± 0.3 | 13.0 ± 0.5 | 13.0 ± 0.2 | 10.0 ± 0.7 | 14.0 ± 0.2 |
| G2 | - | - | - | - | - | - |
| G3 | - | - | - | - | - | - |
| G4 | 10.5 ± 0.2 | 13.0 ± 0.6 | 13.0 ± 0.5 | 15.5 ± 0.2 | 11.3 ± 0.3 | 13.5 ± 0.6 |
| G5 | 14.0 ± 0.6 | 13.0 ± 0.7 | 13.0 ± 0.2 | 18.0 ± 0.5 | 13.0 ± 0.5 | 14.0 ± 0.7 |
| G6 | - | - | - | - | - | - |
| Ampicillin a | 14.0 ± 0.8 | 19.0 ± 0.5 | 24.0 ± 0.7 | 19.0 ± 0.6 | 16.0 ± 0.9 | 18.0 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, L.; Feng, Y.; Liao, Q.; Lei, X.; Hu, X.; Zhou, L.; Zhang, Y. Untargeted Metabolomics Approach for the Discovery of Salinity-Related Alkaloids in a Stony Coral-Derived Fungus Aspergillus terreus. Int. J. Mol. Sci. 2024, 25, 10544. https://doi.org/10.3390/ijms251910544
Liu Y, Wang L, Feng Y, Liao Q, Lei X, Hu X, Zhou L, Zhang Y. Untargeted Metabolomics Approach for the Discovery of Salinity-Related Alkaloids in a Stony Coral-Derived Fungus Aspergillus terreus. International Journal of Molecular Sciences. 2024; 25(19):10544. https://doi.org/10.3390/ijms251910544
Chicago/Turabian StyleLiu, Yayue, Li Wang, Yunkai Feng, Qingnan Liao, Xiaoling Lei, Xueqiong Hu, Longjian Zhou, and Yi Zhang. 2024. "Untargeted Metabolomics Approach for the Discovery of Salinity-Related Alkaloids in a Stony Coral-Derived Fungus Aspergillus terreus" International Journal of Molecular Sciences 25, no. 19: 10544. https://doi.org/10.3390/ijms251910544
APA StyleLiu, Y., Wang, L., Feng, Y., Liao, Q., Lei, X., Hu, X., Zhou, L., & Zhang, Y. (2024). Untargeted Metabolomics Approach for the Discovery of Salinity-Related Alkaloids in a Stony Coral-Derived Fungus Aspergillus terreus. International Journal of Molecular Sciences, 25(19), 10544. https://doi.org/10.3390/ijms251910544





